Commentary - Anesthesiology and Clinical Science Research (2023) Volume 7, Issue 1
Clinical practice guideline on anaesthesia and perioperative blood transfusion management
Yilkal Tadesse Desta*
Department of Anaesthesia, Critical Care and Pain Medicine. Bahirdar University, Ethiopia
- Corresponding Author:
- Yilkal Tadesse Desta
Department of Anaesthesia Critical care and Pain Medicine
Bahirdar University, Bahirdar, Ethiopia
E-mail: destayilkal999@gmail.com
Received: 27-Dec-2022, Manuscript No. AAACSR-23-84386; Editor assigned: 30-Dec-2022, PreQC No. AAACSR-23-84386(PQ); Reviewed: 13-Jan-2023, QC No. AAACSR-23-84386; Revised: 18-Jan-2023, Manuscript No. AAACSR-23-84386(R); Published: 25-Jan-2023, DOI:10.35841/aaacsr-7.1.132
Citation: Desta YT. Clinical practice guideline on anaesthesia and perioperative blood transfusion management. Anaesthesiol Clin Sci Res. 2023;7(1):134
Abstract
Hemorrhage may account for a third of in-hospital deaths, particularly in the first 24 h after admission. In massive hemorrhage, mortality exceeds 50%, such figures emphasize that the importance of early recognition of major blood loss and the need for effective action to prevent shock and its consequences. Surgical control to arrest the cause of the hemorrhage is the main intervention, but even when surgery is performed aggressively, hemorrhage portends low survival, more over; Currently, the ideal transfusion ratios or models for transfusion and coagulation management of patients with hemorrhage are unclear or controversial. Additionally, the overall rate of appropriate use of blood was 40.7%; it was inappropriate in 19.2%of cases (hemoglobin>11 g dl±1). Therefore this review will address new information regarding trauma-induced coagulopathy, priorities for intervention, Anesthetics considerations and not to take extra risk comments for management of operative massive bleeding and traumatic patients. To obtain a systematically developed guideline statements that assists in decision?making about appropriate health care and safe use of intra-operative blood transfusion, depending on local circumstances and accessibility at the point of clinical activity. To examine sound and update scientific evidences, which are intended to supplement current resuscitation guidelines and are specifically directed at improving Anaesthetic management of pre-operative anemia, pre-operative hemorrhagic shock and peri-operative acute massive blood loss events. To adapt with discussions and recommendations on why to take extra risk and priorities for treatment, from those revised literatures and professional consensus. To take part in the Hospital Transfusion Committee members and facilitate the development of protocols for the management of safe transfusion and coagulopathies. To take in to account simple template guidelines for acute blood loss which may be modified with local circumstances and displayed in clinical areas.
Keywords
Anesthesia, Blood transfusion.
Introduction
Massive operative blood loss still represents a major challenge for the operating team members / Hemorrhagic shock accounts for 80% of deaths in the operating theatre and up to 50% of deaths in the first 24 h after injury, and still trauma is the leading cause of death in all ages from 1 to 44 years. Cornerstones of sufficient management are the early recognition of massive bleeding, the prompt initiation of a goal-directed therapy, the anticipation of forth- coming therapeutic requirements and efficient interdisciplinary communication to provide mandatory logistic resources because the management of massive hemorrhage is usually only one component of the management of a critically unwell patient. [1].
Elements and phases for the development of guidelines
I. Systemic review on different literatures via PICO
approach and on three phases;
a) Critical bleeding /massive transfusion
b) Medical and intensive care
c) Obstetric and paediatric/neonates
II. Clinical direction or steering provided by tutors and under guidance of my seniors.
III. Clinical /Consumer Reference Groups (CRG):- experts working and colleagues.
Clinical research/background questions and material for the level of evidences
Systemic review questions
Question 1: In patients with undergoing surgery what is the effect of a multidisciplinary, multimodal programmatic approach to perioperative patient blood management on patient outcome?
Question 2: In patients undergoing surgery what is the effect of perioperative strategies that minimise blood loss on morbidity, mortality and RBC transfusion?
Question 3: In patients undergoing surgery, is anaemia an independent risk factor for adverse outcomes?
Clinical guidance and some of Anaesthetic considerations
Recommendations-Perioperative Patient Blood Management
R1 C: Health-care services should establish a multidisciplinary, multimodal perioperative PBM program (Grade C). This should include preoperative optimization of red cell mass and coagulation status, meticulous attention to surgical homeostasis and minimization of perioperative blood loss.
