Research Article - Current Pediatric Research (2021) Volume 25, Issue 9
Clinical characteristics and outcomes of Multisystem Inflammatory Syndrome in Children (MIS-C): A national multicenter cohort in Saudi Arabia.
Samah Al-Harbi1*, Yasser M Kazzaz2, Mohammed Shahab Uddin3, Fidaa Maghrabi1, Abeer A Alnajjar1, Mohammed Muzaffer1, Bayan M Alnahdi1, Shimaa M Abusaif1, Abdulrahman Alhejaili4, Reham Aljohnei4, Seham Arishi5, Nabil Dhayhi5, Adeeb Khawaji6, Mahmoud Abdulla Majeed6, Eyad Albenayan6, Hanin Alsini6, Israa Baeasa6, Entesar A Batouk7Maha Azzam7, Roah A Merdad8, Ali Alshehri2, Wafaa Alsewairi2, Abdulrhman Alrasheed2, Musaed Alharbi2, Amal Ahmed2, Fayhan Alrogi2, Jubran Alqanatish2
1 Department of Pediatrics, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
2 Department of Pediatrics, King Abdullah Specialist Children’s Hospital, College of Medicine, King Saud Bin Abdulaziz University for Health Sciences; King Abdullah, International Medical Research Center, Riyadh, Saudi Arabia
3 Department of Pediatrics, Ministry of National Guard Health Affairs, Dammam, Saudi Arabia
4 Department of Pediatrics, Maternity and Children Hospital, Madinah, Saudi Arabia
5 Department of Pediatrics, King Fahad Central Hospital, Jizan, Saudi Arabia
6 Department of Pediatrics, King Fahd Armed Forces Hospital, Jeddah, Saudi Arabia
7 Department of Pediatrics, King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Jeddah, Saudi Arabia
8 Department of Community Medicine, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
- Corresponding Author:
- Samah Al-Harbi
Department of Pediatrics
King Abdulaziz University
Jeddah
Kingdom of Saudi Arabia
E-mail: smhalharbi@kau.edu.sa
Accepted date: 17th September, 2021
Abstract
Background: In children, Coronavirus Disease 2019 (COVID-19) is usually mild. However, children can be seriously impacted in rare situations, and clinical manifestations may differ from those seen in adults. One rare consequence associated with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in children is a Multisystem Inflammatory Syndrome (MIS-C). We sought to describe the clinical, laboratory and radiological characteristics, as well as the outcomes of children with MISC. Methods: A multicenter, retrospective cohort study was conducted in seven pediatric intensive care units spanning four regions in Saudi Arabia, from April to December 2020. Patients under 14 years of age who met MIS-C diagnostic criteria were included consecutively. Results: Among 54 patients, 55.6% were boys, 40.7% were 2-5 years old, and 75.9% were Arab. Only four (7.4%) had some comorbidity. The median BMI was 15.6 kg/m2. Contact with another COVID-19 case was reported by 31.5%, and 87% had recent SARS-CoV-2 infection confirmed by RTPCR. Serology was performed in 53.7%, but only positive in three patients (5.3%). Gastrointestinal symptoms were present in 63%. Severe respiratory symptoms were apparent in 48%, but 92.6% of the patients had an abnormal chest X-ray and 83.3% had abnormal echocardiographic findings. Almost all patients (92.6%) received immunoglobulin, but only 37% needed invasive mechanical ventilation, with a median duration of ventilation-free days of 28 days (IQR 9.75-28). The median duration of the PICU stay was seven days, during which nine deaths occurred (16.7%). Conclusion: Most of the current MIS-C patients had characteristics similar to other, previously reported cohorts. Several factors, we believe, played a role in the higher than expected rate of mortality, including high PRISM scores and presentation with acute COVID19 symptoms in many patients, and most being under five years old. There also is no standardized national protocol for MISC therapy in Saudi Arabia.
Keywords
Coronavirus disease 2019-COVID-19, Multi-system inflammatory syndrome in children, PICU, SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2, Children, Saudi Arabia.
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infections is reported less commonly in children than adults. To date, laboratory-confirmed SARS-CoV-2 is estimated to have occurred in 2%-5% of individuals less than 18 years old [1]. The World Health Organization (WHO) dashboard states that children under 14 years old account for 0.2% of all COVID-19 cases [2]. In Saudi Arabia, children’s are 14 years and younger account for 4.8% of all COVID-19 infections [3]. As of June 28th, 2021, the number of patients meeting the MIS-C case definition in the United States, as reported by the Centers for Disease Control (CDC), exceeded 4,500, with 41 deaths [4]. In children, SARS-CoV-2 infection exhibits marked clinical heterogeneity that spans from asymptomatic or mild to severe, life-threatening disease [5-7].
Early in the pandemic, the cumulative COVID-19-associated hospitalization rate among those <18 years old in 14 states across the USA was 8.0 per 100,000 [8]. The first report of severely ill children, describing hyper inflammatory shock and multi organ involvement mimicking Kawasaki disease shock syndrome, came from London, UK, in May 2020, published to alert the pediatric healthcare community about this severe phenotype in children [9]. Subsequently, studies conducted in Europe and the USA have characterized a COVID-19-linked syndrome labeled Multisystem Inflammatory Syndrome in Children (MIS-C) [9,10]. Several systematic reviews have now been published describing MIS-C. One systematic review summarized epidemiological and clinical data from 68 studies, encompassing 953 patients diagnosed with MIS-C [11].
