Research Article - Biomedical Research (2022) Volume 33, Issue 6
Clinical diagnostic value of static CT myocardial perfusion for ischemia with non-obstructive coronary artery disease.
Shihao Yan1, Deyue Yan1, Xiaoshuang Che2, Chenglin Xu1, Baijie Li1, Qigan Xia1, Xiaoyan Wang1, Yubo Tian1, Hairong Yu1, Xiaomei Luan3, Peiji Song1*, Huating Wang1
1Central Hospital Affiliated to Shandong First Medical University, Jinan City, Shandong Province, China
2Liaocheng People's Hospital, Liaocheng City, Shandong Province, China
3Weifang Medical College, Weifang City, Shandong Province, China
- Corresponding Author:
- Peiji Song
Central Hospital Affiliated to Shandong First Medical University
Jinan City
Shandong Province
China
Accepted date: August 11, 2022
Abstract
Background: Currently coronary anomalies mainly rely on anatomical examination (coronary angiography, CAG and coronary CTA, CCTA), but it cannot observe arteries with an inner diameter of <300 um, such as, Ischemia with Non-Obstructive Coronary Artery Disease (INOCA), resulting in insufficient diagnosis and treatment. So, we used static CT Myocardial Perfusion Imaging (sCTMPI) and CAG to evaluate the distribution and diagnosis of ischemic lesions of INOCA patients and test their consistency.
Methods: 35patients with INOCA received sCTMPI, CCTA and CAG. Taking the Right Coronary Artery (RCA), Left Anterior Descending (LAD), and Left Circumflex Artery (LCX) for the target vessel, analyzed all patient images with target blood vessels and vascular perfusion areas in sCTMPI, CAG and CCTA. The iodine content and CT value parameters of the resting myocardial perfusion defect area were determined. Statistical analysis was performed for the CCTA abnormality is determined according to the ischemic area of the coronary artery blood supply and compared with the CAG.
Results: There were 172 segments of myocardial ischemia in 35 patients with INOCA received sCTMP. CCTA assessment of stenosis changes were 73 branches in LAD, LCX and RCA, where lesions were found by sCTMPI. while CAG 77 branches, the Kappa value between CCTA and CAG was 0.478, 0.943, 0.935 respectively.
Conclusion: The relationship between the degree of coronary artery stenosis and myocardial ischemia was nonlinear. Patients with suspected myocardial ischemia-related symptoms, even if the degree of CCTA stenosis is less than 50%, should undergo further sCTMPI to exclude the possibility of INOCA.
Keywords
Coronary angiography, Coronary CTA, Ischemia with non-obstructive coronary artery disease, Static CT myocardial perfusion imaging.
Abbreviation
AHA: American Heart Association; LAD: Left Anterior Descending; LCX: Left Circumflex Artery; RCA: Right Coronary Artery; sCTMPI: static CT Myocardial Perfusion Imaging; INOCA: Ischemia with Non-Obstructive Coronary Artery Disease; CCTA: Coronary Computed Tomography Angiography; CAG: Coronary Angiography.
Introduction
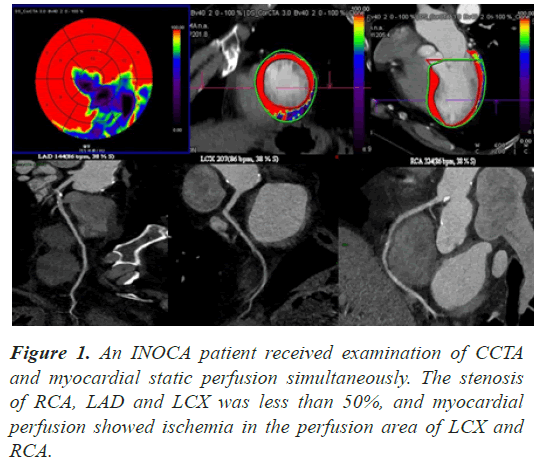
Angina is a common symptom of coronary heart disease, mostly caused by restrictive-flow ischemic heart disease, and in patients with angina, about 10-30% of patients shows normal or non-obstructive coronary arteries on angiography [1-3]. These diseases, in which blood vessels stenosis by less than 50% and are associated with ischemia, are known clinically as Ischemia with Non- Obstructive Coronary Artery Disease (INOCA)[4]. The most important diagnostic technique for ischemic heart disease is the reliance on invasive Coronary Angiography (CAG) with noninvasive Coronary CTA(CCTA)[3]. At present, CAG is the gold standard for diagnosing coronary heart disease [3], CCTA is now close to maturity [5]. But CAG and CCTA could be used for anatomical evaluation of coronary artery disease to determine whether the three major coronary arteries are obstructed, while it lacks sensibility for small blood vessels (inner diameter <300 um) [6]. These examinations have great limitations for the diagnosis of INOCA patients. However, functional examination has a unique advantage in terms of detection rate of INOCA, sCTMPI and CAG were used to evaluate the distribution of ischemic lesions and diagnosis INOCA patients and test their consistency (Figure 1).
