- Biomedical Research (2015) Volume 26, Issue 1
Clinical Evaluation of Intra-Arterial Thrombolysis Treatment of Wake-Up Stroke under CT Perfusion Imaging Guidance.
Peng Wang1, Hong-Jian Guan2*, Zhi-Min Wang1 and Cheng-Hua Xu11Department of Neurology, Taizhou First People's Hospital, Taizhou 318020, China
2Department of Neurology, the Affiliated Hospital of Yanbian University Hospital, Yanbian 133000, China Peng Wang and Hong-Jian Guan are co-first authors
- *Corresponding Author:
- Hong-Jian Guan
Department of Neurology
The Affiliated Hospital of Yanbian University Hospital
No. 1327 Juzi Street, Yanbian 133000, China
Accepted date: November 14 2014
Abstract
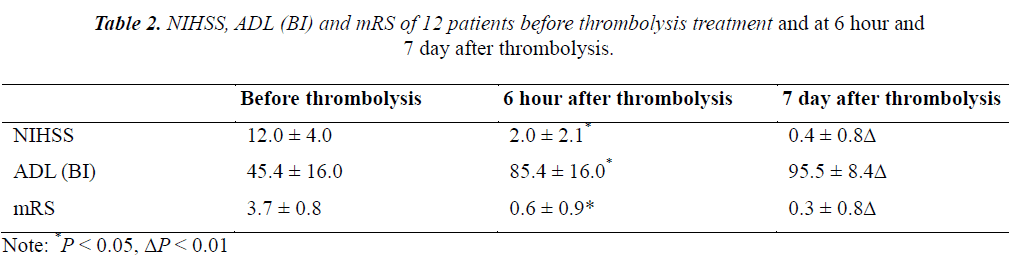
This study aims to assess the efficiency and safety of intra-arterial thrombolysis treatment for wake-up stroke under the supervision of CT perfusion (CTP). 12 wake-up stroke patients fit for intra-arterial thrombolysis were checked with CTP before thrombolysis treatment; cranial CTP was checked after treatment. Neural function defect scores (NIHSS), Barthel Index (BI) of activity of daily life (ADL) and mRS scores were evaluated at 6 hour and 7 day after thrombolysis treatment. Results found that, the cranial CT at 24 hour after thrombolysis showed HI-1 type hemorrhage in 2 patients and HI-2 hemorrhage in 1 patient. No PH-1 or PH-2 hemorrhage was observed. At 7 day after thrombolysis the hypoperfusion was greatly improved. Before thrombolysis, the NIHSS, ADL (BI) and mRS of 12 patients were 12.0 ± 4.0, 45.4 ± 16.0 and 3.7 ± 0.8, respectively. At 6 hour after thrombolysis, the NIHSS, ADL (BI) and mRS were 2.0 ± 2.1, 85.4 ± 16.0 and 0.6 ± 0.9, respectively, which were significantly different with before thrombolysis (P < 0.05). At 7 day after thrombolysis, the NIHSS, ADL (BI) and mRS of 12 patients were 0.4 ± 0.8, 95.5 ± 8.4 and 0.3 ± 0.8, respectively, which were highly significantly different with before thrombolysis (P < 0.01). CTP can be applied for intra-arterial thrombolysis treatment of wake-up stroke, with definite efficacy and safety.
Keywords
Cerebral stroke, intra-arterial thrombolysis treatment, CT perfusion imaging
Introduction
Cerebral stroke is a common global neural system disease, and 67% to 80% of cerebral stroke cases are acute ischemic cerebral vascular diseases. Thrombolysis treatment is one of the effective treatment measures for acute cerebral infarction. Some patients with acute cerebral infarction present the wake-up symptom, also known as wake-up stroke. These patients have no thrombolysis treatment time window under the criterion of “normal appearance”. However, there is a possible thrombolysis time window for partial wake-up stroke cases. A previous study found that, there was no significant difference of safety or clinical prognosis between wake-up stroke patients treated with thrombolysis and treated with tissue plasminogen activator (t-PA) within 3 hours [1].