Fever, gastrointestinal, and cardiovascular symptoms were the prominent manifestations, and the mortality rate was 1.9%. Another systematic review from the United States pooled 662 patients with MIS-C who presented predominantly with fever and gastrointestinal symptoms and had a mortality rate of 1.7% [12]. In Saudi Arabia, few studies have described COVID-19 in children. The largest Saudi Arabian cohort of COVID-19 in children reported on 742 patients, but only five among this cohort were diagnosed with MIS-C, all admitted to PICU [13]. Another multi-center study described 88 children who required hospitalization due to COVID-19, among whom 8% needed ICU admission; among the four mortalities, three were diagnosed with MIS-C [14].
Ten patients diagnosed with MIS-C less than 14 years of age were characterized from two centers in the eastern province; all had fever, gastrointestinal symptoms, and shock and were admitted to an ICU; two died [15]. In another case series of five prospectively enrolled MIS-C patients, one died [16]. As knowledge regarding MIS-C continues to evolve, we conducted this multicenter cohort study to describe the clinical characteristics, laboratory findings, and outcomes of children diagnosed with COVID-19-related MIS-C spanning several different healthcare systems in Saudi Arabia.
Materials and Methods
Study design
This was an observational, multicenter retrospective cohort study conducted in seven tertiary care hospitals in Saudi Arabia between April 1st and December 31st, 2020. All children younger than 14 years of age who were admitted to Pediatric Intensive Care Units (PICUs) and met the Centers for Disease Control and prevention (CDC) case definition for MIS-C were included consecutively. The CDC case definition for MIS-C includes: (1) Fever >38.0 C0 for more than 24 hours; (2) Laboratory evidence of inflammation, including an elevated C-Reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR), fibrinogen, procalcitonin, d-dimer, ferritin, Lactic Acid Dehydrogenase (LDH), or Interleukin 6 (IL-6); elevated neutrophils, reduced lymphocytes, or low albumin; (3) No plausible alternative diagnosis; and (4) Current or recent SARS-CoV-2 infection diagnosed by a positive Reverse Transcription Polymerase Chain Reaction (RT-PCR) or positive serological test (IgM, IgG or IgA), or exposure to a suspected or confirmed COVID-19 case within the four weeks prior to the onset of symptoms [17].
Data collection
Demographic data and clinical characteristics including exposure history; severity illness score (PRISM IV); comorbid conditions; disease severity; admission weight, height, and Body Mass Index (BMI), expressed as an age-adjusted percentile; and radiological, laboratory, treatment, and outcome data were collected retrospectively using electronic case report forms [18]. Laboratory data of interest included inflammatory markers C-Reactive Protein (CRP); Erythrocyte Sedimentation Rate (ESR); procalcitonin; ferritin and markers of cardiac disease troponin and Brain Natriuretic Peptide (BNP). Electro Cardiographic (ECG) and echocardiographic findings also were collected. Clinical outcome indicators included mortality rate, Ventilation Free Days (VFDs), and Length of Hospital Stay (LOS) [19]. All the centers used a real-time Reverse Transcription Polymerase Chain Reaction (rRT-PCR) test for the qualitative detection of nucleic acid from SARS-COV-2 from upper and lower respiratory specimens (e.g., nasopharyngeal or oropharyngeal swabs, or bronchoalveolar lavage) to confirm COVID-19 infection.
Statistical analysis
Statistical analysis was performed using R statistical software version 4.03. Continuous variables were described as means with standard deviations if normally distributed or as medians with interquartile ranges (25th–75th percentile) if non-normally distributed. Categorical variables were described as counts and percentages. Patients who survived and patients who died were compared using Pearson X2 analysis for categorical data and Analysis of Variance (ANOVA) for continuous data. The in-hospital survival rate among children diagnosed with MIS-C was estimated using a Kaplan Meier curve. A two-tailed p-value <0.05 was considered statistically significant for all analyses.
Results
Enrolling the study population
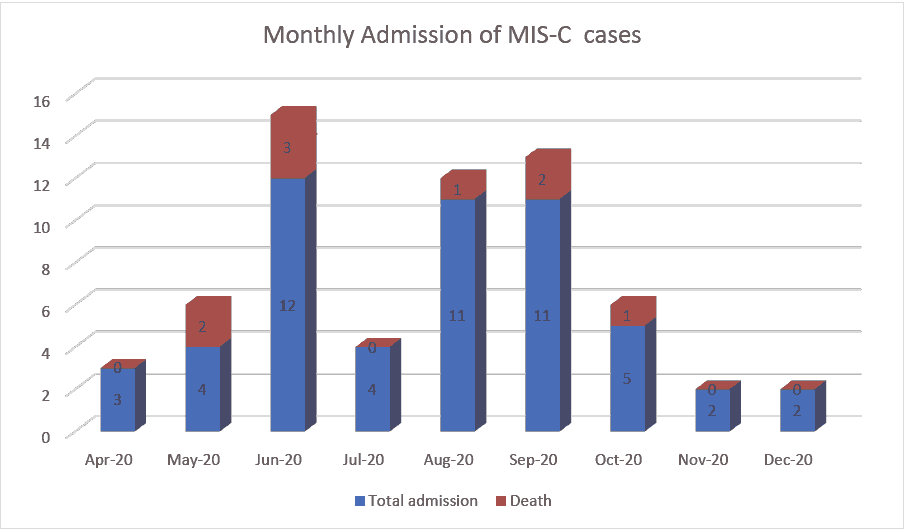
During the study period, a total of 54 children diagnosed with MIS-C were enrolled. The largest number of patients was recruited at King Abdullah Specialist Children’s Hospital in Riyadh (KASCH, n=16, 29.3% of the sample), followed by Maternity and Children’s Hospital in Madinah (MCH, 14, 25.9%), king abdulaziz university hospital in Jeddah (KAUH, 13, 24.1%), and King Fahd Hospital in Jazan (KFHJ, 8, 14.1%). All the remaining centres enrolled one patient (1.9%) each in Figure 1.