Materials and Methods
General materials
This study protocol was approved by the Ethics Committee of our Hospital. Written informed consent was obtained from all participants. All total of 401 patients, from April 1, 2019 to April 1, 2021 in XXX Hospital, who received CCTA and sCTMPI at the same time were enrolled. 35 patients who received CAG within one week and were diagnosed with INOCA were selected, including 16 males and 19 females. Age ranged from 50 to 82 years, with an average of 66 ± 3.2 years. Inclusion criteria: (1) Symptoms of myocardial ischemia, (2) ECG showed ST-T changes, (3) CCTA and CAG showed mild stenosis or stenosis <50%. Exclusion criteria: (1) hypertrophic cardiomyopathy, (2) severe valvular heart disease, (3) severe hypertension or hypotension, systolic blood pressure >180 mmHg or <90 mmHg, (4) severe arrhythmia, (5) patients with critical condition or important organ failure, such as: severe infection, liver and kidney failure, etc. (6) spasm diseases of the airway, such as: asthma, (7) no recent use of theophylline drugs, (8) severe allergic reactions.
Examination method
CCTA+sCTMPI: Preparation before examination: sign informed consent, heart rate should be controlled, if heart rate over 90 bpm, metoprolol (dosage depends on product specification) should be taken. The non-ionic contrast medium iopromide (370 mgI/ml) was used at a flow rate of 5.0 ml/s via the anterior elbow vein, and the double barrel high-pressure syringe was used for injection, followed by 30 ml of normal saline to reduce right ventricular contrast agent artifacts. The patient is taken in a supine position with advanced feet and arms raised and straight, so as not to bend and cause the blood vessels at the injection site to rupture. Electrocardiogram (ECG) monitoring connection, reconstructing ECG gating images. Do breathe-holding training and radiation protection.
Scanning range: 1-2 cm below the upper tracheal rumble, down to the cardiac diaphragm level, left and right bilateral larger than the heart margin 10-20 mm. The voltage is 120 KV, the tube power supply is 300 mAS, the speed of spherical tube rotation is 0.33 s/rpm, the detector acquisition is 32 × 0.625 mm, the pitch is 0.2, the reconstruction function is B25f, the thickness of the reconstruction layer is 0.6 mm, and the distance between layers is 0.6 mm.
Coronary angiography
The double antiplatelet drugs are applied before surgery, the patient is taken to the supine position, and the patient is locally anesthetized through the right radial artery. The needle is punctured through the radial artery, and the pulsatile blood flow is obtained and sent in Duct wire, then insert 6F arterial sheath, Heparin injection was injected at 70-100 U/kg, 6F JL4, JR4 catheters are sent to the left and right coronary artery openings, Bolus Contrast Agent Classic Myron 400, Multi-position projection (head, right shoulder, liver, foot, spider, left shoulder, left front oblique, head). Images were acquired using Philips digital X-ray angiography machines (PHILIPS, Allura Xper FD10). After the operation, there was an electronic computer imaging sheet, which successfully compressed the blood vessels with radial artery compression devices and ECG monitoring closely monitored changes in vital signs.
Image analysis
Two consultant radiologists independently analyzed all patient images with target blood vessels and vascular perfusion areas in sCTMPI, CAG and CCTA. At rest 2.5 mg/mL is the optimal threshold to distinguish between diseased myocardium and normal myocardium (AUC 1.00).
