Research Article - Current Pediatric Research (2019) Volume 23, Issue 2
Circulating nitric oxide, malondialdehyde and total antioxidant capacity levels among term septic neonates
Amira MM Hamed1, Alaa-Eldin A Hassan1, Mohammed Abo-Alwafa Aladawy1 and Mohammed H Hassan2*
1Faculty of Medicine, Department of Pediatrics, Al-Azhar University, Assiut branch, Egypt
2Faculty of Medicine, Department of Medical Biochemistry, South Valley University, Qena, Egypt
- *Corresponding Author:
- Mohammed H. Hassan
Lecturer of Medical Biochemistry
Faculty of Medicine
South Valley University
Qena, Egypt
Tel: +201098473605
E-mail: Mohammedhosnyhassaan@yahoo.com
Accepted on June 17th, 2019
Abstract
Background: Neonatal sepsis with its high mortality rate still remains a diagnostic and treatment challenge for neonatal health care providers. Sepsis represents an oxidative stress condition when occurs in neonates due to rapid changes in tissue oxygen concentrations with immature antioxidant mechanisms. We aimed to study the serum levels of oxidants and antioxidants among term neonates with sepsis.
Patients and methods: A case-control study included 60 full term newborns with sepsis in addition to 30 healthy newborns as controls, recruited from NICU of Pediatric Department, Al-Azhar University Hospital, Assiut, Egypt. Full history, thorough clinical examination, Complete blood count (CBC), Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and blood culture were performed to all included patients. Serum levels of nitric oxide, malondialdehyde (MDA) and total antioxidant capacity (TAO) were assayed in all included neonates using colorimetric methods.
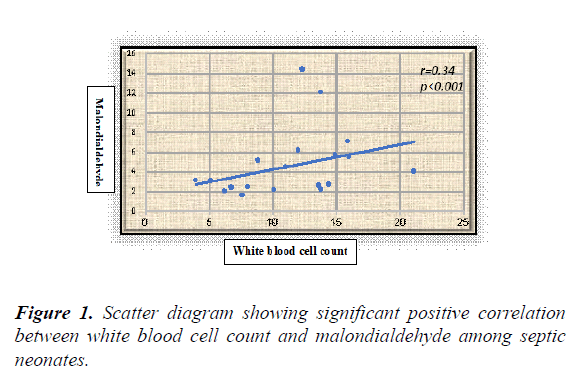
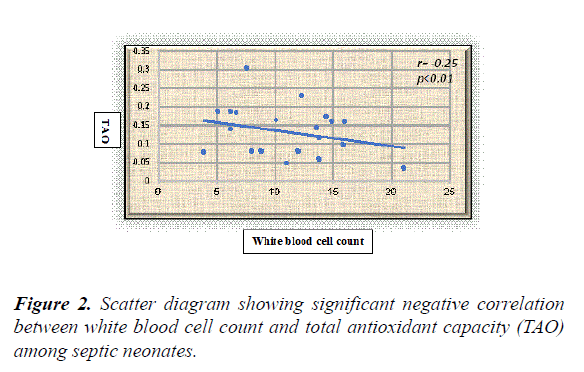
Results: The overall results showed statistically significant higher serum levels of MDA and NO with lower TAO levels among septic neonates when compared with the controls (p value<0.05) for both. Additionally, all the previously measured biochemical oxidative stress markers didn't show significant differences between early and late onset septic groups. Serum NO, MDA and TAO levels at cut-off points (0.514 μmol/l, 0.399 nmol/ml, and 0.591 mm/l respectively) showed high sensitivity (96.4%, 95%, and 98.8% respectively) and specificity (93%, 92% and 94% respectively). White blood cell counts were negatively correlated to TAO (r=-0.25, p<0.01) and positively correlated to MDA in a statistically significant manner among septic neonates (r=0.34, p<0.001).
Conclusion: There is strong evidence of involvement of oxidative stress in neonatal sepsis, mainly NO, MDA and TAO, and could be helpful in its diagnosis. Also, targeting of therapeutic strategies towards the pro-oxidant pathways may be beneficial in neonatal sepsis.