Blood conservation strategies-Preoperative
Recommendation–Preoperative Autologous Donation (PAD)
R2 C The routine use of PAD is not recommended because,
it reduces the risk of allogeneic RBC transfusion, it increases
the risk of receiving any RBC transfusion (allogeneic and autologous) (C).
Blood conservation strategies-Intraoperative
Recommendation–Prevention of hypothermia
R2 A: In patients undergoing surgery measures to prevent hypothermia should be used (A).
Recommendation for Deliberate induced hypotension
R3 C: In patients undergoing radical prostatectomy or major joint replacement, if substantial blood loss is anticipated, deliberate induced hypotension (MAP 50-60 mmHg) should be considered, perfusion (C).
Recommendation–Acute Normovolaemic Haemodilution
(ANH)
R4 C: In adult patients undergoing surgery in which substantial blood loss is anticipated, the use of ANH should be considered (C).
Anaemia management
Recommendations–Preoperative anemia assessment
R5 C: In patients undergoing non-cardiac surgery, preoperative
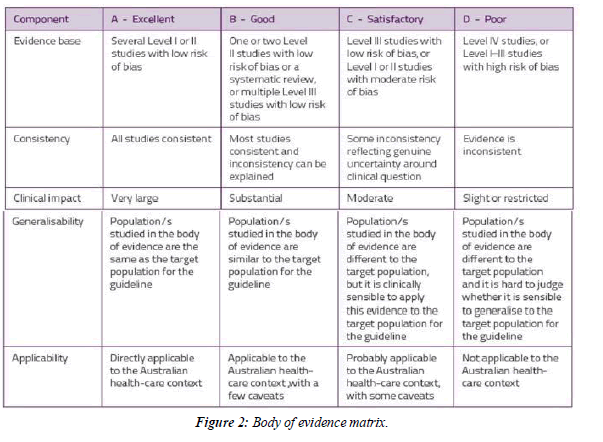
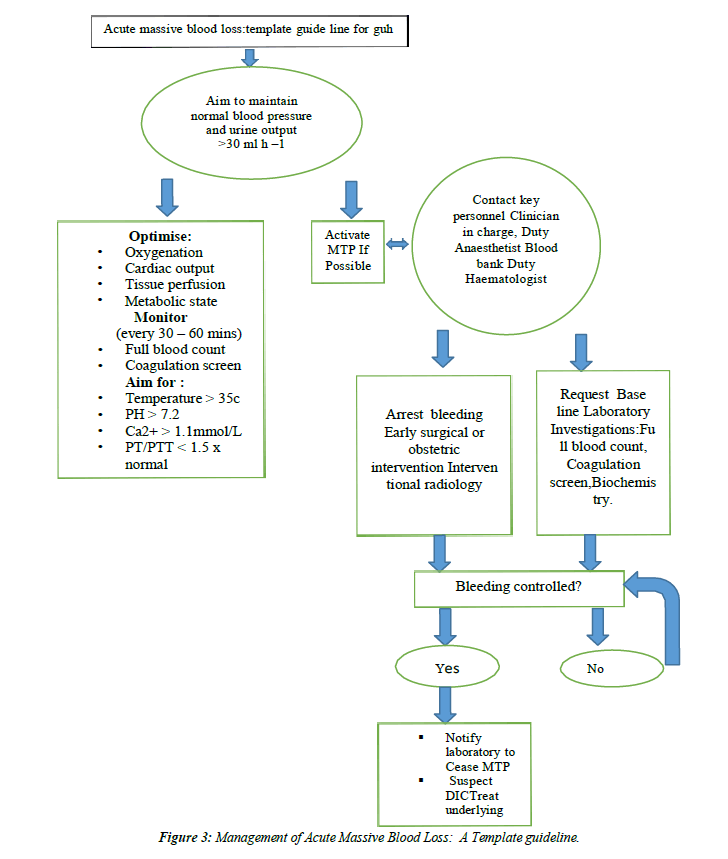
anaemia should be identified, evaluated & managed to minimize RBC transfusion, which may be associated with an increased risk of morbidity, mortality, ICU length of stay & hospital length of stay (C)(Figure 1,Figure 2 and Figure 3).
R6 C: RBC transfusion should not be dictated by a Hb ‘trigger’ alone, but should be based on assessment of the patient’s clinical status. In the absence of acute myocardial or cerebrovascular ischemia, postoperative transfusion may be inappropriate for patients with an Hb level of >80g/L.(Table 1,Table 2 and Table 3)
Some of Anaesthesiological techniques for saving blood
• Avoid hypertension and tachycardia.
• Avoid coughing, straining, and patient maneuvers.
