Research Article - Journal of Clinical Endocrinology Research (2018) Volume 1, Issue 1
Childhood hypopituitarism: Etiological pattern in a major teaching hospital in Saudi Arabia.
Nasir A-M Al-Jurayyan*, Reem A H AlKhalifah, Sharifah D A Al-Issa, Hessa M N Al-Otaibi
Department of Pediatrics, College of Medicine and King Khalid University Hospital, Riyadh, Saudi Arabia
- *Corresponding Author:
- Nasir A-M. Al-Jurayyan
Professor and Consultant Pediatric of College of Medicine,
Endocrinologist, Division of Endocrinology, Department Pediatrics (39),
P.O Box 2925, Riyadh 11461, Saudi Arabia
Tel: 00966505400592
Fax: 00966-11-4679463
E-mail: njurayyan@gmail.com
Accepted date: July 06, 2018
Citation: Al-Jurayyan NAM, AlKhalifah RAH, Al-Issa SDA, et al. Childhood hypopituitarism: Etiological pattern in a major teaching hospital in Saudi Arabia. J Clin Endocrinol Res. 2018;1(1):1-5.
Abstract
Background: Childhood hypopituitarism is a clinical syndrome of deficiency in pituitary hormone production. It might be partial or complete. Presentation varies from asymptomatic to acute collapse depending on the etiology, rapidity of onset, and predominant hormone involved.
Design and setting: A retrospective hospital based study was conducted at the pediatric endocrine service, King Khalid University Hospital (KKUH) Riyadh, Saudi Arabia during the period of January 1989 and December 2017.
Material and methods: The medical records of patients with the diagnosis of hypopituitarism were retrospectively reviewed. Data included age, sex, clinical presentation, and results of relevant laboratory investigations and radiological imaging.
Results: During the period under review, a total of 202 patients were diagnosed to have hypopituitarism. Mean age at diagnosis was 8.9 years, range 0-18 years. A brain MRI was helpful in identifying the cause. A diversity of causes was encountered with a non-tumor causes being the commonest.
Conclusion: Childhood hypopituitarism is not that rare. A brain MRI scan is critical in attempting to determine the specific cause and plan the management.
Keywords
Hypopituitarism, cause, children, Saudi Arabia.
Introduction
Childhood hypopituitarism is a clinical syndrome of deficiency in pituitary hormone production. It might be a life threating condition. It can result from disorders involving the pituitary gland, the hypothalamus, or the surrounding structures, such as tumor, inflammation, or infection, destruction, surgical, radiation, traumatic or vascular insult. Hypopituitarism could be partial or complete insufficiency of pituitary hormone. Panhypopituitarism refer to involvement of two or more pituitary hormone. However, involvement of one hormone only refers to isolated or partial hypopituitarism. The younger the child is at the time of presentation the more likely the etiology is to be congenital. However, on occasions, congenital forms may present or diagnosed well after birth and conversely, some children with acquired form are discovered relatively early in life [1-5].
This article reports on the clinical pattern of childhood hypopituitarism as seen in a major teaching hospital, King Khalid University Hospital (KKUH) in Saudi Arabia over more than 25 years, January 1989 to December 2017.
Material and Methods
The medical records of patients who were diagnosed to have hypopituitarism were retrospectively reviewed. Data included age, sex, clinical presentation, and results of the relevant laboratory investigations and radiological images. The diagnoses of hypopituitarism was based on clinical suspicion suggested by the appropriate hormonal testing Magnetic resonance imaging (MRI) scan was done when appropriate.
The various hormonal testing to assess both the anterior and posterior pituitary gland functions, were performed following the specific protocols. Unfortunately, no genetic studies were done in any of our patients. Pituitary function was evaluated after 1-2 months of any neurological procedure. Thyroid function was periodically evaluated as to monitor for thyroid status.
Statistical Analysis
Statistical packages for social service (SPSS version 21) were used for the statistical analysis of the data.