Keywords
Nitric oxide; Malondialdehyde; Total antioxidant capacity; Neonatal sepsis.
Introduction
Sepsis is one of the common leading factors of neonatal morbidity and mortality. Oxidative stress together with inflammation is involved in neonatal sepsis with subsequent impairment in organ functions and death [1]. Early onset neonatal sepsis (EOS) defined as a positive culture (blood, urine obtained by suprapubic tab or in- and- out-catheterization or cerebrospinal fluid) occurring on day of life 1, 2 or 3, while late onset sepsis (LOS) defined as a positive culture occurring between day of life 4 and 120 [2].
The oxidative stress (OS) was previously defined as an imbalance between reactive oxygen species (ROS) and endogenous antioxidant power with subsequent lipid peroxidation, DNA damage and protein oxidation [3], but recently OS has been referred to the imbalance between the oxidants and antioxidants in favor of oxidants that results in molecular damage or redox signaling arrest [4].
Oxidative damage from pathologic conditions such as sepsis have serious effects in pediatric patients more than in older people due to the lower functional reserve and the requirement for subsequent growth of tissue to follow normal development [5].
The profile of oxidative stress and antioxidant defense during neonatal sepsis is less extensively studied when compared to adult patients [1]. So the current study aimed to assess the serum levels of malondialdehyde (MDA) and nitric oxide (NO) as oxidative stress markers in addition to total antioxidant capacity (TAO) as antioxidant defense marker in full term neonates with sepsis to investigate the possible involvement of oxidative stress and its possible diagnostic value in neonatal sepsis.
Patients and Methods
Study design and participants
The current case-control, hospital-based study has been conducted on 60 full term newborns with sepsis in addition to 30 healthy newborns as controls, recruited from NICU of Pediatric Department, Al-Azhar University Hospital, Assiut, Egypt, in collaboration with Medical Biochemistry Department, Faculty of Medicine, South Valley University, Qena, Egypt. The study has been approved by Al-Azhar University Hospital's Ethics Committee, Egypt. A written informed consent was obtained from all the participants' parents before involvement. The study period was from September 2016 to August 2017.
Selectivity criteria and clinical evaluation
All full term newborns who admitted to NICU and presented with clinical suspicion of sepsis and proved by hematological (leucocytosis, neutrophilia with or without thrombocytopenia, CRP>6 mg/dl, ESR>15 mm after the first hour) and/or bacteriological (positive blood culture) laboratory workup were eligible for the current study, while those with congenital malformation, meconium aspiration, gastro-intestinal obstruction, congenital heart diseases, inborn error of metabolism, or respiratory distress syndrome were excluded. The parents of the included patients were subjected to history taking with special concern on prenatal history (maternal diseases, pre-eclampsia, congenital infections, maternal fever, rupture of membranes more than 18 hour), natal history (mode of delivery, Apgar score 1 min and 5 min, resuscitation measures), post-natal history (respiratory distress symptoms, convulsions and other neurological symptoms, or NICU admission of any of their siblings), gestational age assessment using New Ballard Score method and pregnancy date [6].
Complete general examination including general condition, pulse, blood pressure, respiratory rate, oxygen saturation percent, temperature, and color. Neurological examinations including anterior fontanel, muscle tone, grasp, Moro and suckling reflexes. Clinical diagnosis of sepsis including symptoms and signs suggestive of sepsis in the form of poor feeding, temperature instability, vomiting, diarrhea, constipation, apneic spells, cyanosis, persistent tachycardia or bradycardia, prolonged capillary refilling time, hypotension, apathy, irritability, hypertonia, hyperreflexia, seizures, tremors, jitteriness, poor Moro and suckling reflexes, jaundice, petechiae, purpura, sclerema, omphalitis, or signs of meningitis (in the form of high pitched cry, head retraction, bulging anterior fontanel and convulsions), cranial ultrasound was done and lumbar puncture if meningitis was suspected.
Blood samples and biochemical assays
Two ml of venous blood samples were drawn from every included neonate at the same day of life from both groups and introduced on plain tubes and were centrifuged at 3500 rpm for 15 minutes. Separated serum from each tube was divided into aliquots using 1 ml cryotubes and was stored at -80°C until the biochemical analysis in the form of measuring serum malondialdehyde, nitric oxide and total antioxidant capacity using colorimetric methods (Chem-7, Erba diagnosctics, Germany).
MDA, NO, and total antioxidants assays were done using commercially available colorimetric kits supplied by Biodiagnostic, Egypt (B.D.) according to Nair et al. [7], Liu et al. [8], and Valko et al. [9] respectively and as described in previously published studies [10-13].
Statistical analysis
Data were analyzed using STATA intercooled version 12.1. Quantitative data was represented as mean ± standard deviation. Data was analyzed using student t-test to compare means of two groups. When the data was not normally distributed Kruskal Wallis test for comparison of three or more groups and Mann-Whitney test was used to compare two groups. Qualitative data was presented as number and percentage and compared using either Chi square test or fisher exact test. Pearson correlation analysis was used to identify correlation. Graphs were produced by using Excel or STATA program. P value was considered significant if it was less than 0.05.
Results
Baseline characteristics of the included neonates
The current study has been conducted on 60 term septic neonates, 20 (33.3%) were males and 40 (66.7%) were females, with their mean gestational age was 39 weeks, 1.42 SD and the mean birth weight was 3.18 kg, 0.43 SD. They were compared with 30 healthy neonates, 10 males (33.3%) and 20 (66.7%) were females. Their mean gestational age and birth weight were 38 weeks ± 1.25 SD, 3.26 kg, 0.66 SD respectively, with non-significant differences with the included cases in respect to age, birth weight and sex matching. Apgar score for cases was significantly lower than that of controls at 1st min (5 ± 2 vs. 7 ± 2) with non-significant differences at 5th min (Table 1).
| Demographic characteristics | Term septic neonates (n=60) | Control (n=30) | *P-value |
|---|---|---|---|
| Type of birth ( number and percentage) | |||
| CS | 35 (58.3%) | 21 (70%) | < 0.05 |
| NVD | 25 (41.7%) | 9 (30%) | < 0.05 |
| Maternal risk factors ( number and percentage) | |||
| Fever | 20 ( 33.3% ) | 6 ( 20% ) | < 0.01 |
| PROM | 15 ( 25% ) | 3 ( 10% ) | < 0.01 |
| Maternal UTI | 6 (10%) | 1 (3.3%) | < 0.01 |
| Sex ( number and percentage) | |||
| Male | 20 (33.3%) | 10 (33.3%) | >0.05 |
| Female | 40 (66.7%) | 20 (66.7%) | >0.05 |
| Gestational age (Mean ±SD) | |||
| GA (weeks) | 39 ± 1.42 | 38 ± 1.25 | >0.05 |
| Apgar score | |||
| At 1st minute | 5 ± 2 | 7 ± 2 | < 0.05 |
| At 5th minute | 7 ± 2 | 9 ± 1 | >0.05 |
| Birth weight (Mean ±SD) | |||
| Birth weight (kg) | 3.18 ± 0.43 | 3.26 ± 0.66 | >0.05 |
| Length (Mean ±SD) | |||
| Length (cm) | 47.1 ± 1.9 | 48.15 ± 3.66 | >0.05 |
| Head Circumference (Mean ±SD) | |||
| Head Circumference (cm) | 35.06 ± 0.77 | 34.6 ± 2.06 | >0.05 |
*N.S. (>0.05): Non statistically significant, <0.05: statistically significant, <0.01: highly statistically significant.
CS: Cesarean Section; NVD: Normal Vaginal Delivery; PROM: Premature Rupture of Membranes; UTI: Urinary Tract Infection; GA: Gestational Age.
Table 1. Baseline characteristics of the studied groups.
Pregnancy and obstetric history of neonatal mothers
Twenty-five (41.7%) of cases and 9 (30%) of controls had normal vaginal delivery. It was noted that significant portion of the cases 35 (58.3%) were delivered through Caesarean section. The study revealed that the percentage of mothers who had either fever, premature rupture of membranes, or urinary tract infection was higher in cases [20 (33.3%), 15 (25%) and 6 (10%) respectively] than controls [6 (20%), 3 (10%) and 1 (3.3%) respectively] (Table 1).
Hematological and bacteriological evidence of sepsis
There were significant higher mean leucocytic and granulocytic count, CRP, and ESR (18.32 (109/l) ± 4.35, 68.52 (103/mm3) ± 7.59, 72.65 (mg/dl) ± 46.31, 30 (mm) ±7.07 respectively), among septic neonates when compared with the controls (11.34 ± 3.28, 55.54 ± 4.25, 4 ± 1.59, 10 ± 2.19 respectively), with p value <0.05 for all. Additionally, there were significant lower mean hemoglobin level and platelets count in cases (11.74 (g/dl) ± 3.35, and 109.38 (109/l) ± 43.03 respectively) versus the control group (15.3 ± 2.32, and 290 ± 54.74 respectively), with p value <0.05 for both (Table 2).
| Variables | Septic neonates (n=60) |
Control (n=30) |
*P value |
|---|---|---|---|
| WBC (109/l) | 18.32 ± 4.35 | 11.34 ± 3.28 | < 0.01 |
| GRA (103/mm3) | 68.52 ± 7.59 | 55.54 ± 4.25 | < 0.05 |
| LYM (103/mm3) | 24.73 ± 6.96 | 26.66 ± 2.06 | > 0.05 |
| MON (103/mm3) | 8.15 ± 4.53 | 8.36 ± 2.87 | > 0.05 |
| RBC (106/mm3) | 3.78 ± 1.03 | 4.52 ± 0.32 | > 0.05 |
| HGB (g/dl) | 11.74 ± 3.35 | 15.3 ± 2.32 | < 0.05 |
| PLT (109/l) | 109.38 ± 43.03 | 290 ± 54.74 | < 0.001 |
| HCT (%) | 41.10 ± 9.82 | 47.08 ± 6.53 | < 0.05 |
| MCV (µm3) | 100.04 ± 6.87 | 97 ± 7.59 | > 0.05 |
| MCH (pg.) | 32.72 ± 2.31 | 31.84 ± 2.46 | > 0.05 |
| CRP (mg/dl) | 72.65 ± 46.31 | 4 ± 1.59 | < 0.001 |
| ESR (mm) | 30 ± 7.07 | 10 ± 2.19 | < 0.001 |
*N.S. (> 0.05): Non statistically significant, <0.05: statistically significant, <0.01: highly statistically significant.
WBC: White Blood Cells; GRA: Granulocyte; LYM: Lymphocyte; MON: Monocyte; RBC: Red Blood Cells; HGB: Hemoglobin; PLT: Platelet ; HCT: Hematocrit ; MCV: Mean Corpuscular Volume ;MCH: Mean Corpuscular Hemoglobin; CRP: C-Reactive Protein; ESR: Erythrocyte Sedimentation Rate
Table 2. Routine laboratory investigations in the form of complete blood count (CBC) parameters, CRP and ESR among septic neonates versus controls.