Statistical analysis
All statistical tests were performed using the SPSS v23.0 statistical software package. The diagnostic agreement of INOCA between sCTMPI and CAG was evaluated by kappa test. Kappa values were interpreted as follows: values 0 as no agreement; 0.01 to 0.20 as none to slight; 0.21 to 0.40 as fair; 0.41 to 0.60 as moderate; 0.61 to 0.80 as substantial; and 0.81 to 1.00 as almost perfect agreement. Results were considered significant when P<0.05.
Results
Among the 35 patients, the degree of coronary artery stenosis was less than 50%. In sCTMPI, bull's eye diagram of myocardial blood supply at 595 segments was analyzed according to AHA17 segment segmentation method, and 172 segments of myocardium were found to be ischemic (Table 1). In the ischemic segment of area supplied by LAD, ischemic areas were mainly distributed in 7, 8, 13, 17 segments. LCX and RCA were mainly distributed in 6, 12, 16 segments and 3, 9, 10 segments respectively. LAD was dominant in all ischemic segments.
| Coronary | LAD |
LCX | RCA | Total |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Segment | 1 | 2 | 7 | 8 | 13 | 14 | 17 | 5 | 6 | 11 | 12 | 16 | 3 | 4 | 9 | 10 | 15 | |
| Normal | 35 | 35 | 6 | 31 | 3 | 35 | 25 | 33 | 17 | 34 | 31 | 18 | 13 | 32 | 30 | 12 | 33 | 423 |
| Abnormal | 0 | 0 | 29 | 4 | 32 | 0 | 10 | 2 | 18 | 1 | 4 | 17 | 22 | 3 | 5 | 23 | 2 | 172 |
| Total | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 595 |
Table 1. The findings of 17 segments (AHA) of myocardial ischemia which blood supply of LAD, LCX and RCA on sCTMPI examination among 35 cases of the INOCA.
In 35 patients, there were 32 abnormal arteries in the LAD and 18 arteries in LCX for CCTA examination (Table 2). CAG was similar to CCTA, LAD had a maximum of 34 arteries, and LCX had a minimum of 19 arteries (Table 3).
| LAD | LCX | RCA | Total | |
|---|---|---|---|---|
Normal |
3 | 17 | 12 | 32 |
Abnormal |
32 | 18 | 23 | 73 |
Total |
35 | 35 | 35 | 105 |
Table 2. CCTA Assessment of stenosis changes at lad, lcx and rca, where lesions were found by sCTMPI.
| LAD | LCX | RCA | Total | |
|---|---|---|---|---|
Normal |
1 | 16 | 11 | 28 |
Abnormal |
34 | 19 | 24 | 77 |
Total |
35 | 35 | 35 | 105 |
Table 3. CAG Assessment of stenosis changes at LAD, LCX and RCA among 35 cases of the INOCA.
Three coronary arteries received CCTA and CAG. LAD, LCX and RCA were analyzed separately, and Kappa value of LAD was 0.478 (P<0.05), which was moderately correlated. Kappa values were greater than 0.9 in both LCX and RCA (P<0.05), which are regarded as almost perfect agreement (Table 4).
| No. of cases | (%) | No. of cases | (%) | Kappa | P | ||
|---|---|---|---|---|---|---|---|
| LAD | CCTA | 3 | 0.086 | 32 | 0.914 | 0.478 | <0.05 |
| CAG | 1 | 0.029 | 34 | 0.971 | |||
| LCX | CCTA | 17 | 0.486 | 18 | 0.514 | 0.943 | <0.05 |
| CAG | 16 | 0.457 | 19 | 0.543 | |||
| RCA | CCTA | 12 | 0.343 | 23 | 0.657 | 0.935 | <0.05 |
| CAG | 11 | 0.314 | 24 | 0.686 |
Table 4. Kappa test for the evaluation of the diagnosis agreement between CCTA and CAG Changes at the LAD, LCX, and RCA.
Discussion
Based on the results of this study, we found that the stenosis degree of coronary artery was non-linear in relation to myocardial ischemia. Patients with myocardial ischemia could be well performed in myocardial perfusion images [7].