In that study, 61% cases received intravenous thrombolysis, 30% received intra-arterial thrombolysis, and 9% received combined intravenous and intra-arterial thrombolysis. Up to 4.3% cases presented symptomatic cerebral haemorrhage and the mortality rate increased by 15%. That study contained comparisons of different intraarterial and intravenous thrombolysis with intravenous thrombolysis, and lacked consistency. In addition, the incidence of haemorrhage was higher, whereas the concurrent symptomatic intracranial haemorrhage and the time window of partial cases were actually out of the thrombolysis treatment range. Computed tomography perfusion (CTP) can provide a large area of ischemia perfusion abnormalities in transient ischemic attacks. An early CTP of a large area of infarction was able to identify the perfusion abnormality, evaluate the ischemic penumbra [2], and guide thrombolysis. Since 2009, our hospital has been conducting cranial CTP screening for patients with wake-up stroke, complying with the overall thrombolysis time window and carrying out evaluations through cerebral blood flow, cerebral blood volume, and mean transit time. Intra-arterial thrombolysis was conducted for cases that met the prepared thrombolysis criteria. This study investigates whether CTP can accurately evaluate ischemic penumbra to reduce symptomatic haemorrhage risk. Moreover, treatment efficiency and safety of intra-arterial thrombolysis for wake-up stroke are evaluated.
Materials and Methods
General data
A total of 12 patients (6 males and 6 females; average age of 67 ± 11 years) with wake-up stroke acute cerebral infarction treated in the neurology department of Taizhou First People's Hospital (Taizhou, China) and Affiliated Hospital of Yanbian University Hospital (Yanbian, China) from January 2009 to August 2009 were enrolled in this study. Their clinical manifestations all met the diagnostic criteria of total anterior circulation infarction by the Oxfordshire Community StrokeProject (OCSP-TACI). Their NIHSS scores fell between 7 and 22 points. All patients were hospitalized within 3.5 h after symptoms. The exclusion criteria: 1) more than 80 years; 2) atrial fibrillation and a history of warfarin administration; 3) a history of diabetes or random blood sugar > 11.1 mmol/l; 4) cerebral hemorrhage, tumor, and signs of early cerebral infarction by cranial CT; and 5) ASPEPCTS score ≥ 7. Emergent cervical artery ultrasonography, cardiac color ultrasound, and electrocardiogram examination for baselines such as trial of Org 10172 in acute stroke treatment (TOAS); large-artery atherosclerosis (LAA), cardioembolism (CE), small-artery occlusion lacunar (SAA), acute stroke of other determined etiology (SOE), and stroke of other undetermined etiology (SUE) types, Barthel Index (BI), and modified ranking scale (mRS) scores were performed.
CT perfusion imaging examination
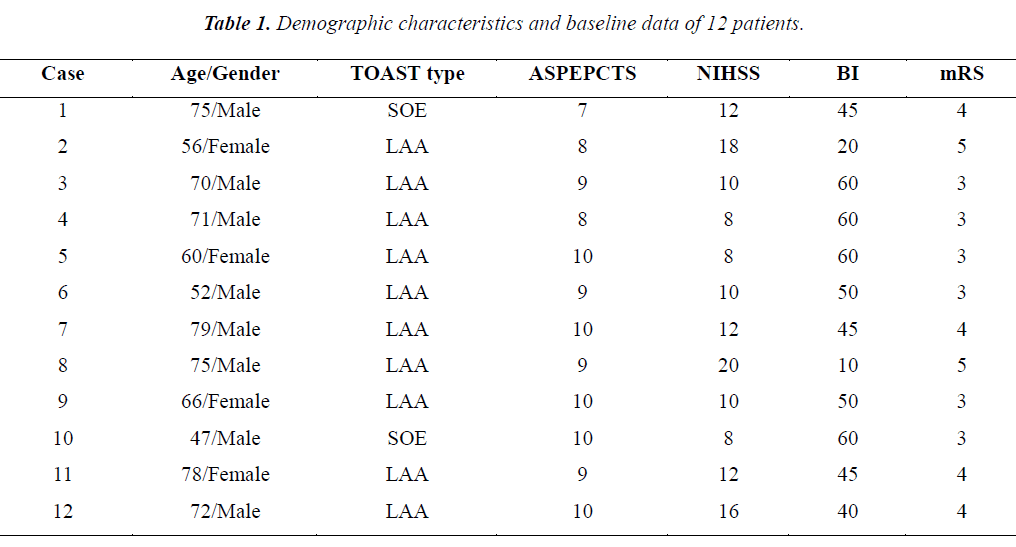
A GE lightspeed ultra eight-layer Spiral CT machine was used to carry out routine plain scanning. The interest layer of CTP dynamic scanning was identified based on the CT plain scanning results to conduct same-layer dynamic scanning. The scanning parameters are as follows: type, cine full 1.0 s; thickness, 5 mm/4i; KV, 80; mA, 200; total exposure time, 50 s; extension time, 5 s; contrast medium, 49 ml of 300 mgI/ml Iohexol; flow rate, 4 ml/s. Synchronous dynamic CT axial scanning was conducted for the interest layer after the contrast medium was injected for 5 s. Anterior cerebral artery or middle cerebral artery and longitudinal sinuses on the same layer were then taken as control to obtain the time density curve (TDC). The CT 2 perfusion software (deconvolution algorithm) of the AW 4.1 image workstation was used for image processing after CT perfusion to obtain CT perfusion images of the corresponding parameters and to symmetrically measure cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) of the bilateral corresponding areas.