Results
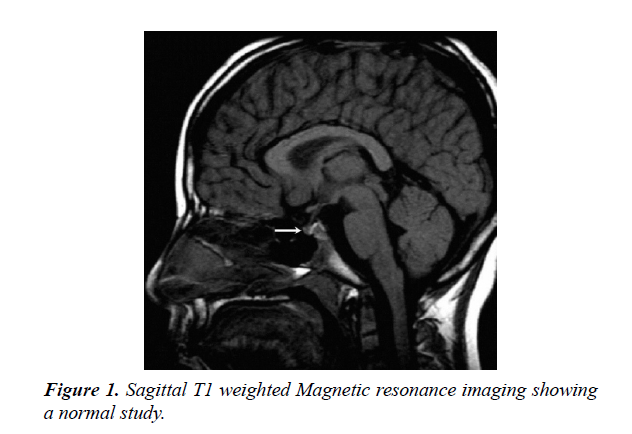
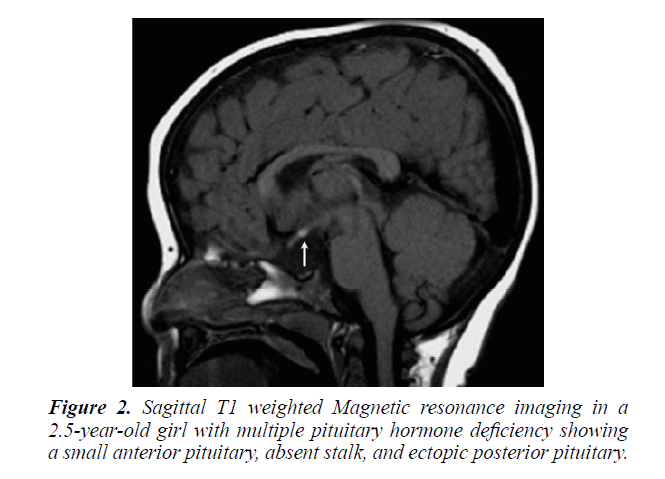
During the period under review, January 1989 and December 2017, There were 202 patients with hypopituitarism, of these, 142 (70.3%) were males and 60 (29.7%) females. the mean age was 8.9 years (range 0-18 years). MRI brain was performed in 173 (85.6%) of patients, Figures 1 and 2.
The etiology of hypopituitarism was variable. Of these, growth hormone deficiency (GHD) was the commonest:- 152 (70.2%) patients with isolated growth hormone deficiency (IGHD) in 123 patients and multiple pituitary hormone deficiency (MPHD) in 29 patients, Table 1. Central adrenal insufficiency was the second most common hormonal deficiency seen in 59 (29.2%), Table 2. Diabetes insipidus associated with Panhypopituitarism seen in 24 (11.9%) patients, Table 3, while hypothyroidism presented in 14 (6.9%) patients of these five patients were isolated central hypothyroidism. Gonadotrophic hormone deficiency was present in 8 (3.9%) patients.
Table 1: Etiology of growth hormone deficiency (GHD) in 152 patients with magnetic resonance imaging (MRI).
| Isolated Growth HormoneDeficiency No. (123): | Multiple Pituitary Hormone Deficiency(MOHD) No. (29): | ||||
|---|---|---|---|---|---|
| Diagnosis | No. | % | Diagnosis | No. | % |
| Idiopathic | 111 | 90.2 | ·Hypothalamic pituitary tumor | 12 | 41.4 |
| ·Birth asphyxia | 9 | 7.4 | ·Congenital hypopituitarism | 6 | 20.7 |
| ·Neurofibromatosis | 2 | 1.6 | ·Septo-optic dysplasia | 3 | 10.3 |
| ·Histocytosis X | 1 | 0.8 | ·Trumatic brain injury | 3 | 10.3 |
| Majority of brain MRI showed normalMRI | ·Histocytosis X | 2 | 6.9 | ||
| ·Infection | 2 | 6.9 | |||
| ·Empty sella | 1 | 3.5 | |||
Majority of brain MRI showedpituitary hypoplasia or aplasia
Table 2: Etiology of central adrenal insufficiency in 25 patients with hypopituitarism.