Patient characteristics
There was almost equal gender distribution, with 30 males (55.6%). Sixteen patients (29.6%) were under 2 years old, while 22 (40.7%) were 2-5 years old, and 16 (29.6%) over 5. Two thirds of the patients (n=37, 68.5%) had previously been healthy with no comorbidity. The most common comorbidities were neuromuscular and cardiovascular diseases, each present in four patients (7.4%). The median duration of symptoms was 5 days. The most common presentations were gastrointestinal in nature, followed by respiratory Table 1. Relative to survivors, patients who died had higher baseline PRISM scores (p<0.001) and more pre-existing comorbidities (p=0.047) and were more often of non-Arabic ethnicity (p=0.031).
| Demographic and clinical characteristics of patients stratified by outcome | |||||
|---|---|---|---|---|---|
| Total | Death | Survive | P value | ||
| N | 54 | 9 | 45 | ||
| Age (%) | <2 Years | 16 (29.6) | 2 (22.2) | 14 (31.1) | 0.867 |
| 2-5 Years | 22 (40.7) | 4 (44.4) | 18 (40.0) | ||
| >5 Years | 16 (29.6) | 3 (33.3) | 13 (28.9) | ||
| Sex (%) | Male | 30 (55.6) | 4 (44.4) | 26 (57.8) | 0.713 |
| Female | 24 (44.4) | 5 (55.6) | 19 (42.2) | ||
| BMI (median (IQR)) | 15.6 (12.8-17.9) | 13.70 (10.40, 14.87) | 16.00 (14, 18) | 0.128 | |
| Ethnicity (%) | Arab | 41 (75.9) | 4 (44.4) | 37 (82.2) | 0.031 |
| Black or African | 4 (7.4) | 1 (11.1) | 3 (6.7) | ||
| South Asian | 2 (3.7) | 0 (0.0) | 2 (4.4) | ||
| Southeast Asian | 3 (5.6) | 2 (22.2) | 1 (2.2) | ||
| West Asian | 4 (7.4) | 2 (22.2) | 2 (4.4) | ||
| Contact with SARS-CoV-2 (%) | 37(69) | 5 (55.6) | 32 (71.1) | 0.6 | |
| RT-PCR for SARs-CoV-2 (%) | Negative | 7 (13.0) | 2 (22.2) | 5 (11.1) | 0.717 |
| Positive | 47 (87.0) | 7 (77.8) | 40 (88.9) | ||
| SARS-CoV-2 serology (%) | Not available | 25 (46.3) | 6 (66.7) | 19 (42.2) | 0.215 |
| Negative | 26 (48.1) | 2 (22.2) | 24 (53.3) | ||
| Positive | 3 (5.6) | 1 (11.1) | 2 (4.4) | ||
| Co-Morbidities (%) | No co-morbidities | 37 (68.5) | 4 (44.4) | 33 (73.3) | 0.047 |
| Neuromuscular | 4 (7.4) | 1 (11.1) | 3 (6.7) | ||
| Heart disease | 4 (7.4) | 2 (22.2) | 2 (4.4) | ||
| Hematology | 2 (3.7) | 0 (0.0) | 2 (4.4) | ||
| Immunodeficiency or | 2 (3.7) | 2 (22.2) | 0 (0.0) | ||
| Malignancy or HIV | |||||
| Liver disease | 1 (1.9) | 0 (0.0) | 1 (2.2) | ||
| Renal disease | 1 (1.9) | 0 (0.0) | 1 (2.2) | ||
| Respiratory | 1 (1.9) | 0 (0.0) | 1 (2.2) | ||
| Other | 2(3.7) | 2 (4.4) | |||
| Clinical features | |||||
| Duration of illness on admission | 5(3,7) | 5.00 (3.00,6.00) | 5.00 (3.00,7.00) | 0.916 | |
| Median (IQR) | |||||
| Fever (%) | 54 (100.0) | 9 (100.0) | 45 (100.0) | NA | |
| Respiratory symptoms | |||||
| Shortness of breath (%) | 33 (61.1) | 8 (88.9) | 25 (55.6) | 0.134 | |
| Cough (%) | 26 (48.1) | 6 (66.7) | 20 (44.4) | 0.394 | |
| Sore throat (%) | 26 (48.1) | 3 (33.3) | 13 (28.9) | 1 | |
| Gastrointestinal symptoms | |||||
| Nausea (%) | 21 (38.9) | 2 (22.2) | 19 (42.2) | 0.454 | |
| Vomiting (%) | 29 (53.7) | 4 (44.4) | 25 (55.6) | 0.807 | |
| Diarrhea (%) | 34 (63.0) | 6 (66.7) | 28 (62.2) | 1 | |
| Abdominal pain (%) | 25 (46.3) | 3 (33.3) | 22 (48.9) | 0.625 | |
| CNS symptoms | |||||
| Headache (%) | 4 (7.4) | 0 (0.0) | 4 (8.9) | 0.816 | |
| Seizure (%) | 6 (11.3) | 1 (11.1) | 5 (11.4) | 1 | |
| Mucocutaneous | |||||
| Lymphadenopathy (%) | 8 (14.8) | 2 (22.2) | 6 (13.3) | 0.864 | |
| Rash (%) | 35 (64.8) | 6 (66.7) | 29 (64.4) | 1 | |
| Conjunctivitis (%) | 19 (35.2) | 3 (33.3) | 16 (35.6) | 1 | |
| Skin peeling (%) | 4 (7.4) | 0 (0.0) | 4 (8.9) | 0.816 | |
| PRISM score (Median (IQR)) | 14 (7,20) | 23 (20,25) | 10(5.50,16.0) | <0.001 | |
| Predicated mortality rate % | 8.20% | ||||
| Organ involvement | |||||
| Respiratory (%) | 42 (77.8) | 9 (100.0) | 33 (73.3) | 0.188 | |
| AKI (%) | 19 (35.2) | 8 (88.9) | 11 (24.4) | 0.001 | |
| DIC (%) | 17 (31.5) | 6 (66.7) | 11 (24.4) | 0.036 | |
| Cardiovascular (%) | |||||
| Arrythmia | 6 (11.1) | 4 (44.4) | 2 (4 . 4 ) | 0.004 | |
| Coronary artery lesion | 3 (5.6%) | 0 (0.00) | 3 (6.7%) | 0.344 | |
| Ventricular dysfunction | 42 (77.8) | 9 (100.0) | 33 (73.3) | 0.188 | |
| MOF (%) | 50 (92.6) | 9 (100.0) | 41 (91.1) | 0.816 | |
Table 1. Demographic, clinical characteristics of patients, and organ involvements stratified by outcome. PRISM: Pediatric Risk of Mortality Score; AKI: Acute Kidney Injury; DIC: Disseminated Intravascular Coagulation; MOF: Multiple Organ Failure.
Laboratory results
Laboratory assessments revealed no co-infections with other viruses. Roughly nine in ten patients had a positive SARS-CoV-2 (rRT-PCR) test (n=47, 87%). Among the 29 patients who had serology testing, only three were positive. Overall, patients had elevated levels of inflammatory markers, including procalcitonin (median=14.5, IQR: 6.7-27.5); CRP (median=39.5, 16-133.5); ESR (median=46.0, 6-64); and ferritin (median=900, 325.75-2207.25). Half of the patients had an increased neutrophile count (median=7.3, 4.19-9.28) associated with thrombocytopenia (median=149, 99.25-257.5]. In addition, coagulation profile results included elevated D-Dimer (median=4.66, 1.42-7.43); INR (median=1.29, 1.13-1.600), and fibrinogen (median=5.40, 2.66-80.0). Hypoalbuminemia was a common feature in the most critically ill patients (median=16, 14-21). Cardiac dysfunction markers were not significantly elevated (troponin median=0.50, 0.06-18.15; BNP median=62.50, 35.58-244.27). Reported echocardiographic findings were abnormal in 45 patients (83.3%), among them ventricular dysfunction in 42 (77.8%) and a coronary artery aneurysm in three patients (5.6%). Relative to survivors, patients who died developed worse Disseminated Intravascular Coagulation (DIC) and Acute Kidney Injury (AKI). In addition, procalcitonin, ferritin, LDH, D-dimer, INR, urea, and creatinine levels were significantly higher in patients who died (Tables 2 and 3).
| Lab Test | Overall | Death | Survival | P value |
|---|---|---|---|---|
| Number | 54 | 9 | 45 | |
| Hb (median (IQR)) (11.3-15.0 × 10^9/L | 10.35 (9,11.8) | 8.70 (7.90,11.80) | 10.5 (9.4,11.8) | 0.186 |
| WBC (median (IQR)) 4.00-12.00 × 10^9/L | 9.5 (6.61,11.93) | 11.6 (8.73,18.48) | 9.27 (6.54,11.0) | 0.291 |
| Neutrophil (median (IQR)) 1.10-7.2 × 10^9/L | 7.3 (4.19,9.28) | 9.23 (8.90,9.72) | 6.52 (3.72,9.17) | 0.032 |
| Lymphocyte (median (IQR)) 1.30-7.20 × 10^9/L | 1.8 (1.02,3.92) | 0.80 (0.70,2.53) | 1.94 (1.20,4.08) | 0.128 |
| Platelet (median (IQR)) 150-400 × 10^9/L | 149 (99.25, 257.5) | 90 (43., 104) | 178 (112, 264) | 0.002 |
| ESR (median (IQR)) 0-20 | 46 (6, 64) | 4.00 (2.00,22.00) | 49 (19.00,64.25) | 0.145 |
| CRP (median (IQR)) <1.2 mg/L | 39.5 (16,133.5) | 30 (11.0,129) | 48 (22,135) | 0.444 |
| Procalcitonin (median (IQR)) | 14.50 (6.70,27.45) | 67.0 (50, 100) | 12. (6, 22) | <0.001 |
| Ferritin (median (IQR)) 4.6-204 ug/L | 900.1 (325.75,2206.25) | 2304 (1926,3035) | 645 (314,1503) | 0.013 |
| LDH (median(IQR))125- 220 U/L | 400 (331,581.5) | 677 (409.0,1580) | 386 (326.0,535) | 0.007 |
| D.dimer (median (IQR)) 0.00-0.5 mg/L | 4.86 (1.70,7.74) | 14 (8.7,26) | 4.02 (1.3,7) | 0.001 |
| INR (median (IQR)) 0.8-1.2 | 1.29 (1.13,1.60) | 1.70 (1.29,2.38) | 1.23 (1.11,1.50) | 0.026 |
| Troponin (median (IQR)) <15.6 pg/L | 0.50 (0.06,19.45) | 2 (0.20,31) | 0.45 (0.05,16.12) | 0.293 |
| BNP (median (IQR)) >28.9 pmol/L | 60 (36.10,216) | 88. (55,348) | 57 (29.02,147.02) | 0.145 |
| Sodium (median (IQR)) 135-145 mmol/L | 136 (130.25,139.75) | 140 (124.0,146) | 136 (131,139) | 0.719 |
| Potassium (median (IQR)) 3.4-4.7 mmol/L | 3.9 (3.6,4.8) | 5 (3.90,5.5) | 3.8 (3.5,4.5) | 0.047 |
| Urea (median (IQR)) 2.5-6 mmol/L | 5 (3.25,12.2) | 14.7 (10.5,23) | 4.4 (3.0,9.7) | 0.002 |
| Creatinine (median (IQR)) 27-62 micmol/L | 47.5 (32,81.5) | 115 (64.0,216) | 44 (29.0,77) | 0.028 |
| Albumin (median (IQR)) 38-54 g/L | 27.5 (21,32) | 16 (14,21) | 30 (23,32.0) | <0.001 |
| ALT (median (IQR)) 5-55 U/L | 40.00 (24.25,90.75) | 34 (26,48) | 41 (24,91) | 0.508 |
Table 2. Laboratory result overall and stratified survival with death cohorts. WBC: White Blood Cells, CRP: C-Reactive Protein, LDH: Lactate Dehydrogenase, BNP: Brain-Natriuretic Peptide, ALT: Alanine Amino Transferase.
| Lab Test | Overall | Death | Survival | P value |
|---|---|---|---|---|
| Number | 54 | 9 | 45 | |
| Anemia (%) <11.3 × 10^9/L | 35 (64.8) | 6 (66.7) | 29 (64.4) | 1 |
| Leukocytosis (%) >12.00 × 10^9/L | 12 (22.2) | 3 (33.3) | 9 (20.0) | 0.661 |
| Neutrophilia (%) >7.2 × 10^9/L | 28 (51.9) | 8 (88.9) | 20 (44.4) | 0.038 |
| Lymphopenia (%) <1.30 × 10^9/L | 17 (31.5) | 5 (55.6) | 12 (26.7) | 0.19 |
| Thrombocytopenia(%) <150 × 10^9/L | 27 (50.0) | 9 (100.0) | 18 (40.0) | 0.003 |
| High ESR (%) >20 | 41 (75.9) | 6 (66.7) | 35 (77.8) | 0.776 |
| High CRP (%) >1.2 mg/L | 52 (96.3) | 8 (88.9) | 44 (97.8) | 0.747 |
| High procalcitonin (%) | 51 (94.4) | 9 (100.0) | 42 (93.3) | 1 |
| High ferritin (%) >204 ug/L | 47 (87.0) | 9 (100.0) | 38 (84.4) | 0.469 |
| High LDH (%) >220 U/L | 50 (92.6) | 9 (100.0) | 41 (91.1) | 0.816 |
| High D-dimer (%) >0.5 mg/L | 53 (98.1) | 9 (100.0) | 44 (97.8) | 1 |
| High INR (%) >1.2 | 31 (57.4) | 8 (88.9) | 23 (51.1) | 0.085 |
| High troponin (%) >15.6 pg/L | 15 (29.4) | 4 (44.4) | 11 (26.2) | 0.492 |
| High BNP (%) >28.9 pmol/L | 44(81.5) | 9 (100.0) | 35(77.8) | 0.252 |
| Hyponatremia (%) <135 mmol/L | 23 (42.6) | 4 (44.4) | 19 (42.2) | 1 |
| Hyperkalemia (%) >4.7 mmol/L | 15 (27.8) | 5 (55.6) | 10 (22.2) | 0.103 |
| High urea (%) >6 mmol/L | 26 (48.1) | 8 (88.9) | 18 (40.0) | 0.021 |
| High creatinine (%) >62 micmol/L | 25 (46.3) | 7 (77.8) | 18 (40.0) | 0.088 |
| Low albumin (%) <38 g/L | 27 (50.0) | 9 (100.0) | 18 (40.0) | 0.003 |
Table 3. Abnormal Laboratory result, overall and stratified with survival with death cohorts. LDH: Lactate Dehydrogenase, BNP: Brain-Natriuretic Peptide.
Clinical course, treatment, and outcomes
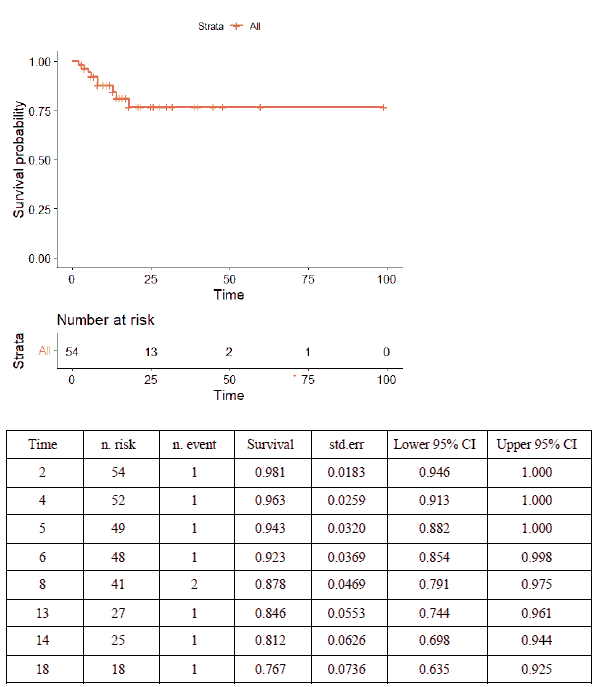
Almost all patients received intravenous immunoglobulin (n=50, 92.6%), with the immunomodulatory medications anakinra or tocilizumab administered to 17 (31.5%) and eight patients (14.8%), respectively. Aspirin and low molecular weight heparin were used in 27 (50%) and 16 (29.6%) patients, respectively. Thirty-two patients (59.3%) required inotropic or vasopressor support, 21 (38.9%) invasive mechanical ventilation, and 21 (38.9%) non-invasive mechanical ventilation. More children who died received inotropes/vasopressors and tocilizumab than survivors did (Table 4). On time-to-event data analysis, the Kaplan-Meier Survival curve (Figure 2) revealed a survival probability of 0.98 on day 2 of admission (98% having survived to day 2), dropping to 0.77 on day 18.
| Treatment | Overall | Death | Survival | P value |
|---|---|---|---|---|
| Number | 54 | 9 | 45 | |
| Antibiotics (%) | 53 (98.1) | 9(100.0) | 44 (97.8) | 1 |
| Macrolides (%) | 26 (48.1) | 6 (66.7) | 20 (44.4) | 0.394 |
| Hydroxychloroquine (%) | 2 (3.7) | 0 (0.0) | 2 (4.4) | 1 |
| Antiviral (%) | 21 (38.9) | 5 (55.6) | 16 (35.6) | 0.454 |
| Intravenous Immunoglobulin (%) | 50 (92.6) | 9 (100.0) | 41 (91.1) | 0.816 |
| Steroid (%) | 47 (87.0) | 7 (77.8) | 40 (88.9) | 0.717 |
| Aspirin (%) | 27 (50.0) | 3 (33.3) | 24 (53.3) | 0.465 |
| Low Molecular Weight Heparin (%) | 16 (29.6) | 1 (11.1) | 15 (33.3) | 0.351 |
| Tocilizumab (%) | 8 (14.8) | 4 (44.4) | 4 (8.9) | 0.026 |
| Anakinra (%) | 17 (31.5) | 3 (33.3) | 14 (31.1) | 1 |
| Inotropes/vasopressor (%) | 32 (59.3) | 9 (100.0) | 23 (51.1) | 0.019 |
| Respiratory Support (%) | 43 (79.6) | 9 (100.0) | 34 (75.6) | 0.227 |
| Outcome (%) | 54 | 9 (16.7) | 45((83.3) |
Table 4. Treatment and Outcome of 54 cohorts’ patients and comparison between death and survival cohorts.
Discussion
In this cohort study, we described the epidemiology, clinical characteristics, and outcomes of 54 patients under 14 years old who met the CDC criteria for MIS-C recruited from seven PICUs spanning four geographic regions in Saudi Arabia. To our knowledge, this is the largest multicenter cohort of patients diagnosed with MIS-C and admitted to a PICU in Middle Eastern/North African (MENA) countries. Major findings were that most of these 54 patients had PCR-confirmed SARS-CoV-2 (87%). The overall mortality rate was 16%, with neutrophilia, thrombocytopenia, AKI and DIC more commonly observed in patients who died. The most common presentation was gastrointestinal, followed by respiratory symptoms. Cardiovascular involvement was common over the course of hospitalization.
In this study, 55.6% of the patients were male, less than the 67% male patients documented in a multicenter observational study of children with Pediatric Inflammatory Multisystem Syndrome: Temporally Associated with SARS-CoV-2 (PIMS-TS) admitted to 21 PICUs in a UK study but similar to the percentage reported for 46 North American PICUs [20,21]. In our cohort, the median age of patients with MIS-C was 3.4 years, with 70% under the age of 5, meaning that our cohort was younger than previously reported elsewhere (e.g., medians of 9 years old in the USA, 7 in Egypt, and 7.8 in Turkey) [21-23]. This might, however, be attributable to the upper age limit of 14 years for pediatric patients in Saudi Arabia. Similar to what was documented in international reports from the United Kingdom and United States, more than 70% of our affected children had previously been healthy, 68.5% having no pre-existing comorbid conditions [20,21]. Ethnic minorities have been reported as having significantly greater risks for COVID-19 infection, severe disease, and mortality; and, in our study, more than 55% of the deaths occurred in non- Arabic children. The vast majority (87%) in our cohort was positive for SARS-CoV-2 by PCR. However, because the serological antibody assay was unavailable at most of the PICUs during the study period, only half of our patients underwent serologic testing, and only three were positive (one of whom also was positive for SARS-CoV-2 by PCR). Four patients were negative for both SARS-CoV-2 PCR and serology but had been in close contact with someone with confirmed COVID19 infection. In contrast, studies conducted in the UK identified a low incidence of positive SARS-CoV-2 PCR tests, and the presence of SARS-CoV-2 antibodies in 24 (96 percent) of 25 patients tested after a negative SARSCoV- 2 PCR, indicating that MIS-C is a post-COVID-19 immunological disease, clinically distinct from acute COVID-19 in children [20]. The clinical presentations of our patients were similar to those previously reported. Gastrointestinal involvement was the most prominent presentation, followed by respiratory manifestations. Previous studies also revealed GI symptoms (abdominal pain, vomiting, and diarrhea) as the predominant presenting symptoms, occurring in 78%-92% of all patients, often mimicking common gastrointestinal infections or even inflammatory bowel disease [20,23,24]. Cardiovascular involvement was common in our cohort, with 81.5% having an abnormal BNP, but only 29.4% elevated troponin. Among 52 patients who had an echocardiogram, 42 exhibited ventricular dysfunction and three coronary artery lesion/ectasia. Roughly six in ten (59.2%) patients required an inotrope or vasopressor. The 80% ventricular dysfunction observed among the echocardiograms performed in our sample is higher than percentages reported in Egypt (35.5%), Turkey (30.5%), and the USA (41.7%) [22-24]. Normal to high neutrophil counts and lymphopenia have been reported in children with MIS-C [20,22-24]. Neutrophilia >7.2 × 109/L, thrombocytopenia <150 × 109/L, and hypoalbuminemia <38 g/L occurred in 50% of our cohort and were found to be statistically associated with mortality. Previous studies have implicated lymphopenia as a marker of MIS-C severity [22,25]. Although lymphopenia was identified in one third of our cohort, the percentage of patients with lymphopenia was not different between patients who survived and those who died.
One fourth of the cohort was composed of ethnic minorities with associated higher mortality rate. These findings are congruent with reports coming from UK [20,26]. Although a Royal decree mandated free health care for all patients with COVID-19, socioeconomic factor may have led to delayed seeking of health care and potentially worse outcomes [27]. In addition, genetic susceptibility and pathophysiological differences been proposed as potential etiologies for differences in incidence and outcome among ethnicities [26]. The mortality rate of 16% in our cohort was higher than the previously reported rate of 2% [28]. This said, it is similar to the 13.3% mortality rate reported in Egypt and the 10% detected in a previous studies conducted in Saudi Arabia [13,15,23]. Previous studies included MIS-C patients who were admitted for both critical and general ward care [23,24], whereas our cohort was limited to patients admitted to a PICU and were critically ill, as reflected by high PRISM scores. A sizeable proportion also exhibited ventricular dysfunction and were very young. In addition, Extracorporeal Membrane Oxygenation (ECMO) is not currently offered routinely in any of the PICUs included in our analysis, and this is a potentially life-saving modality that has been offered for severe MIS-C at other centres [12]. All these factors could have contributed to the higher than usual mortality rate we observed. For MIS-C treatment, IVIG was the most frequently used agent, administered either alone or in combination with one or more other agents, including steroids in 87%, antiviral therapy in 38.9%, anakinra in 31.5%, and tocilizumab in 14.8%. Based upon the initial recommendations of the American College of Rheumatology, tocilizumab was given to MIS-C patients early on during the pandemic, but subsequently found to be of no benefit [29]. Our patients who received tocilizumab were critically ill, all with PRISM scores >20, and all developed ARDS. Half the patients treated with tocilizumab died within the first week of illness. Our study has several limitations. Foremost among them is the retrospective nature of data collection, which led to missing data and increased the risk of bias. In addition, there currently is no agreed-upon management protocol for MIS-C in Saudi Arabia. For this reason, there likely was considerable variability in the approach to both the work up and treatment of MIS-C at the different participating centers.
Conclusion
In a cohort of 54 children under 14 admitted to one of seven PICUs across Saudi Arabia, some of the clinical, laboratory, and echocardiographic characteristics of MIS-C associated with SARS-CoV-2 in severely ill children were found to be similar to those described in other recently published international studies. The higher mortality rate we observed could be explained by our patients’ high baseline PRISM scores, greater disease severity upon presentation, and younger age, as well as the lack of any national guidelines for MIS-C management. To better understand the variations we identified, prospective studies are needed to compare the various approaches currently being employed for early recognition, as well as the differences in the availability of resources and types of treatment used, and how all these factors impact patient outcomes, especially survival.