According to the relevant research conclusions, haemorrhage risk caused by thrombolysis treatment greatly increased for infarction of over 1/3 of the middle cerebral artery supply area [3]. CT perfusion research confirmed the existence of ischemic penumbra when CBF>CBV. MTT extension indicated ischemic hypoperfusion. Therefore, preparation of the screening criteria of intravenous thrombolysis treatment for cases with MTT extension, normal CBV or CBV<1/3CBF, and ratio of bilateral CBF raging from 0.2 to 0.35 is necessary. The outcomes were evaluated by two associate chief physicians at the department of radiology. Only if their results were found consistent, thrombolysis could then be performed.
Treatment method
Patients meeting the criteria of wake-up stroke acute cerebral infarction were subjected to emergent cerebral digital subtraction angiograph (DSA) for responsible artery identification. For local thrombosis without complete occlusion (TIMI II), micro guide wire perfusion of alteplase (5 mg) was performed. For a completely-occluded arterys, a micro guide wire was used to damage the blood clots. If micro guide wire angiography demonstrated residual stenosis < 70% after occluded artery recanalization, surgery was stopped. However, if the residual stenosis was more than 70%, intracranial Wingspan stenting and extracranial Abbott Vascular ACCLINK stenting were performed. If the micro guide wire successfully destroyed blood blots but angiography showed the occluded artery was not recanalized, PTA balloon catheter technique was employed. In a case that the micro guide wire could not pass through the thrombus or of over 6 h after disease occurrence, the operation attempt was given up. If patients exhibited severe headache or extravasation of contrast media during thrombolysis, thrombolysis was stopped immediately and cranial CT was then performed for review. For patients subjected to intraoperative stenting, 100 mg of aspirin, 300 mg of clopidogrel, and 40 mg of atorvastatin were administered. For those not subjected to such a treatment, 100 mg of aspirin, 300 mg of clopidogrel, and 40 mg of atorvastatin were administered 6 h after operation. Then, 100 mg of aspirin and 75 mg of clopidogrel were given for seven successive days. After that, the medication was changed into 100 mg of aspirin or 75 mg of clopidogrel combined with 40 mg of atorvastatin. For patients with blood pressure > 185/105 mmHg, urapidil was intravenously pumped for controlled blood pressure of 140/90 mmHg. The middle cerebral artery (TCD) was monitored during operation. If the mean blood flow velocity in the TCD increased by 100% compared with that before operation, blood pressure was continuously reduced until a level of 120/80 mmHg was reached.
Evaluation criteria and observation indicators
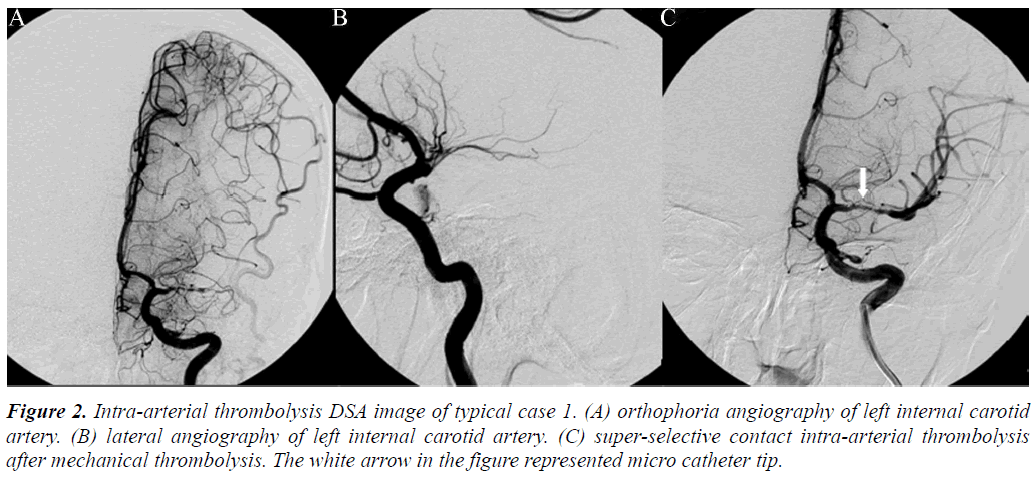
Neural function defect scores (NIHSS) and BI were evaluated at 6 hour and 7 day after thrombolysis. At 3 months, NIHSS, BI, and mRS were evaluated. Cranial CT examination was conducted within 24 h after thrombolysis to confirm whether cerebral haemorrhage occurred. Mucocutaneous and visceral haemorrhage situations were also recorded. Moreover, CTP intracranial perfusion situations after 7 d were evaluated.