| Diagnosis | Number | Percentage % |
|---|---|---|
| ·Hypothalamic pituitary tumor | 12 | 20.3 |
| ·Congenital hypopituitarism | 19 | 32.2 |
| ·Isolated ACTH deficiency | 3 | 5.1 |
| ·Histocytosis-X | 3 | 5.1 |
| ·Septo-optic dysplasia | 6 | 10.2 |
| ·Infection : streptococcal meningitis | 2 | 3.4 |
| · Post-trumatic brain injury | 3 | 5.1 |
| ·Cushing’s syndrome | 1 | 1.7 |
| ·Drug induced glucocorticoid deficiency | 10 | 16.9 |
ACTH: Adrenocorticotrophic Hormone
Table 3: Etiology of Central Diabetes Insipidus (CDI) in 24 patients with hypopituitarism.
| Diagnosis | Number | Percentage % |
|---|---|---|
| ·Brain Tumor | 11 | 45.8 |
| ·Congenital hypopituitarism | 4 | 16.7 |
| ·Post-trumatic brain injury | 3 | 12.5 |
| ·Histocytosis-X | 3 | 12.5 |
| ·Infection : streptococcal meningitis | 2 | 8.3 |
| · Septo-optic dysplasia | 1 | 4.1 |
There were few cases of hypopituitarism which we came across. Various tumors in the hypothalamic pituitary region, the majority of which is craniopharyngioma, constitute 12 (5.9%) patients in our series, while traumatic brain injury (TBI), in three patients and subarachnoid hemorrhage (SAH), in one patient. Central nervous system infections were documented in two and histocytosis in three with empty sella syndrome in one patient.
Discussion
Hypopituitarism is not that rare in children. It follows a smoldering course, unless it has an onset with pituitary apoplexy. Hypopituitarism is often associated with increased mortality [1-6].
Magnetic resonance imaging (MRI) scan remains the modality of choice for assessing the hypothalamic pituitary region in patient with hypopituitarism. MRI scan precisely diagnose abnormality of the adenohypophysis and neurohypophysis usually the stalk. Pituitary stalk interruption syndrome seems to be more strongly associated with perinatal adverse events and has been reported more frequently in patients with multiple pituitary hormone deficiency than in those with isolated GHD. Nevertheless, the existence of significant relationships between neuroradiological findings and either perinatal events or endocrine function in hypopituitarism has not been confirmed by others and the subject remains controversial. A midline syndrome is suggested by absence of the septum pellucidum or the corpus callosum. Different central nervous system malformation, such as optic nerve hypoplasia, hydrocephalus and vascular abnormalities, also brain tumors in the hypothalamic pituitary region such as craniopharyngioma, can be detected. Such findings indicate the importance and the role of pituitary and hypothalamus MRI scan in determining the cause. Our study supports this [7-12].
The clinical presentation varies from asymptomatic to acute collapse, depending on the etiology, rapidity of onset, and predominant hormones involved. Children with birth trauma, midline defect, optic nerve atrophy (suggesting septo-optic dysplasia), or in boys with micropenis (suggesting gonadotropin hormone deficiency) suggest hypopituitarism. It may also be associated with diabetes insipidus due to posterior pituitary gland abnormalities [1,13-17].
A variety of diseases may cause hypopituitarism, Growth hormone deficiency (GHD), the most common hormonal deficiency, and present with a wide spectrum of findings. The endocrine abnormality of GHD manifest either as an isolated deficiency (IGHD) or it may be present as multiple hormone deficiency (MPHD). A spectrum of MRI findings were observed in our series ranging from normal to small or complete absent of anterior or posterior pituitary together with absence or thin stalk [3,18-20].
Secondly, central adrenal insufficiency accounts for the second most commonly encounters in this study, however, it is the most lethal. All except one had multiple anterior pituitary hormone deficiency. This was present in 59 (29.3%) patients [21,22].
Thirdly, central diabetes insipidus (CDI) or anti-diuretic hormone (ADH) deficiency may be associated with hypopituitarism, due to impairment of posterior pituitary gland or hypothalamus in 24 (11.9%) patients. Patients presented with excessive water drinking, thirst, excessive urination, and had a high serum sodium of more than or equal 150 mmol\l with diluted urine, a water deprivation test was confirmed this [23,24].
Secondary hypothyroidism due to various causes was present in 14 (6.9%) [25]. Congenital isolated hypogonadotrophin was extremely rare in this study, was reported in 8 (3.9%) patients out of 202 patients, and that is similar to what had been reported before. However, secondary hypogonadism is not that rare [26].