• Controlled hypotension (a reduction of systolic blood pressure down to 80-90 mmHg, a reduction in mean blood pressure down to 50-65 mmHg, or a 30% reduction of the mean baseline blood pressure) can be obtained pharmacologically or by spinal or epidural anesthesia.
• Avoid intraoperative hypothermia
• Positioning /trendlenerg
• Vasoconstrictor infiltrations over the incision site
• Post-operative adequate analgesia
| Evidence statement- for perioperative patient blood management | Evidence | Consistency | Clinical-impact | Generalizability | Applicability |
|---|---|---|---|---|---|
| A multidisciplinary, multimodal programmatic approach to perioperative patient blood management is associated with a reduction in transfusion requirements on non-cardiac surgeries. | x/D | √√/B | √√/B | √√/B | √/C |
Table 1: Effect of perioperative patient blood management program.
| Evidence statement- Preoperative Autologus Donation | Evidence | Consistency | Clinical‑impact | Generalisablity | Applicablity |
|---|---|---|---|---|---|
| In adult patients undergoing surgery in which substantial blood loss is anticipated, PAD may reduce the volume of allogenic blood transfusion. | √/C | NA | √√/B | √/C | √√/B |
| In adult patients undergoing surgery in which substantial blood loss is anticipated, PAD reduces preoperative haemoglbin concentration. | √/C | √√/B | √/C | √√/B | √√/B |
| In adult patients undergoing surgery in which substantial blood loss is anticipated, PAD does not appear to have an effect on the overall volume of blood transfusion. | √/C | NA | √√/B | √/C | √√/B |
Table 2: Effect of perioperative strategies that minimize blood loss.
| Evidence statement- anaemia | Evidence | Consistency | Clinical‑impact | Generalizability | Applicablity |
|---|---|---|---|---|---|
| In patients undergoing non-cardiac surgery, preoperative anaemia is associated with an increased risk of postoperative morbidity and mortality. | √√/B | √√/B | √/C | √√/B | √/C |
| In patients undergoing non-cardiac surgery, preoperative anaemia is associated with an increased likelihood of transfusion and increased hospital length of stay. | √/C | √√√/A | √√/B | X | √/C |
| In patients undergoing non-cardiac surgery, postoperative anaemia is associated with an increased risk of postoperative morbidity and mortality. | √√/B | √√/B | √√/B | X | √/C |
| In patients undergoing non-cardiac surgery, postoperative anaemia is associated with an increased likelihood of transfusion | √/C | NA | √/C | √/C | √/C |
Table 3: Effect of anaemia assessment on outcomes.
Anaesthetic methods
• Volatile or total IV general anesthesia
• Neuraxial and other techniques c/w general anesthesia
• Type of ventilation.
Further considerations and controversies yet
The optimal amounts of plasma, platelet, cryoprecipitate, and other coagulation factors in relationship to the RBC transfusion volume are currently unknown, but current data support the use of plasma: RBC: platelet ratio of 1:1:1. Future prospective clinical trials will hopefully assist in continuing to improve the transfusion management of massively transfused patients with hemorrhage. Additionally there is a need for further studies to clarify these issues and provide firm evidence on which future recommendations can be based.
Conclusion
It is emphasized that, if avoidable deaths are to be prevented, Surgeons, Anesthetists, Hematologists and blood bank staff need to improve communications closely in order to achieve the goals of secure homeostasis, restoration of circulating volume, and effective management of blood loss replacement while avoiding for extra risks. Blood is a scarce resource. A critical approach to the use of blood is needed to minimize the risk to the patient from transfusion. The objective of blood transfusion, as specified by the ASA guidelines, is to improve inadequate oxygen delivery secondary to anemia.