Statistical analysis
Data were expressed as mean±SD. Statistical analysis was performed using SPSS 12.0 statistical software. F-test was performed for analyzing the measurement data, and SNKq test was used for multiple comparisons. P < 0.05 was considered as statistically significant.
Results
General data
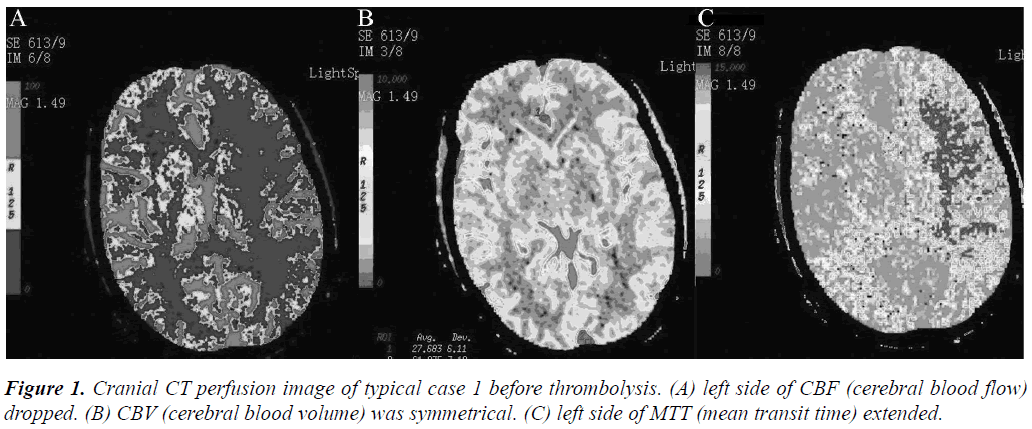
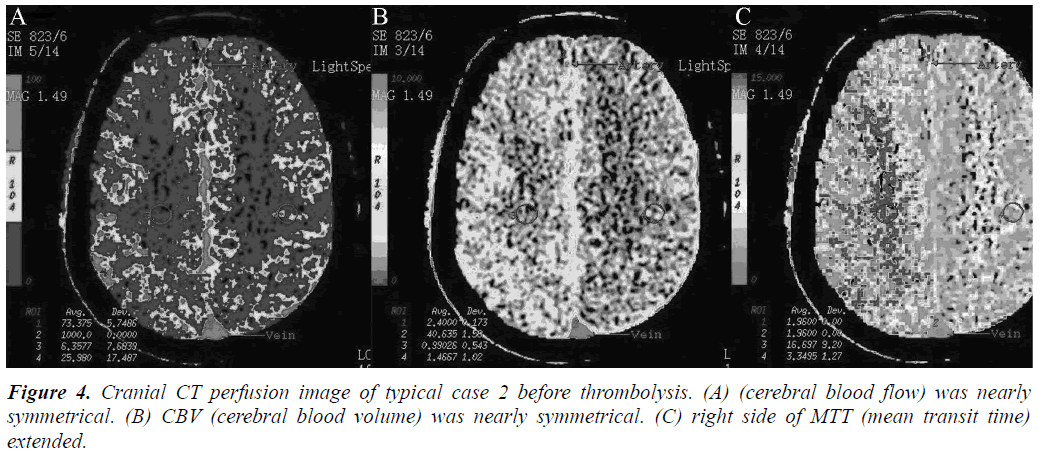
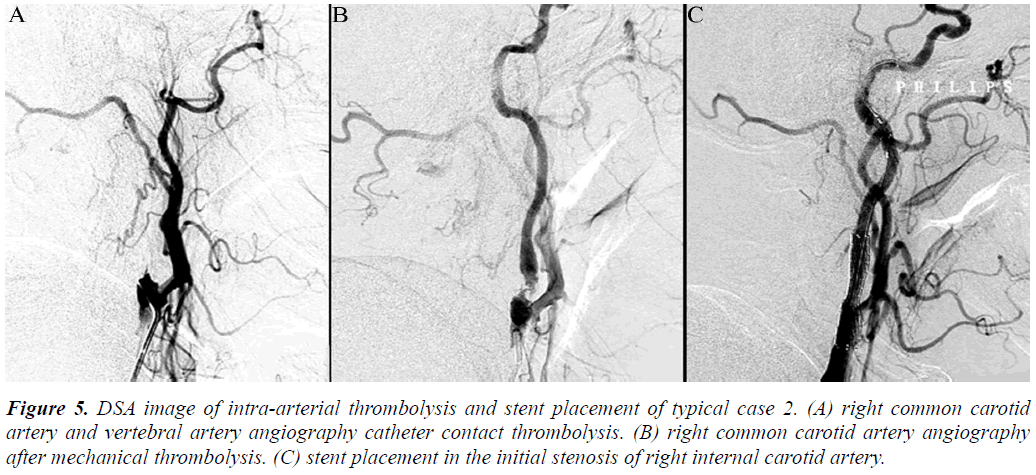
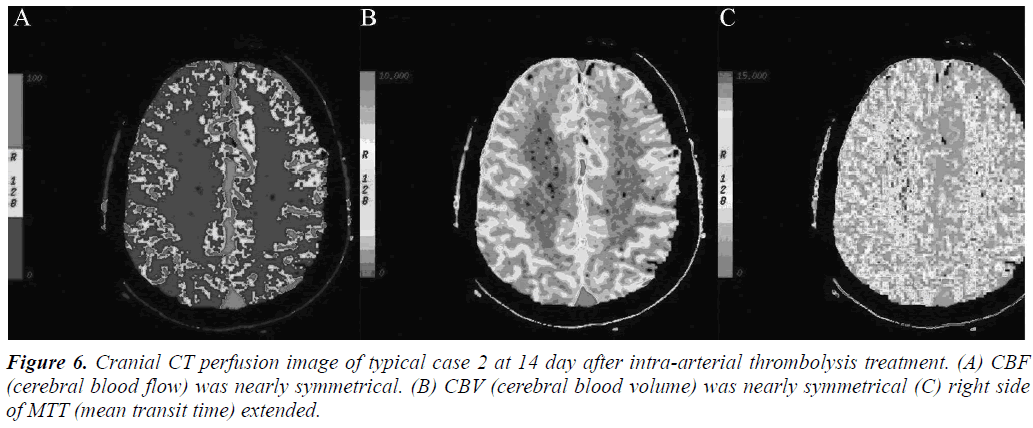
Arterial thrombolysis was performed for 12 patients. The demographic characteristics and baseline of 12 patients were shown in Table 1. Among these patients, 7 had the responsible artery at the TCD M1 segment, 3 at the TCD M2 segment, 1 at the C1 segment of the internal carotid artery initial, and 1 at the C2 segment. Arterial recanalization by t-PA perfusion thrombolysis was done for 4 patients, micro guide wires for thrombosis destruction for 10, PTA catheter dilation for 5, Wingspan for 2, and ACCLINK stenting with the employment of RX Accunet protection system for 1. The anterior and posterior cranial CTP images and DSA contrastographic pictures of two patients are shown in (Figures 1-6). Cranial CT at 24 hour after thrombolysis showed HI-1 type hemorrhage in 2 patients, HI-2 hemorrhage in 1, gingival hemorrhage in 1, and subcutaneous ecchymosis in 1. No PH-1 or -2 occurred. No contrast allergy during examination as well as within the following one week after the examination occurred. Cranial CT was rechecked 7 day later and the result showed that the hypoperfusion was greatly improved.
Figure 2: Intra-arterial thrombolysis DSA image of typical case 1. (A) orthophoria angiography of left internal carotid artery. (B) lateral angiography of left internal carotid artery. (C) super-selective contact intra-arterial thrombolysis after mechanical thrombolysis. The white arrow in the figure represented micro catheter tip.
Figure 5: DSA image of intra-arterial thrombolysis and stent placement of typical case 2. (A) right common carotid artery and vertebral artery angiography catheter contact thrombolysis. (B) right common carotid artery angiography after mechanical thrombolysis. (C) stent placement in the initial stenosis of right internal carotid artery.
Changes of NIHSS, ADL (BI) and mRS
Before thrombolysis, the NIHSS, ADL (BI) and mRS of 12 patients were 12.0 ± 4.0, 45.4 ± 16.0 and 3.7 ± 0.8, respectively. At 6 hour after thrombolysis, the NIHSS, ADL (BI) and mRS were 2.0 ± 2.1, 85.4 ± 16.0 and 0.6 ± 0.9, respectively, which were significantly different with before thrombolysis (P < 0.05). At 7 day after thrombolysis, the NIHSS, ADL (BI) and mRS of 12 patients were 0.4 ± 0.8, 95.5 ± 8.4 and 0.3 ± 0.8, respectively, which were highly significantly different with before thrombolysis (P < 0.01) (Table 2).
Discussion
Studies confirm that the symptoms of 16% to 28% patients with acute cerebral infarction are seen after waking up [4-9], and the overall incidence time of this part of the case is still within the thrombolysis treatment time window. Therefore, thrombolysis treatment is useful for the patients. However, thrombolysis will increase haemorrhage complication risk if the overall incidence time is out of the range of the thrombolysis treatment time window. Thus, selecting an appropriate evaluation method to assess cases that comply with thrombolysis is important. Our preliminary thrombolysis treatment was decided according to the time window and the clinical symptoms, without considering the intracranial hemodynamic changes and assessing the presence of ischemic penumbra, which was incomprehensive [8]. All current structural imaging methods, such as CT and MRI, cannot reliably describe the progress of ischemic damage at the initial 6 h to 12 h; they require a special image description system. Before morphological changes of acute cerebral ischemia, CT perfusion is an important method to find the location, scope, and extent of the ischemic lesion of early cerebral infarction [10-15]. CT perfusion can show acute cerebral ischemic lesion at an early stage and quantitatively analyse the cerebral blood flow perfusion. The mechanism of ischemic brain damage is complex and cerebrovascular bases, ischemic tolerance extents, and collateral circulation situations of different individuals are different; thus, treatment time windows are different [16-18].
Conclusion
Our preliminary study shows that the twelve cases all presented ischemic penumbra confirmed by preoperative CTP evaluation. After opening the occluded arteries, the NIHSS, BI, or mRS score indicated that a good prognosis was obtained and no severe complications, such as intracranial haemorrhage, occurred. This study showed that CT perfusion screening can reduce the ratio of hyperperfusion-caused intracranial haemorrhage complication. Therefore, CT perfusion imaging is a safe and effective method that can be used to examine patients with acute cerebral infarction wake-up stroke before thrombolysis. Studies reported that the other indicators of CT perfusion (MTT, TTP) were more sensitive to ischemia. In two cases listed in this study, CBF and CBV did not exhibit obvious change and MTT presented asymmetry, indicating super-early ischemia. Our study on individual cases showed that CTP evaluation of wake-up stroke is feasible and intraarterial thrombolysis treatment of wake-up stroke is safe and effective. Although this study only focused on individual cases, it is a preliminary test of larger samples, defining the best evaluation method and thrombolytic regimen for largesample size multicenter studies.
References
- Barreto AD, Martin-Schild S, Hallevi H, Morales MM, Abraham AT, Gonzales NR, Illoh K, Grotta JC, Savitz SI. Thrombolytic therapy for patients who wake-up with stroke. Stroke 2009; 40: 827-832.
- Gasparotti R, Grassi M, Mardighian D, Frigerio M, Pavia M, Liserre R, Magoni M, Mascaro L, Padovani A, Pezzini A. Perfusion CT in patients with acute ischemic stroke treated with intra-arterial thrombolysis: predictive value of infarct core size on clinical outcome. AJNR Am J Neuroradiol 2009; 30: 722-727.
- Singer OC, Kurre W, Humpich MC, Lorenz MW, Kastrup A, Liebeskind DS, Thomalla G, Fiehler J, Berkefeld J, Neumann-Haefelin T. MR Stroke Study Group Investigators: Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI-ASPECTS. Stroke 2009; 40: 2743-2748.
- Cortijo E, Calleja AI, Garcia-Bermejo P, Perez-Fernandez S, Del Monte JM, Tellez N, Campos-Blanco DM, Garcia-Porrero MA, Fernandez-Herranz MR, Arenillas-Lara JF. Perfusion computed tomography makes it possible to overcome important SITS-MOST exclusion criteria for the endovenous thrombolysis of cerebral infarction. Rev Neurol 2012; 54: 271-276.
- Kang DW, Kwon JY, Kwon SU, Kim JS. Wake-up or unclear-onset strokes: are they waking up to the world of thrombolysis therapy? Int J Stroke 2012; 7: 311-320.
- Kuruvilla A, Norris GM, Xavier AR. Acute endovascular recanalization therapy in wake-up stroke. J Neurol Sci 2011; 300: 148-150.
- Natarajan SK, Snyder KV, Siddiqui AH, Ionita CC, Hopkins LN, Levy EI. Safety and effectiveness of endovascular therapy after 8 hours of acute ischemic stroke onset and wake-up strokes. Stroke 2009; 40: 3269-3274.
- Roveri L, La Gioia S, Ghidinelli C, Anzalone N, De Filippis C, Comi G. Wake-up stroke within 3 hours of symptom awareness: imaging and clinical features compared to standard recombinant tissue plasminogen activator treated stroke. J Stroke Cerebrovasc Dis 2013; 22: 703-708.
- Silva GS, Lima FO, Camargo EC, Smith WS, Singhal AB, Greer DM, Ay H, Lev MH, Harris GJ, Halpern EF, Sonni S, Koroshetz W, Furie KL. Wake-up stroke: clinical and neuroimaging characteristics. Cerebrovasc Dis 2010; 29: 336-342.
- Ferreira RM, Lev MH, Goldmakher GV, Kamalian S, Schaefer PW, Furie KL, Gonzalez RG, Sanelli PC. Arterial input function placement for accurate CT perfusion map construction in acute stroke. AJR Am J Roentgenol 2010; 194: 1330-1336.
- Hopyan J, Ciarallo A, Dowlatshahi D, Howard P, John V, Yeung R, Zhang L, Kim J, MacFarlane G, Lee TY, Aviv RI. Certainty of stroke diagnosis: incremental benefit with CT perfusion over noncontrast CT and CT angiography. Radiology 2010; 255: 142-153.
- Inoue M, Mlynash M, Straka M, Lansberg MG, Zaharchuk G, Bammer R, Albers GW. Patients with the malignant profile within 3 hours of symptom onset have very poor outcomes after intravenous tissue-type plasminogen activator therapy. Stroke 2012; 43: 2494-2496.
- Payabvash S, Souza LC, Kamalian S, Wang Y, Passanese J, Kamalian S, Fung SH, Halpern EF, Schaefer PW, Gonzalez RG, Furie KL, Lev MH. Location-weighted CTP analysis predicts early motor improvement in stroke: A preliminary study. Neurology 2012; 78: 1853-1859.
- Todo K, Moriwaki H, Saito K, Tanaka M, Oe H, Naritomi H. Early CT findings in unknown-onset and wake-up strokes. Cerebrovasc Dis 2006; 21: 367-371.
- Wang XC, Gao PY, Xue J, Liu GR, Ma L. Identification of infarct core and penumbra in acute stroke using CT perfusion source images. AJNR Am J Neuroradiol 2010; 31: 34-39.
- García-Bermejo P, Calleja AI, Pérez-Fernández S, Cortijo E, del Monte JM, García-Porrero M, Fe Muñoz M, Fernández-Herranz R, Arenillas JF. Perfusion computed tomography-guided intravenous thrombolysis for acute ischemic stroke beyond 4.5 hours: A case-control study. Cerebrovasc Dis 2012; 34: 31-37.
- Saake M, Goelitz P, Struffert T, Breuer L, Volbers B, Doerfler A, Kloska S. Comparison of conventional CTA and volume perfusion CTA in evaluation of cerebral arterial vasculature in acute stroke. AJNR Am J Neuroradiol 2012; 33: 2068-2073.
- Wang XC, Gao PY, Lin Y, Ma L, Guan-ruiLiu, Xue J, Sui BB, Wang CJ, Wang YJ. Clinical value of computed tomography perfusion source images in acute stroke. Neurol Res 2009; 31: 1079-1083.