Among the included cases, 40 (66.6%) have early onset sepsis (EOS) with positive blood culture in 17 (42.5%) of them, while the remaining 20 (33.3%) have late onset sepsis (LOS) and blood cultures were positive in 8 (40%). Group B. Streptococci and E. coli were the most frequent causative organisms in EOS group (59%, and 23.5% respectively), while, in LOS group, Staphyloccus aureus and Klebsiella were the frequently causative organisms (50% and 37.5% respectively) (Table 3).
| Blood cultures (No.,%) |
Early onset sepsis, (EOS) (n=40, 66.6%) | Late onset sepsis, (LOS) (n=20, 33.3%) | ||
| Positive blood culture | Negative blood culture | Positive blood culture | Negative blood culture | |
| 17 (42.5%) | 23 (57.5%) | 8 (40%) | 12 (60%) | |
| Causative organisms (No.,%) |
Early onset sepsis, (EOS) (n=17) | Late onset sepsis, (LOS) (n=8) | ||
| Group B. Streptococci | 10 (59%) | Staph. aureus | 4 (50%) | |
| E. coli | 4 (23.5%) | Klebsiella | 3 (37.5%) | |
| Listeria monocytogens | 3 (17.6%) | Pseudomonas | 1 (12.5%) | |
Table 3. Blood culture and causative organisms among septic newborns (EOS and LOS).
Oxidative stress status of the included neonates
There were significant higher mean serum level of NO and MDA (33.16 (μmol/l) ± 17.82, and 4.57 (nmol/ml) ± 3.26 respectively) with significant lower mean TAO level (0.130 (mm/l) ± 0.067) among neonates with sepsis when compared with the control group, with p value <0.05 for all, while, there were non-significant differences between oxidative stress biomarkers among EOS and LOS groups, with p value >0.05 for all (Table 4).
| Variables | Neonatal sepsis group (n=60) |
Control group (n=30) |
*P value | |
|---|---|---|---|---|
| Oxidants | Nitric oxide (µmol/l) | 33.16 ± 17.82 | 26.49 ± 2.32 | <0.05 |
| Malondialdehyde (nmol/ml) | 4.57 ± 3.26 | 1.83 ± 0.47 | <0.01 | |
| Total antioxidant capacity (mm/l) | 0.130 ± 0.067 | 0.44 ± 0.24 | <0.01 | |
| Variables | Early onset septic neonates (n=40) | Late onset septic neonates (n=20) | *P value | |
| Oxidants | Nitric oxide (µmol/l) | 33.72 ±19.15 | 33.74 ±18.67 | >0.05 |
| Malondialdehyde (nmol/ml) | 2.23 ± 0.55 | 2.44 ± 0.05 | >0.05 | |
| Total Antioxidant (mm/l) | 0.13 ± 0.078 | 0.11 ± 3.61 | >0.05 | |
*N.S. (>0.05): Non-statistically Significant, <0.05: Statistically significant, <0.01: highly statistically significant.
Table 4. Comparison between serum oxidants (Nitric oxide & Malondialdehyde) and total antioxidant capacity among the study groups.
Serum NO, MDA and TAO levels at cut-off points (0.514 μmol/l, 0.399 nmol/ml, and 0.591 mm/l respectively) showed high sensitivity (96.4%, 95%, and 98.8% respectively) and specificity (93%, 92%, and 94% respectively) (Table 5).
| Variables | Area under the curve (95% CI) | Cutoff | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|---|
| Nitric oxide (µmol/l) | 0.514 (15.3 – 82.8) |
0.39 | 96.40% | 93% | 98.2 | 74 |
| Malondialdehyde (nmol/ml) | 0.399 (0.11 – 15.4) |
0.27 | 95% | 92% | 95.7 | 75 |
| Total antioxidant capacity (mm/l) | 0.591 (0.0 – 1.0) |
0.46 | 98.70% | 94% | 100 | 77 |
Table 5. Sensitivity and specificity of Nitric oxide, malondialdehyde (MDA), and total antioxidant capacity in diagnosing sepsis among term
White blood cell counts were negatively correlated to TAO (r=-0.25, p=0.01) and positively correlated to MDA in a statistically significant manner among septic neonates (r=0.34, p<0.001) (Figures 1 and 2).
Discussion
Morbidity and death from bacterial infections represent significant portion among neonates [14]. In Egypt, neonatal sepsis is prevalent due to the defective infection control measures, insufficient nursing staff and exposure to adult infection with underestimated incidences [15], in addition to birth condition, pregnancy control or maternal morbidities.