In recent evidences, low hemoglobin was the primary trigger (72%). The other two common but inappropriate triggers were hypovolemia and the anesthetist's or surgeon's choice. Prevention of peri-operative hypothermia, controlled hypotension in experienced hands, and a reduction in central venous pressure (1-5 mmHg)/for liver resection/ can increasingly contribute to reducing intra-operative bleeding in selected patients. Now recommended criteria from recent ASA guidelines used to evaluate the rate of appropriate transfusion are hemoglobin greater than 8 g dl±1, hemoglobin greater than 10 g dl±1 in patients with medical co-morbidities and blood loss greater than 20% of blood volume when more than 1000 ml. On a recent study medical co-morbidities were found in 30 of the 140 patients transfused (21.4%). These patients had a significantly higher rate of appropriate transfusion of (80%) compared with the other patients (P<0.05, c2 test). Medical co morbidities such as coronary artery disease, renal dysfunction, left ventricular dysfunction, and chronic obstructive airway disease were recorded to have more appropriateness for transfusion. Operative blood loss estimation is subjective and often unreliable, because of inaccuracies in measurement from drains and swabs, inter-compartmental fluid shifts during surgery, and the delusional effects of crystalloid therapy. This can result in an overestimation of blood loss, provoking an excessive response. In studies, intraoperative hemoglobin estimation was found to be the more appropriate one /the primary trigger (72.1%)/transfusions in these patients were often appropriate, with a low incidence of inappropriate use (10.3%). Which can be made by; Objective laboratory Ixs for an intraoperative Hgb concentration. Or Subjective assessment for clinical sign and symptoms of adequate tissue oxygenation, & hemoglobin concentration (stable/unstable);
i.e. In the presence of adequate cardiorespiratory function, there is evidence that a hemoglobin concentration of 9±10 g dl±1 improves capillary perfusion, reduces viscosity and improves tissue oxygenation. A hemoglobin level of 8 g dl±1 seems an appropriate threshold for transfusion in surgical patients with no risk of ischemia, whereas a threshold of 10 g dl±1 can be just increased for patients who have compromised cardiorespiratory function. In massive hemorrhage, mortality exceeds 50%. Coagulopathy after hemorrhage is thought to be a secondary event because of depletion and dilution of coagulation factors however recently its more likely from acute trauma induced coagulopathies, therefore early MTP protocols should be considered. In selected patients, allogenic transfusion complications can be reduced by autologous blood transfusion, intraoperative blood salvage, or intraoperative isovolaemic haemo-dilution. A Massive Transfusion Protocol (MTP) is necessary in treating the massively hemorrhaging patient undergoing massive transfusion. To mitigate the lethal triad of acidosis, hypothermia, and coagulopathy; and the two goals of the MTP should be; Earlier and more aggressive transfusion intervention and Resuscitation with blood components that approximate whole blood as a part of damage control resuscitation. But inappropriate use of blood squanders a limited resource, causes unwanted side effects, and raises the cost of patient care. Inappropriate transfusions can be prevented by using strict preset criteria for triggering homologous blood administration, encouragement in Hospital Transfusion Committee members for formulating audits and seminars and also to take in to account simple template guidelines which can be displayed in clinical areas of concerns.
References
- Feltracco P, Brezzi ML, Barbieri S, et al. Blood loss, predictors of bleeding, transfusion practice and strategies of blood cell salvaging during liver transplantation. World J Hepatol. 2013;5(1):1.
- Lin FQ, Li C, Zhang LJ, et al. Effect of rapid plasma volume expansion during anesthesia induction on haemodynamics and oxygen balance in patients undergoing gastrointestinal surgery. Int J Medical Sci. 2013;10(4):355.
- Stainsby D, MacLennan S, Hamilton PJ. Management of massive blood loss: a template guideline. Br J Anaesth. 2000;85(3):487-91.
- Sisak K, Manolis M, Hardy BM, et al. Epidemiology of acute transfusions in major orthopaedic trauma. J Orthop Trauma. 2013;27(7):413-8.
- Banerjee M, Bouillon B, Shafizadeh S, et al. Epidemiology of extremity injuries in multiple trauma patients. Injury. 2013;44(8):1015-21.
- Barbosa Neto JO, Moraes MF, Nani RS, et al. Hemostatic resuscitation in traumatic hemorrhagic shock: case report. Rev Bras Anestesiol. 2013;63:103-6.
- Lockey DJ, Weaver AE, Davies GE. Practical translation of hemorrhage control techniques to the civilian trauma scene. Transfusion. 2013;53:17S-22S.
- Bonnet MP, Deneux‐Tharaux C, Dupont C, et al. Transfusion practices in postpartum hemorrhage: a population‐based study. Acta Obstet Gynecol Scand. 2013;92(4):404-13.
- Abdelmalak BB, Cata JP, Bonilla A, et al. Intraoperative tissue oxygenation and postoperative outcomes after major non-cardiac surgery: an observational study. Br J Anaesth. 2013;110(2):241-9.
- Arya VK. Basics of fluid and blood transfusion therapy in paediatric surgical patients. Indian J Anaesth. 2012;56(5):454.
- Amr YM, Amin SM. Effects of preoperative β-blocker on blood loss and blood transfusion during spinal surgeries with sodium nitroprusside–controlled hypotension. Saudi J Anaesth. 2012;6(3):263.
- Nielsen K, Dahl B, Johansson PI, et al. Intraoperative transfusion threshold and tissue oxygenation: a randomised trial. Transfus Med. 2012;22(6):418-25.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref