Research Article - Biomedical Research (2017) Volume 28, Issue 3
Clinical analysis of 14 cases of community-acquired Pseudomonas aeruginosa bacteraemia in children
Tao Zhang1, Jiujun Li1*, Xiaoshi Zheng1, Lijie Wang1 and Zhijie Zhang21Pediatric Intensive Care Unit (PICU), Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
2Department of Clinical Laboratory, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
- *Corresponding Author:
- Jiujun Li
Paediatric Intensive Care Unit
Shengjing Hospital of China Medical University, China
Accepted date: August 3, 2016
Abstract
This aims to study the clinical features and outcomes of Community-Acquired Pseudomonas aeruginosa Bacteraemia (CAPAB) in children. Retrospectively analysed the conditions of patients diagnosed with sepsis over nearly 6 years and screened out 14 cases of CAPAB in children to statistically analyse the related clinical features, biochemical tests, susceptibility results, and treatment outcomes. There were 7 boys and 7 girls among the 14 Community-Acquired Pseudomonas aeruginosa Bacteraemia (CAPAB) children with an average age of 21.8 months. Community-Acquired Pseudomonas aeruginosa Bacteraemia (CAPAB) occurred mainly in spring and winter, and all participants exhibited fever symptoms with an average course of 6.1 days. Most cases were associated with digestive symptoms, and 4 exhibited necrotic abscess-like rash changes. No child received effective antibiotic treatment before admittance, 9 progressed to septic shocks, and 7 died; the mortality rate was 50%. Community- Acquired Pseudomonas aeruginosa Bacteraemia (CAPAB) incidence in children was low and was more associated with fever and digestive symptoms. Necrotic abscess-like rash was the specific change, and shock might have progressed rapidly and resulted in high mortality.
Keywords
Pseudomonas aeruginosa, Community acquired, Septic shock, Children.
Introduction
In developing countries and rural areas, septic shock remains one of the major causes of child mortality [1]. Knowing how to identify septic shock early and perform appropriate anti-shock treatment is essential for the prognosis of affected children [2,3]. However, due to the sporadic features of septic shock and the fact that children with septic shock more commonly exhibit "warm shock," which differs substantially from that in adults [4], it is difficult for clinicians to identify in its early stage, thus missing the best treatment timing. We summarized the data of children diagnosed with sepsis over nearly 6 years and combined the final blood culture results, screening out certain bacterial infections caused by septic shock. The hope was to retrospectively analyse the disease characteristics and biochemical tests and help clinicians to recognize septic shock and perform targeted antibiotic therapy in the early stage. As a rare community-acquired pathogen, Pseudomonas aeruginosa might exhibit specific clinical manifestations and high mortality rate after infection, so it attracted our attention.
Pseudomonas aeruginosa is also known as Bacillus aeruginosus and widely exists in soil, water, air, normal skin, and respiratory and intestinal tracts as one of the most common clinical conditional pathogens. Patients with lower body resistance, congenital immune deficiencies, blood diseases, and malignant cancers as well as postoperative or long-term administrated antibiotics might be susceptible to these bacteria [5]. Foreign studies have shown that the incidence rate of Pseudomonas aeruginosa sepsis in populations was 3.16/100,000 to 4.70/100,000 per year, about 1/5th of which was community-acquired Pseudomonas aeruginosa sepsis [6]. This disease occurs mainly in adults over the age of 60, and the proportion in children is very low. Community-acquired Pseudomonas aeruginosa bacteraemia (Community-Acquired Pseudomonas Aeruginosa Bacteraemia (CAPAB)) in healthy children is even less common, so the current clinical reports in China and abroad are rare [7-9]. This study collected 14 cases of Community-Acquired Pseudomonas Aeruginosa Bacteraemia (CAPAB) in children admitted into the Paediatric Intensive Care Unit (PICU), Shengjing Hospital of China Medical University, from April 2009 to May 2015, and summarized and analysed their clinical characteristics and treatment outcomes.
Materials and Methods
Subject source and diagnostic criteria
The 14 cases enrolled were selected from the children treated in the Shengjing Hospital of China Medical University PICU from April 2009 to May 2015 and met the following 3 criteria. 1. Clinical manifestations and laboratory tests were in line with the sepsis diagnostic criteria. The diagnostic criteria of sepsis and septic shock referred to the Guidelines of International Sepsis Treatment in 2012 [10] and the criteria developed in the 2006 International Paediatric Sepsis meeting [11] (sepsis pus cardiovascular dysfunction: exhibited blood pressure reduction after intravenously infused isotonic liquid for more than 40 ml/kg within 1 h and less than 5% of that in this age group, or the systolic blood pressure was less than 2 standard deviations from the normal value in this age group, or vasoactive drugs were needed to maintain the blood pressure within normal dopamine range >5 μg/(kg•min) or any dose of dobutamine, epinephrine, or norepinephrine. Met 2 of the 5 following items: (1) unexplainable metabolic acidosis, base deficit>5 mmol/L; (2) arterial blood lactate level was increase more than 2 times the normal upper limit; (3) without urine: urine output<0.5 ml/ (kg•h); (4) capillary refilling was prolonged (>5s); (5) centerto- surrounding temperature difference was>3˚C). 2. Within 48 h of admission, blood bacterial culture exhibited positive results of Pseudomonas aeruginosa at least once, with or without positive results of humoral Pseudomonas aeruginosa culture from the lesion sites. 3. The following high-risk situations were excluded: admitted within nearly 1 month and had surgical history; required dialysis or needed long-term stay in nursing unit; had long-term catheter indwelling, intravenous, or other percutaneous devices. This study was conducted in accordance with the Declaration of Helsinki. This study was conducted with approval from the Ethics Committee of China Medical University. Written informed consent was obtained from all participants’ guardians.
General information
The 14 cases included 7 male and 7 female patients with a mean age of 21.8 months. The youngest was 2 months old, and the eldest was 11 years old; 12 patients were aged<3 years old. Seven cases lived in urban areas, and the rest lived in rural areas. Thirteen cases were physically healthy previously without history of major diseases; 1 case had once been hospitalized in our hospital’s department of paediatric general surgery for 2 months for "perineal anoplasty and fistula closing surgery." The operations and healing went well. Season of onset: There were 10 cases in winter and 4 in summer. Eleven had a history of outpatient medication, 7 of which were applied penicillin or cephalosporins (first or second generation), and the longest treatment duration was a 6-day outpatient intravenous infusion of second-generation cephalosporins (Ceftezole). The remaining 4 cases had once received antiviral drugs or macrolides as shown in Table 1.
| No | Gender | Age | Blood type | Fever duration (days) | Symptoms in digestive system | Symptoms in nerve system | Rash | Previous history | Outpatient medication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 1 year | B-RH (-) | 15 | N/A | N/A | N/A | Healthy | Azithromycin for 5 days |
| 2 | M | 1 year | O-RH (+) | 4 | Abdominal distension, diarrhoea | Irritable | N/A | Healthy | Penicillin for 1 day |
| 3 | M | 2 months | B-RH (+) | 4 | Diarrhoea | Convulsion | N/A | Healthy | Ceftezole, Xiyanping for 1 day |
| 4 | F | 4 months | B-RH (+) | 3 | Diarrhoea, vomiting | N/A | N/A | Performed surgery 2 months age | No |
| 5 | F | 1 year | B-RH (+) | 5 | Diarrhoea, abdominal distension | Lethargy | yes | Healthy | Ceftezole, Xiyanping for 6 days |
| 6 | M | 3 months | A-RH (+) | 7 | N/A | Convulsion | N/A | Healthy | Erythromycin, Shaduolika for 3 days |
| 7 | M | 5 months | O-RH (+) | 7 | Diarrhoea | N/A | Yes | Healthy | N/A |
| 8 | F | 4 months | B-RH (+) | 4 | N/A | Convulsion | N/A | Healthy | Xiyanping, complex coenzymes for 2 days |
| 9 | M | 6 months | AB-RH (+) | 6 | N/A | N/A | Yes | Healthy | Second-generation cephalosporins for 3 days |
| 10 | F | 7 years | B-RH (+) | 3 | N/A | N/A | Yes | Healthy | Erythromycin, second-generation cephalosporins for once |
| 11 | M | 6 months | B-RH (+) | 5 | Abdominal distension | N/A | N/A | Healthy | N/A |
| 12 | F | 11 years | B-RH (+) | 13 | N/A | N/A | Yes | Healthy | Second-generation cephalosporins, Xiyanping for 6 days |
| 13 | M | 5 months | O-RH (+) | 5 | N/A | Drowsiness | N/A | Healthy | Erythromycin, Xiyanping for 3 days |
| 14 | M | 19 months | O-RH (+) | 5 | N/A | N/A | N/A | Healthy | Second-generation cephalosporins, Xiyanping for 3 days |
Table 1: General information of the 14 patients (N=14).
Results
Clinical manifestations
All patients exhibited fever before admission with durations ranging from 3 to 15 days (average 6.1 days). Six cases were associated with gastrointestinal symptoms (diarrhoea, abdominal distension, or vomiting), 4 had idiopathic rash appearance, and 6 exhibited nervous system involvement (drowsiness and convulsion). In 4 cases, soft tissue purulencelike changes were found in physical examination when admitted (2 cases of facial cellulitis, 1 case of perianal abscess, and bi-lower limb abscess in all). Nine patients were diagnosed with "septic shock" when admitted.
Blood routine, CRP, and primary biochemical tests
Twelve cases exhibited White blood cell (WBC)<4.0 × 109/L, 1 case was normal, and 1 case was>10.0 × 109/L. Among WBC proportions, only 4 cases exhibited neutrophils as the main proportion (>50%); the remaining 10 cases exhibited lymphocytes as the main proportion (>50%). Seven cases had varying degrees of anaemia (Hb<90 g/L), 6 exhibited significantly decreased platelets with the lowest at 20 × 109/L, and 4 exhibited reduction in all 3 lines. The C-Reactive Protein (CRP) levels were significantly increased; 5 cases were within 8 ~ 100 mg/l, and 9 cases were>100 mg/l. 9 patients received Procalcitonin (PCT) assay with the results>5 ηg/ml, and 9 were associated with albumin reduction (ALB<30 g/L) as shown in Table 2.
| No | Amikacin | Aztreonam | Ceftriaxone | Ceftazidime | Cefotaxime | Cefuroxime | Cefaclor | Cefoperazone | Cefepime | Cefoperazone/ sulbactam |
Piperacillin | Gentamicin | Imipenem | Levofloxacin | Ciprofloxacin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | R | S | I | S | I | R | R | S | S | S | S | R | S | S | S |
| 2 | S | S | R | S | R | R | R | S | S | S | S | S | S | S | S |
| 3 | S | S | I | S | I | R | R | S | S | S | S | S | S | S | S |
| 4 | S | S | I | I | I | R | R | S | S | S | I | R | S | S | S |
| 5 | S | S | I | S | I | R | R | S | S | S | S | S | S | S | S |
| 6 | S | S | S | S | S | R | R | S | S | S | S | S | S | S | S |
| 7 | S | S | I | S | I | R | R | S | S | S | S | S | S | S | S |
| 8 | S | S | I | S | I | R | R | S | S | S | S | S | S | S | S |
| 9 | S | S | S | S | I | R | R | S | S | S | S | S | S | S | S |
| 10 | R | S | R | S | I | R | R | S | S | S | I | I | S | S | S |
| 11 | S | S | I | S | R | R | R | S | S | S | S | I | S | S | S |
| 12 | S | S | S | S | S | R | R | S | S | S | S | S | S | S | S |
| 13 | S | S | I | S | R | R | R | S | S | S | R | R | S | R | R |
| 14 | S | S | S | S | I | R | R | S | S | S | S | R | S | S | S |
| Sensitivity rate (%) | 85.7 | 100 | 28.6 | 92.9 | 14.3 | 0 | 0 | 100 | 100 | 100 | 78.6 | 57.1 | 100 | 92.9 | 92.9 |
Table 2: Results of sensitivity assay (N=14).
Immunologic tests
Eleven patients received immunoglobulin assay, 9 of which exhibited IgG<2.5 g/L with or without IgM and IgA reduction and could be diagnosed with "temporary hypogammaglobulinemia". 7 patients received lymphocyte subsets assay, and 4 exhibited significantly reduced NK% (<5%) (Normal range: 7 ~ 36%).
Bacterial culture and sensitivity results
France bioMerieux API system and VITEK 2-COM-PACT system were used for strain identification. The agar disc diffusion method (K-B method) was used for in vitro sensitivity assay, strictly following the method recommended by the Clinical and Laboratory Standards Institute (CLSI). Quality control strain was PAATCC27853. The sensitivity criteria were divided into 3 levels: sensitive, intermediary sensitive, and resistant. A total of 16 Pseudomonas aeruginosa positive strains were tested (14 in blood culture and 2 in pus culture). Fourteen patients received drug sensitivity assay as shown in Table 2, the results of which indicated the strains were generally resistant to second-generation cephalosporins, ceftriaxone, and cefotaxime. While highly sensitive to aztreonam, amikacin, ceftazidime, cefoperazone, cefepime, carbapenems, and quinolones, all strains showed no ESBLs (Extended-Spectrum β-Lactamase Strains) and multi-drug resistance.
Treatment and outcomes
Twelve patients received a "high-strength, wide-coverage, combined antibiotic treatment strategy" based on clinical experience after admittance, which included sodium cefoperazone/sodium sulbactam, cefepime, or carbapenems plus vancomycin or linezolid for the anti-inflammatory treatment. The remaining 2 patients were admitted into the general ward for "pneumonia, diarrheal disease" because of their atypical clinical symptoms, and, after receiving secondgeneration cephalosporins for 2-day anti-inflammatory treatment, they were quickly transferred to our department for the associated occurrence of septic shock. Although they were given aggressive anti-shock treatment, they both died within 24 hours.
Nine patients received ventilator-assisted therapy, 2 received blood purification, 2 received bowel resection and anastomosis after intestinal perforation, and 3 received necrotic tissue debridement and skin eschar grafting after eschar removal.
Among the total 14 patients, 7 died, 1 was discharged from hospital, and 6 survived. The reason for the deaths was refractory septic shock, which was eventually merged with DIC, pulmonary haemorrhage, and refractory hypotension, and the patients died within 72 hours of admission. Among the 6 patients who survived, 5 underwent surgery, with an average length of hospital stay of 42.7 days as shown in Table 3.
| No. | WBC (*109/L) | Hb (g/L) | PLT (*109/L) | CRP (mg/L) | PCT (ng/ml) | Septic shock | Mechanical ventilation (day) | Surgery | Hospital stay (day) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.9 | 66 | 32 | 36.8 | N/A | Yes | 1 | N/A | 2 | Died |
| 2 | 1.3 | 82 | 158 | 163 | N/A | Yes | 1 | N/A | 1 | Died |
| 3 | 9.6 | 113 | 280 | 337 | N/A | N/A | N/A | N/A | 16 | improved |
| 4 | 1.6 | 67 | 227 | 171 | N/A | Yes | 1 | N/A | 2 | Died |
| 5 | 3.5 | 89 | 20 | 171.59 | 9.7 | N/A | N/A | Bowel resection, anastomosis | 64 | cured |
| 6 | 3 | 92 | 186 | 31.3 | N/A | N/A | N/A | Bowel resection, anastomosis | 42 | improved |
| 7 | 1.1 | 62 | 25 | 53.4 | 6.32 | yes | 6 | skin eschar grafting | 56 | Improved |
| 8 | 0.6 | 95 | 66 | 314 | 21.96 | Yes | 6 | Facial debridement | 53 | Improved |
| 9 | 3.7 | 83 | 69 | 183 | 7.7 | N/A | N/A | Necrotic tissue debridement | 25 | Improved |
| 10 | 3 | 92 | 313 | 96.2 | 8.91 | Yes | 1 | N/A | 1 | Died |
| 11 | 17 | 115 | 22 | 202 | >100 | N/A | N/A | N/A | 6 | Discharged |
| 12 | 0.2 | 111 | 281 | 330 | 26.1 | Yes | 1 | N/A | 1 | Died |
| 13 | 1.5 | 73 | 151 | 10.2 | 57.53 | Yes | 1 | N/A | 2 | Died |
| 14 | 3.4 | 130 | 212 | 112 | 88.42 | Yes | 1 | N/A | 1 | Died |
Table 3: Biochemical markers and clinical outcomes of the patients (N=14).
Discussion
Sepsis is a serious state of infection, which often leads to higher mortality and enormous health care costs. In many hospitals, especially in ICU, Pseudomonas aeruginosa has become a very important hospital-acquired pathogen. Many studies [12-16] have shown Pseudomonas aeruginosa bacteremia mortality to be more than 20%, and that of the patients infected with Multidrug-Resistant Pseudomonas aeruginosa (MDRPA, at least resistant to 3 kinds of β- lactamase, carbapenems, aminoglycosides, and quinolones) was even higher [17,18]. The early application of antibiotics as well as the occurrence of respiratory failure and septic shock was significantly correlated with mortality [12,16,19-21].
Obritsch et al. monitored an American ICU ward and found that MDRPA incidence rose from 4% in 1993 to 14% in 2002 and the antibiotics with the highest resistance rates were β- lactams and ciprofloxacins, while aminoglycosides, fluoroquinolones, and piperacillin/tazobactam exhibited the lowest resistance rates [22]. With the rapid developments of paediatric intensive medicine, the increased infection status of Pseudomonas aeruginosa in PICU wards has attracted more and more attention. Yang et al. collected information from 62 cases of hospital-acquired PAB in children over nearly 10 years and studied their clinical characteristics and treatment outcomes, finding that 59 patients had basic diseases. Longterm PICU stay (>1 month) was a significant risk factor for Pseudomonas aeruginosa infection, and respiratory failure was one risk factor of death [23]. Long-term PICU stay and respiratory failure often predicted MDRPA infection and higher mortality, and, in this study, the incidence of MDRPA was 11.3% with a mortality of 14.5%. The PICU department of Shengjing Hospital of China Medical University performed statistical analysis towards antibiotic-resistance cases of Pseudomonas aeruginosa over nearly 5 years [24] and found that MDRPA incidence did not fluctuate much with an average of 7.3%, exhibiting high sensitivity to cefoperazone/sulbactam, meropenem, and ceftazidime with a mortality rate of 24%.
Differing from those with hospital-acquired Pseudomonas aeruginosa infections, patients with CAPA infections had no long history of hospitalization and long-term applications of antibiotics; thus, MDRPA incidence would be low. However, because of the infections’ delitescence as well as the application delay of sensitive antibiotics, its mortality rate is often high. Some studies have shown [13,25] that CAPA infections in adults occurred more in patients with cancers with the mortality as high as 39%. Currently, there are few articles about large-sample CAPA infections in children. Only some small-sample studies have been reported [10,26], most occurring in previously healthy children with fulminant septic shock as the main cause of death and mortality rates from 30% to 62%.
Among the 14 Community-Acquired Pseudomonas aeruginosa Bacteraemia (CAPAB) cases in this study, most patients were previously healthy, and infants accounted for the majority (85.7%), suggesting that it might be related with infants’ immature immune systems and low intestinal barrier functions. 11 patients received immunoglobulin detection, and 9 (81.8%) exhibited varying degrees of immunocompromised status, consistent with those in foreign studies finding that the occurrence of Community-Acquired Pseudomonas aeruginosa Bacteraemia (CAPAB) in previously healthy infants had a certain relationship with potential immunodeficiency [27].
Clinical manifestation of CAPAB infections in children varied, while fever and digestive system-involved performance might occur in most cases, appearing as abdominal distension, diarrhoea, vomiting, and other symptoms, and could progress to toxic intestinal paralysis and intestinal obstruction, even intestinal perforation. It was considered to be related with retrograde infection of certain bacteria. Among the 6 surviving infants in this study, 2 received bowel resection and anastomosis and showed multiple intestinal perforation and ulcer-like changes during surgery. Because the positions of early hyperaemia, oedema, multiple ulcer, and possible perforation in lots of intestinal canals in toxic intestinal paralysis were difficult to determine, the surgical timing was often difficult to choose, which was not mentioned in literature. Based on the experience of paediatric surgery in our hospital, we recommended that early dynamic observation be carried out with daily abdominal plain film examination. Once intestinal perforation was discovered, emergency surgical treatment should be performed.
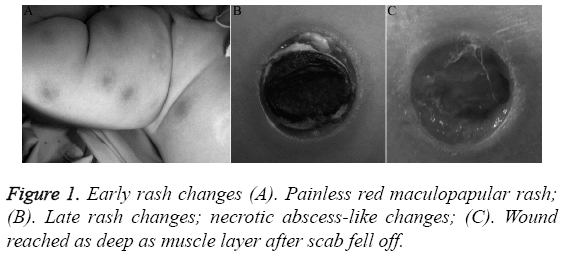
Necrotic abscess-like rash was the specific change of Community-Acquired Pseudomonas aeruginosa Bacteraemia (CAPAB) and an important basis for judging Pseudomonas aeruginosa infections early [8,28], but its incidence rate was low. The initial appearance of rash was painless red rash or pimples, which then became nodules, blisters, or pustules and eventually evolved into center-depressed black or gray necrotic tissues with red projections around, similar to the changes of crater, and the survivors would need elective necrotic tissue removal surgery shown in Figure 1. When such changes are found during physical examination, it should be highly suspected as caused by this disease, and clinicians must pay a close attention as early antibiotic treatment, bacterial culture, and related assay of infection indexes is critical [29,30].
Twelve patients in this study exhibited decreased leukocyte levels when admitted (85.7%), and most were associated with the reduction of haemoglobin and platelets, which was considered to be related with bone marrow suppression after severe infections. Simultaneously, the levels of CRP and procalcitonin were significantly increased, supporting the diagnosis of severe sepsis. The above infection indicators could be obtained more rapidly than blood bacterial culture; in particular, procalcitonin could much more accurately reflect sepsis severity. Therefore, once infants exhibited such symptoms as fever and diarrhoea and infection indexes appeared the above changes, it should attract our vigilance for early empirical applications of antibiotic treatment that could cover Pseudomonas aeruginosa. If early antibiotic application does not cover these bacteria, this disease will progress rapidly, and most patients will soon develop into septic shock. In this paper, 9 patients (64.2%) appeared to experience septic shock, and because of early delitescence of shock performance, clinicians could not easily find these symptoms. Among these patients, 7 died of refractory hypotension, DIC, and pulmonary haemorrhage.
Obritsch et al. summarized the literature in 1966-2005 and published a review about epidemiology and antibiotic treatment strategy of hospital-acquired MDRPA infections [7]. The paper emphasized the importance of joint antibiotic applications towards critically ill patients with MDRPA infections. It recommended the selection of β-lactams joint with aminoglycosides or quinolones; even if the sensitivity assay indicated resistance, a certain study still found synergistic effects of joint application, which could still achieve better therapeutic effects. If wide-range resistance caused the limited selection of antibiotics, polymyxin could be considered for the treatment, but it is still necessary to inform the patients of the possible side effects. Within the current limited reports [9,26,31,32], MDRPA stain was rarely found in CAPA infections in children, but the patients with suspected infection of this bacteria should be recommended the initial antibiotic treatment that could cover Pseudomonas aeruginosa, such as β-lactams or aminoglycosides. While ceftriaxone and cefotaxime were not included, the sensitivity results of blood culture in this study also supported the above conclusions. Quinolones also exhibited high sensitivity against Pseudomonas aeruginosa, but due to the impacts on children’s safety, they were currently not used as first-line therapy.
Recent studies have shown that the ABO blood type has certain relevance with Pseudomonas aeruginosa infections in children; children with B-type blood are more vulnerable to the infection, and its principle might be related with humoral immunity and clinical susceptibility [33]. In this study, 8 patients had B-type blood (57.1%), but the case number was too small to effectively prove the above assertion. Further large-sample studies are still needed.
CAPA infections in children were clinically rare, and mostly occurred in small infants and previously healthy children with such symptoms as fever and diarrhoea. The initial general state was acceptable with atypical clinical manifestations, and the laboratory blood routine assay might exhibit low leukocytes in most cases, so it is easily misdiagnosed by clinicians as common viral or bacterial infection. If in-time effective antibiotics and other supportive care are not performed, this disease might progress rapidly and develop quickly into septic shock, so its mortality rate is high and should attract extensive attention by clinical front-line physicians. Empirical early effective antibiotic treatment and early shock judgment was key towards successful rescue.
Conflicts of Interest
Tao Zhang, Jiujun Li, Xiaoshi Zheng, Lijie Wang and Zhijie Zhang declare that they have no conflicts of interest regarding this paper.
Acknowledgement
Liaoning Provincial Natural Science Foundation of China (2014021077): Interventional mechanisms of bone marrow stem cells towards acute lung injury in young mice.
References
- Linde-Zwirble WT, Angus DC. Severe sepsis epidemiology: sampling, selection, and society. Crit Care 2004; 8: 222-226.
- Dohna-Schwake C, Felderhoff-Muser U. Early recognition of septic shock in children. KlinPadiatr 2013; 225: 201-205.
- Sagy M, Al-Qaqaa Y, Kim P. Definitions and pathophysiology of sepsis. CurrProblPediatrAdolesc Health Care 2013; 43: 260-263.
- Aneja R, Carcillo J. Differences between adult and pediatric septic shock. Minerva Anestesiol 2011; 77: 986-992.
- Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 2013; 67: 159-173.
- Parkins MD, Gregson DB, Pitout JD, Ross T, Laupland KB. Population-based study of the epidemiology and the risk factors for Pseudomonas aeruginosa bloodstream infection. Infection 2010; 38: 25-32.
- Obritsch MD, Fish DN, MacLaren R, Jung R. Nosocomial infections due to multidrug-resistant pseudomonas aeruginosa: epidemiology and treatment options. Pharmacother 2005; 25: 1353-1364.
- Goolamali SI, Fogo A, Killian L, Shaikh H, Brathwaite N. Ecthymagangrenosum: an important feature of pseudomonal sepsis in a previously well child. ClinExpDermatol 2009; 34: e180-182.
- Zhang Q, Smith JC, Zhu Q, Guo Z, MacDonald NE. A five-year review of Pseudomonas aeruginosabacteremia in children hospitalized at a single center in southern China. Int J Infect Dis 2012; 16: e628-632.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign Guidelines Committee including the Paediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41: 580-637.
- Goldstein B, Giroir B, Randolph A. International Consensus Conference on Paediatric Sepsis. International paediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in paediatrics. PediatrCrit Care Med 2005; 6: 2-8.
- Vidal F, Mensa J, Almela M, Martínez JA, Marco F, Casals C, Gatell JM, Soriano E, Jimenez de Anta MT. Epidemiology and outcome of Pseudomonas aeruginosa bacteraemia, with special emphasis on the influence of antibiotic treatment: analysis of 189 episodes. Arch Intern Med 1996; 156: 2121-2126.
- Chatzinikolaou I, Abi-Said D, Bodey GP, Rolston KV, Tarrand JJ, Samonis G. Recent experience with Pseudomonas aeruginosa in patients with cancer: retrospective analysis of 245 episodes. Arch Intern Med 2000; 160: 501-509.
- Wisplinghoff H, Seifert H, Tallent SM, Bischoff T, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in paediatric patients in United States hospitals: epidemiology, clinical features, and susceptibilities. Pediatr Infect Dis J 2003; 22: 686-691.
- Osmon S, Ward S, Fraser VJ, Kollef MH. Hospital mortality for patients with bacteremia due to Staphylococcus aureus or Pseudomonas aeruginosa. Chest 2004; 125: 607-616.
- Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother 2005; 49: 1306-1311.
- Wolska K, Kot B, Piechota M, Frankowska A. Resistance of Pseudomonas aeruginosa to antibiotics. PostepyHig Med Dosw 2013; 67: 1300-1311.
- Ali Z, Mumtaz N, Naz SA, Jabeen N, Shafique M. Multi-drug resistant pseudomonas aeruginosa: a threat of nosocomial infections in tertiary care hospitals. J Pak Med Assoc 2015; 65: 12-16.
- Kang CI, Kim SH, Kim HB, Park SW, Choe YJ, Oh MD, Kim EC, Choe KW. Pseudomonas aeruginosa bacteraemia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis 2003; 37: 745-751.
- Dantas RC, Ferreira ML, Gontijo-Filho PP, Ribas RM. Pseudomonas aeruginosa bacteraemia: independent risk factors for mortality and impact of resistance on outcome. J Med Microbiol 2014; 63: 1679-1687.
- Gonzalez AL, Leal AL, Cortes JA, Sanchez R, Barrero Li, Castillo JS, Alvarez CA. Effect of adequate initial antimicrobial therapy on mortality in critical patients with Pseudomonas aeruginosa bacteraemia. Biomedica 2014; 34: 58-66.
- Obritsch MD, Fish DN, MacLaren R, Jung R. National surveillance of antimicrobial resistance in Pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrob Agents Chemother 2004; 48: 4606-4610.
- Yang MA, Lee J, Choi EH, Lee HJ. Pseudomonas aeruginosa bacteraemia in children over ten consecutive years: analysis of clinical characteristics, risk factors of multi-drug resistance and clinical outcomes. J Korean Med Sci 2011; 26: 612-618.
- Wang LJ, Sun Y, Song WL, Zhang ZJ, Liu CF. Changes of drug-resistance of Pseudomonas aeruginosa in paediatric intensive care unit. ZhonghuaErKeZaZhi 2012; 50: 657-663.
- Kang CI, Kim SH, Park WB, Lee KD, Kim HB. Clinical features and outcome of patients with community-acquired Pseudomonas aeruginosa bacteraemia. ClinMicrobiol Infect 2005; 11: 415-418.
- Huang YC, Lin TY, Wang CH. Community-acquired Pseudomonas aeruginosa sepsis in previously healthy infants and children: analysis of forty-three episodes. Pediatr Infect Dis J 2002; 21: 1049-1052.
- Aliaga L, Mediavilla JD, Cobo F. A clinical index predicting mortality with Pseudomonas aeruginosa bacteraemia. J Med Microbiol 2002; 51: 615-619.
- Wu CT, Huang JL. Multiple ecthymagangrenosum in a healthy infant with community-acquired Pseudomonas aeruginosa sepsis. PediatrEmerg Care 2010; 26: 750-751.
- Bowers DR, Tam VH. Pseudomonas aeruginosa treatment and transmission reduction. Expert Rev Anti Infect Ther 2013; 11: 831-837.
- Gonzalez AL, Leal AL, Cortes JA, Sanchez R, Barrero LI, Castillo JS, Alvarez CA. Effect of adequate initial antimicrobial therapy on mortality in critical patients with Pseudomonas aeruginosa bacteraemia. Biomedica 2014; 34: 58-66.
- Su TY, Ye JJ, Hsu PC, Wu HF, Chia JH, Lee MH. Clinical characteristics and risk factors for mortality in cefepime-resistant Pseudomonas aeruginosa bacteremia. J MicrobiolImmunol Infect 2015; 48: 175-182.
- Zhang Y, Chen XL, Huang AW, Liu SL, Liu WJ, Zhang N, Lu XZ. Mortality attributable to carbapenem-resistant Pseudomonas aeruginosabacteremia: a meta-analysis of cohort studies. Emerg Microbes Infect 2016; 5: e27.
- Kuo KC, Kuo HC, Huang LT, Lin CS, Yang SN. The clinical implications of ABO blood groups in Pseudomonas aeruginosa sepsis in children. J MicrobiolImmunol Infect 2013; 46: 109-114.