Advances in computed tomography of the heart have made it possible to perform, in addition to obtaining conventional anatomical results of coronary arteries, CCTA can also be functional studied of coronary artery microcirculation [8]. Previous studies show that only 40% of CAGs are positive when there is evidence of myocardial ischemia in patients with suspected coronary heart disease, and there is a mild stenosis, lesions of single and small branches of the arteries, the diagnostic sensitivity is lower and it is easier to miss the diagnosis [9,10]. Among our study 401 patients with ischemia, 35 patients were found to have coronary artery stenosis by CAG examination, and the positive rate was significantly lower than 40%.
The advantage of qualitative assessment of myocardial perfusion is that it could visually detect the location and extent of myocardial ischemia using grayscale and color images, reflects myocardial microvascular perfusion and cardiomyocyte activity, improves the diagnostic accuracy of stenosis and ischemia [11,12]. We could detect ischemic areas if these lesions were small and confined to the subendocardial region. sCTMPI has an advantage over CCTA and CAG in assessing myocardial ischemia in all three coronary artery supplied regions, including RCA, LAD, and LCX [13]. In patients with clinically significant assessment of coronary circulatory function, sCTMPI may be performed at the same time as diagnostic CCTA [12]. Expert consensus suggests that sCTMPI should be carried out for patients at high risk of obstructive coronary artery disease, including stenosis of uncertain functional significance patient [3].
In this study, three major coronary arteries and their blood supplied areas were analyzed according to American Heart Association standards [14]. The results showed, LAD ischemia areas were mainly distributed in the 7 and 13 segments, while LCX in the 6 and 16 segments and RCA in the 3 and 10 segments. The current test results are consistent with the Cardiology Association Standard Guidelines, our results of studies demonstrated that coronary artery feeding myocardium lesion areas are consistent with lesions in all blood supply areas following abnormal coronary arteries [15]. Contrary to the standard, the major ischemic areas were uneven distribution and mostly distributed in specific areas, which may be related to the distribution of coronary arteries and blood supply.
In the UK, current National Institute for Health and Care Excellence(NICE) guidelines recommend the use of CCTA as a first-line test for angina patients (before any functional testing),[16] but patients with INOCA are likely to be missed by this anatomical examination approach. The latest European Society of Cardiology (ESC) guidelines for the management of chronic coronary syndrome indicate that sCTMPI should be determined by the physician [3], so sCTMPI combined with CCTA is necessary for the initial diagnosis of INOCA patients [17]. This study revealing, in a total of 105 in target coronary arteries, including LAD, LCX and RCA, there are 77 branches stenosis, and the positive rate of abnormal arteries is about 73%. Most blood vessels have some degree of coronary atherosclerosis, consistent with previous studies [18,19].
There are some limitations in our study. Firstly, the sample size is small. Secondly, the prevalence of coronary artery spasm in patients with INOCA is 3% to 95%, which was ignored in this study [20]. Thirdly, there is some overlap between patients with INOCA and patients with MINOCA, which was not distinguished in detail. In the future study, we are planning to establish animal models with coronary arteries stenosis less than 50% to confirm appearance of myocardial ischemia.
Conclusion
INOCA image manifestation: the stenosis degree of coronary artery is nonlinear with myocardial ischemia. Patients with suspected myocardial ischemia-related symptoms, even if degree of CCTA stenosis is less than 50%, should undergo further myocardial perfusion functional examination to exclude the possibility of INOCA, so as to avoid misdiagnosis and missed diagnosis. The combination of CCTA and sCTMPI is one of the commonly used noninvasive methods to effectively exclude epicardial coronary artery disease and evaluate microvascular diastolic function.
Acknowledgement
HTW, PJS contributed to the study conception and design. SHY, DYY, HRY, XSC, CLX, BJL, XML performed the Material preparation, data collection and analysis. SHY was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Sources of Funding
This work was supported by the Jinan Clinical Medical Science and Technology Innovation Program Project [202019133]; the Jinan Central Hospital's 2020 first-batch research fund for introducing talents [YJRC2020005]; the Jinan Government 5150 Project for Innovative Talents.
Conflict of Interest
None to report
References
- Timmis A, Townsend N, Gale CP, Torbica A, Lettino M. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur Heart J 2020; 41: 12-85.
[Crossref] [Google Scholar] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax J. Fourth Universal Definition of Myocardial Infarction. Circulation 2018; 138: e618-e51.