The current study revealed significantly higher percent of neonates with sepsis were delivered through cesarean section; in addition, significant portion of the included neonates with sepsis had their mothers with associated co-morbidities in the form of fever, premature rupture of membranes and urinary tract infection. Our findings were in agreement with many investigators [16-20]. In addition, neonates with sepsis exhibited significantly lower Apgar score at first min than the controls which were in line with many studies [19-22]. This could be explained that neonates with low Apgar score may indicates poor accommodation with the extrauterine life resulting from labour stress and therefore more susceptible to infection as stated by Adatara et al. [19].
A complete blood count (CBC) is frequently used to evaluate the likelihood of infection [23]. In the current study, there were significant higher white blood cell and granulocytes counts, with lower hemoglobin level and platelet counts among septic neonates when compared with controls. These results were in agreement with many researchers [24-26]. Surapanenik and Vishnu [26], reported that the membrane of the erythrocytes in the neonates is frequently subjected to oxidative stress due to pro-oxidant predominance. Cellular products of the micro-organisms enhance platelet clumping and adherence with subsequent destruction and thrombocytopenia [27]. The significantly higher CRP and ESR noticed among the included septic neonates in our study were in agreement with many studies [28-30].
The sensitivity of blood culture is low in neonatal sepsis and many factors may affect is accuracy e.g. timing and numbers of cultures taken, organism concentration, culture medium, temperature, technique, and blood volume [31]. Current study revealed that 42.5% of neonates with EOS and 40% of the included neonates with LOS have positive blood culture with Group B. Streptococci, Staph Aureus, Klebsiella, and E. coli among the most frequent causative organisms. These results were in line with many investigators [32-34].
Immune activation induced by sepsis, will initiate the redox cascade with subsequent production of reactive oxygen and nitrogen species [35], e.g. NO. The present study revealed occurrence of oxidative stress in neonatal sepsis evidenced by significant higher oxidants (NO and NDA) associated with significant lower total antioxidant capacity among the included neonates with sepsis versus the controls, with non-significant difference in the oxidative stress status regarding to onset of sepsis (EOS and LOS). These results were in accordance with many researchers [36-41]. Chuang et al. [42], reported that serum TAO could be used as marker for clinical severity of sepsis, suggesting that its lower levels in patients with severe sepsis could explained by the associated excessive pro-inflammatory cytokines and immune activation in such patients.
No previous studies could be traced in literature regarding the possible cutoff point for serum levels of oxidative stress markers in diagnosing neonatal sepsis. In the current study, serum oxidants (NO, MDA) at cut-off points (0.514 μmol/l and 0.399 nmol/ml respectively) showed sensitivity (96.4% and 95%, respectively) and specificity (93%, 92%, respectively), while serum level of TAO at cutoff 0.591 mm/l exhibited 98.8% sensitivity and 94% specificity in diagnosing sepsis among neonates.
Conclusion
The present study revealed significant correlations between the oxidative stress markers and the immune system activation represented by the white blood cell count as evidenced by the presence of significant positive correlation between WBCs and MDA with significant negative correlation between WBCs and TAO among the included septic neonates. The results of the current study confirm the involvement of oxidative stress in neonatal sepsis with its possible use in sepsis diagnosis when combined with other standard diagnostic tools. We call for trials using antioxidants as adjunct therapy in neonatal sepsis.
Limitations of the study
One of the main limitations of this study is that it does not contain any preterm infancy group. Because due to the deficient and immature antioxidant system, NO, MDA and TAO levels should be compared with septic term and preterm infants. Additionally, the study was performed in term infants and we only evaluated these parameters during sepsis, it would be more useful if these parameters were evaluated at 48-72 hours and 7-10 days of therapy.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Authors contribution
• AMMH, AAH, YTK equally contribute in the study concept, design, supervision, methodology, statistical analysis and data collection.
• MHH performed the investigations and laboratory workup and writes the first draft of the manuscript.
• All authors reviewed and approved the final draft of the manuscript.