Authors’ contributions
Samah Al-Harbi conceptualized and designed the study and designed the data collection form. Mohammed Shahabuddin, Fidaa Maghrabi, Bayan M. Alnahdi, Shimaa M. Abusaif, Reham Aljohnei, Seham Arishi, Nabil Dhayhi, Hanin Alsini, Entesar Batook, Amal Ahmed, Ali Alshehri, Wafaa Alsewairi, Abdulrhman Alrasheed, Musaed Alharbi; Fayhan Alrogi collected the data. Samah Al-Harbi and Mohammed Shahabuddin coordinated data collection and editing. Mohammed Shahabuddin performed all statistical analyses; Samah Al-Harbi and Jubran Alqanatish drafted the initial manuscript, while Yasser M. Kazzaz and Jubran Alqanatish edited and reviewed the final manuscript. All authors read and approved the submitted manuscript.
Acknowledgements
We want to thank all health care providers in all centers for being dedicated and hardworking during this pandemic.
Funding
The authors received no financial support for the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Ethics approval
This study was approved by the Research Ethics Committees of all the participating institutions and the requirement for informed consent was waived, since it was an observational, retrospective study (reference No.513-20, REC 411, and RC20/541/R).
References
- Wiersinga W, Rhodes A, Cheng A, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19). JAMA. 2020; 324(8): 782.
- https://covid19.who.int/
- Alsofayan Y, Althunayyan S, Khan A, et al. Clinical characteristics of COVID-19 in Saudi Arabia: A national retrospective study. J Infect Public Health 2020; 13(7): 920-925.
- https://www.cdc.gov/mis/index.html
- Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in china. Pediatrics 2020; 145(6): e20200702.
- He J, Guo Y, Mao R, et al. Proportion of asymptomatic coronavirus disease 2019: A systematic review and meta-analysis. J Med Virol 2020; 93(2): 820-830.
- Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19. Pediatr Infect Dis J 2020; 39(5): 355-368.
- Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. (MMWR) Morbidity and mortality weekly report. 2020; 69(15): 458-464.
- Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyper inflammatory shock in children during COVID-19 pandemic. The Lancet. 2020; 395(10237): 1607-1608.
- Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. The Lancet 2020; 395(10239): 1771-1778.
- Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: A systematic review. Eur J Pediatr 2021; 180(7): 2019-2034.
- Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: A systematic review. E Clinical Medicine 2020; 26: 100527.
- Alharbi M, Kazzaz Y, Hameed T, et al. SARS-CoV-2 infection in children, clinical characteristics, diagnostic findings and therapeutic interventions at a tertiary care center in Riyadh, Saudi Arabia. J Infect Public Health 2021; 14(4): 446-453.
- Kari J, Shalaby M, Albanna A, et al. Coronavirus disease in children: A multicentre study from the Kingdom of Saudi Arabia. J Infect Public Health 2021; 14(4): 543-549.
- Almoosa Z, Al Ameer H, AlKadhem S, et al. Multisystem inflammatory syndrome in children, the real disease of COVID-19 in pediatrics-a multicenter case series from al-ahsa, Saudi Arabia. Cureus. 2020.
- Asseri A, AlHelali I, Elbastawisi E, et al. Multi-system inflammatory syndrome in children during the coronavirus disease 2019 in Saudi Arabia Medicine. 2021; 100(22): e25919.
- https://emergency.cdc.gov/han/2020/han00432.asp
- Pollack MM, Holubkov R, Funai T, et al. The pediatric risk of mortality score. Pediatric Critical Care Medicine. 2016; 17(1): 2–9.
- Yehya N, Harhay MO, Curley MAQ, et al. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med 2019; 200(7): 828-36.
- Davies P, Evans C, Kanthimathinathan H, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: A multicentre observational study. Lancet Child Adolesc Health 2020; 4(9): 669-677.
- Shekerdemian L, Mahmood N, Wolfe K, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to us and Canadian pediatric intensive care units. JAMA Pediatrics. 2020; 174(9): 868.
- Alkan G, Sert A, Oz S, et al. Clinical features and outcome of MIS-C patients: An experience from central Anatolia. Clin Rheumatol 2021.
- Mahmoud S, Fouda E, Kotby A, et al. The “Golden Hours” algorithm for the management of the Multisystem Inflammatory Syndrome in Children (MISC). Glob Pediatr Health 2021; 8: 2333794X2199033.
- Feldstein L, Rose E, Horwitz S, et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med 2020; 383(4): 334-346.
- Lee P, Day-Lewis M, Henderson L, et al. Distinct clinical and immunological features of SARS–CoV-2–induced multisystem inflammatory syndrome in children. J Clin Investig 2020; 130(11): 5942-5950.
- Khunti K, Singh A, Pareek M, et al. Is ethnicity linked to incidence or outcomes of covid-19? BMJ 2020;1548.
- Kazzaz Y, Alkhalaf H, Alharbi M, et al. Hospital preparedness and management of pediatric population during COVID-19 outbreak. Ann Thorac Med 2020; 15(3): 107.
- Guimarães D, Pissarra R, Reis-Melo A, et al. Multisystem Inflammatory Syndrome In Children (MISC): A systematic review. Int J Clin Pract 2021.
- Henderson L, Canna S, Friedman K, et al. American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS–CoV-2 and hyper inflammation in pediatric COVID-19: Version 1. Arthritis Rheumatol 2020; 72(11): 1791-1805.