[Crossref] [Google Scholar] [PubMed]
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41: 407-477.
[Crossref] [Google Scholar] [PubMed]
- Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017; 135: 1075-1092.
[Crossref] [Google Scholar] [PubMed]
- Muscogiuri G, Van Assen M, Tesche C, De Cecco CN, Chiesa M. Artificial Intelligence in Coronary Computed Tomography Angiography: From Anatomy to Prognosis. Biomed Res Int 2020; 2020: 6649410.
[Crossref] [Google Scholar] [PubMed]
- Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology and Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J 2020; 41: 3504-3520.
[Crossref] [Google Scholar] [PubMed]
- Takx RAP, Celeng C, Schoepf UJ. CT myocardial perfusion imaging: ready for prime time?. Eur Radiol 2018; 28: 1253-1256.
[Crossref] [Google Scholar] [PubMed]
- Caruso D, Schoepf UJ, Zerunian M, Eid M. CT myocardial perfusion: state of the science. Minerva Cardioangiol 2017; 65: 252-264.
[Crossref] [Google Scholar] [PubMed]
- Sucato V, Novo S, Manno G, Coppola G, Caronna N. Ischemia with no obstructive coronary artery disease: microvascular angina and vasospastic angina. G Ital Cardiol (Rome) 2020; 21: 954-960.
[Crossref] [Google Scholar] [PubMed]
- Schuijf JD, Matheson MB, Ostovaneh MR, Arbab-Zadeh A, Kofoed KF. Ischemia and No Obstructive Stenosis (INOCA) at CT Angiography, CT Myocardial Perfusion, Invasive Coronary Angiography, and SPECT: The CORE320 Study. Radiology 2020; 294: 61-73.
[Crossref] [Google Scholar] [PubMed]
- Varga-Szemes A, Meinel FG, De Cecco CN, Fuller SR, Bayer RR. CT myocardial perfusion imaging. AJR Am J Roentgenol 2015; 204: 487-497.
- Branch KR, Haley RD, Bittencourt MS, Patel AR, Hulten E. Myocardial computed tomography perfusion. Cardiovasc Diagn Ther 2017; 7: 452-462.
[Crossref] [Google Scholar] [PubMed]
- Kono T, Uetani T, Inoue K, Nagai T, Nishimura K. Diagnostic accuracy of stress myocardial computed tomography perfusion imaging to detect myocardial ischemia: a comparison with coronary flow velocity reserve derived from transthoracic Doppler echocardiography. J Cardiol 2020; 76: 251-258.
[Crossref] [Google Scholar] [PubMed]
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002; 105: 539-542.
[Crossref] [Google Scholar] [PubMed]
- Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2018; 72: e91-e220.
[Crossref] [Google Scholar] [PubMed]
- Carrabba N, Berteotti M, Taborchi G, Ciatti F, Acquafresca M. Integration of CTA in the Diagnostic Workup of New Onset Chest Pain in Clinical Practice. Biomed Res Int 2019; 2019: 2647079.
[Crossref] [Google Scholar] [PubMed]
- Seitun S, De Lorenzi C, Cademartiri F, Buscaglia A, Travaglio N. CT Myocardial Perfusion Imaging: A New Frontier in Cardiac Imaging. Biomed Res Int 2018; 2018: 7295460.
[Crossref] [Google Scholar] [PubMed]
- Pelliccia F, Pasceri V, Niccoli G, Tanzilli G, Speciale G. Predictors of Mortality in Myocardial Infarction and Nonobstructed Coronary Arteries: A Systematic Review and Meta-Regression. Am J Med 2020; 133: 73-83.
[Crossref] [Google Scholar] [PubMed]
- Zandecki L, Janion-Sadowska A, Kurzawski J, Piatek L, Zabojszcz M. Clinical presentation and 3-year outcomes of patients with acute coronary syndromes and non-obstructive coronary arteries on angiography. PLoS One 2020; 15: e0234735.
[Crossref] [Google Scholar] [PubMed]
- Scalone G, Niccoli G, Crea F. Editor's Choice- Pathophysiology, diagnosis and management of MINOCA: An update. Eur Heart J Acute Cardiovasc Care 2019; 8: 54-62.
[Crossref] [Google Scholar] [PubMed]