References
- Poggi C, Dani C. Sepsis and oxidative stress in the newborn: From pathogenesis to novel therapeutic targets. Oxid Med Cell Longev. 2018; 2018: 9390140.
- Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum. Dev. 2012; 88: 69-74.
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014; 24: 453-462.
- Sies H. On the history of oxidative stress: concept and some aspects of current development. Curr Opin. Toxicol. 2018; 7: 122-126.
- Tsukahara H. Biomarkers for oxidative stress: Clinical application in pediatric medicine. Curr Med Chem. 2007; 14: 339-351.
- Ballard JL, Khoury JC, Wedig K, et al. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991; 119: 417-423.
- Nair V, O'Neil CL, Wang PG. Malondialdehyde encyclopedia of reagents for organic synthesis, John Wiley and Sons, New York. 2008.
- Liu B, Gao HM, Wang JY, et al. Nitric oxide: Novel actions, deleterious effects, and clinical potential. Ann N Y Acad Sci. 2012; 962: 318-331.
- Valko M, leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human diseases. Int j biochem cell boil. 2007; 39: 44-84.
- Abdel-Maged WM, Hassan MH, Mostafa MA, et al. Lesional levels of superoxide dismutase and malondialdehyde in paucibacillary and multibacillary leprosy patients. Journal of the Egyptian Women's Dermatologic Society. 2017; 14: 156-160.
- El-Masry HMA, Sadek AA, Hassan MH, et al. Metabolic profile of oxidative stress and trace elements in febrile seizures among children. Metab Brain Dis . 2018; 33: 1509-1515.
- Saleem TH, Abo El-Maali N, Hassan MH, et al. Comparative protective effects of n-acetylcysteine, n-acetyl methionine, and n-acetyl glucosamine against paracetamol and phenacetin therapeutic doses-induced hepatotoxicity in rats. Int J Hepatol. 2018; 2018: 7603437.
- Ahmed AE, Hassan MH, Rashwan NI, et al. Myocardial injury induced by scorpion sting envenoming and evidence of oxidative stress in Egyptian children. Toxicon.2018; 153 ; 72–77.
- McIntire DD, Bloom SL, Casey BM, et al. Birth weight in relation to morbidity and mortality among newborn infants. N Eng J Med. 2003; 35: 1234-1238.
- Youssry I, Edris A, Tawfik N, et al. Monocytes expressing tissue factor as a diagnostic marker of neonatal sepsis. Pediatrics. 2008; 816: 121-124.
- Ladfors L, Tessin I, Mattsson LA, et al. Risk factors for neonatal sepsis in offspring of women with prelabor rupture of the membranes at 34–42 weeks. J of perinatal med. 1998; 26: 94–101.
- Shah G, Budhathoki S, Das BK, et al. Risk factors in early neonatal sepsis. Kathmandu University Medical Journal. 2006; 4: 187-191.
- Utomo MT. Risk factors of neonatal sepsis: A preliminary study in Dr. Soetomo hospital. Indonesian Journal of Tropical and Infectious Disease. 2010; 1: 23–26.
- Adatara P, Afaya A, Salia SM, et al. Risk factors for neonatal sepsis: A retrospective case-control study among neonates who were delivered by caesarean section at the trauma and specialist hospital, Winneba, Ghana. Biomed Res Int. 2018; 2018: 6153501.
- Adatara P, Afaya A, Salia SM, et al. Risk factors associated with neonatal sepsis: A case study ata specialist hospital in ghana. The Scientific World Journal. 2019; 2019.
- Sundaram V, Dutta S, Ahluwalia J, et al. Score for neonatal acute physiology II predicts mortality and persistent organ dysfunction in neonates with severe septicemia. Indian Pediatrics. 2009; 46: 775-780.
- Jabiri A, Wella HL, Semiono A, et al. Prevalence and factors associated with neonatal sepsis among neonates in Temeke and Mwananyamala Hospitals in Dar es Salaam, Tanzania. Tanzania Journal of Health Research. 2016; 18: 1-6.
- Newman TB, Puopolo KM, Wi S, et al. Interpreting complete blood counts soon after birth in newborns at risk for sepsis. Pediatrics. 2010; 126: 903-909.
- Kuboyama RH, DeOliveria HB, Moretti-Branchini MX. Molecular epidemiology of systemic infection caused by enterobacter cloacae in a high risk neonatal intensive care unit. Infect control Hosp Epidemiol. 2003; 24: 490-494.
- Ng PC, Lam HS. Diagnostic markers for neonatal sepsis. Curr Opin Pediatr. 2006; 18: 125-131.
- Surapaneni KM, Vishnupriya V. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in neonatal jaundice patients. Journal of Clinical and Diagnostic Research. 2008; 3: 827-832.
- Guida JD, Kunig AM, Leef KH. Platelet count and sepsis in very low birth weight neonates: Is there an organism specific response? Pediatrics. 2003; 111: 1411-1415.
- Stoll BJ. Infections of the Neonatal Infant. In: Kliegman RM, Behrman RE, Jenson HB and Stanton BF (eds.,). Nelson Textbook of Pediatrics, 18th ed. Elsevier. 2008; 109: 794-811.
- Marrekchi Z. Early-onset neonatal infection. Experience from Yunisia. 19th scientific congress of the Egyptian Society for Neonatal and Preterm Care (ESNPC). 2007.
- Joram N, Boscher C, Denizot S, et al. Umbilical cord blood procalcitonin, C-reactive protein concentrations and ESR as markers for early diagnosis of very early-onset neonatal infection. Arch Dis Child Fetal Neonatal Ed. 2006; 91: 65-66.
- Kumar Y, Qunibi M, Neal TJ, et al. Time to positivity of neonatal blood cultures. Arch Dis Fetal Neonatal Ed. 2001; 85: 182-6.
- Boraey NF, Sheneef A, Mohammad MA, et al. Procalcitonin and C-reactive protein as diagnostic markers of neonatal sepsis. Aust J Basic Appl Sci. 2012; 6: 108–14.
- Hisamuddin E, Hisam A, Wahid S, et al. Validity of C-reactive protein (CRP) for diagnosis of neonatal sepsis. Pak J Med Sci .2015; 31: 527–531.
- Rashwan NI, Hassan MH, Mohey El-Deen ZM, et al. Validity of biomarkers in screening for neonatal sepsis - A single center -hospital based study. Pediatr Neonatol. 2018.
- Spasojević I, Obradović B, Spasić S. Bench-to-bedside review: Neonatal sepsis - redox processes in pathogenesis. Critical Care. 2012; 16: 221.
- Kumar R, Mandal RN, Tandon A, et al. Serum TNF-alpha and free radical scavengers in neonatal septicemia. The Indian Journal of Pediatrics. 1999; 66: 511-516.
- Batra S, Kumar R, Kapoor AK, et al. Alterations in antioxidant status during neonatal sepsis. Annals of Tropical Paediatrics. 2000; 20: 27–33.
- Marom D, Yuhas Y, Sirota L, et al. Nitric oxide levels in preterm and term infants and in premature infants with bacteremia. Biol Neonate. 2004; 86: 160-164.
- Mahmoud AT, Tawfik MM, Alshafey MK, et al. Study on nitric oxide level in septicemic neonates. Menoufia Med J. 2014; 27: 474-477.
- Annagür A, Örs R, Altunhan H, et al. Total antioxidant and total oxidant states, and serum paraoxonase-1 in neonatal sepsis. Pediatr Int. 2015; 57: 608-613.
- Asci A, Surmeli-Onay O, Erkekoglu P, et al. Oxidant and antioxidant status in neonatal proven and clinical sepsis according to selenium status. Pediatr Int. 2015; 57: 1131-1137.
- Chuang CC, Shiesh SC, Chi CH, et al. Serum total antioxidant capacity reflects severity of illness in patients with severe sepsis. Crit Care. 2006;10: R36.