Various tumors in the hypothalamic pituitary region where encountered in our study. Most likely is the development of mass effect of the tumor on the neighboring structures. However, surgical procedure to remove the tumor may lead to hypopituitarism. Radiotherapy treatment for the tumors in the head and neck region may cause damage to the pituitary. Different hypothalamic pituitary axis has different sensitivity to radiation [27-30].
Traumatic brain injury is very important cause in this study, with subarachnoid hemorrhage. The incidence of TBI is 100- 150 in 100,000. Post-traumatic hypopituitarism is observed in 5.4 to 40% of patient with history of TBI usually presenting as isolated deficiency in most cases. Hypopituitarism has been observed in 19% of patient with ischemic stroke and 47% of patient with SAH presenting as an isolated deficiency in most cases. This could be due to lack of awareness [31-35]. Efforts need to be made to sensitize the clinicians about the existence of hypopituitarism in such patients.
Langerhans cell histocytosis (LCH) is a rare and heterogeneous disease, characterized by accumulation and cloned proliferation of immature dendritic cells in different organs, present in 3 (1.5%) of patients [36,37].
Central nervous system infections known to have high incidence in developing countries are known to cause hypopituitarism. In this study hypopituitarism was observed in 2 (0.5%) of patients with streptococcal infection [38,39]. And finally, empty sella is not that rare in our study as reported by others [40,41].
Conclusion
This is the largest report of childhood hypopituitarism from Saudi Arabia. Hypopituitarism is not that rare in children. A brain MRI scan is critical in attempting to determine the specific cause and plan the management. It needs to be diagnosed early to prevent associated mortality and morbidity. The etiology differs in childhood as non-tumor cause are common than in adults.
Acknowledgement
The authors would like to thank Mr. Abdulrahman N Aljurayyan for his help in preparing the manuscript.
Conflict of Interest
The authors have no Conflict of Interest to declare
Funding
None
References
- Geffner ME. Hypopituitarism in Childhood. Cancer Control. 2002;9(3):212-22.
- Kim SY. Diagnosis and Treatment of Hypopituitarism. Endocrinal Metab (Seoul). 2015;30(4):443-55.
- Gundgurthi A, Garg MK, Bhardwaj R, et al. Clinical spectrum of hypopituitarism in India. A single centre experiene. Indian J Endocrinol Metab. 2012;16(5):803-8.
- Van Aken MO, Lamberts SW. Diagnosis and treatment of hypopituitarism: an update. Pituitary. 2005;8(3-4):183-91.
- Higham C E, Johannsson G, Shalet SM. Hypopituitarism. Lancet. 2016;388(10058):2403-15.
- Regal M, Páramo C, Sierra SM, et al. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol (Oxf). 2001;55(6):735-40.
- Dutta P, Bhansali A, Singh P, et al. Congenital hypopituitarism: clinico-radiological correlation. J Pediatr Endocrinol Metab. 2009;22(10):921-8.
- AlJurayyan RNA, Al Jurayyan NAM, Omer HG, et al. Pituitary imaging in 129 children with growth hormone defeciency. A spectrum of finding. Sudan J Paediatr. 2017;17(1):30-5.
- Maghnie M, Lindberg A, Koltowska-Haggstrom M, et al. Magnetic resonance Imaging of CNS in 15 043 children with GH deficiency in KIGS (Pfizer International Growth Database). Eur J Endocrinal. 2013;168:211-7.
- Di Iorgi N, Allergri AE, Napoli F, et al. The use of neuroimaging for assessing disorders of pituitary development. Clin Endocrinol (Oxf). 2012;76(2):161-76.
- Maghnie M, Ghirardello S, Genovese E. Mangnetic resonance imaging of the hypothalamus-pituitary unit in children suspected of hypopituitarism: who, how and when to investigate. J Endocrinol Invest. 2004;27(5):496-509.
- Garel C, Leger J. Contribution of magnetic resonance imaging in non-tumoral hypopituitarism in children. Horm Res. 2007;67(4):194-202.
- Lustig RH, Rose SR, Burghen GA, et al. Hypothalamic obesity caused by cranial insult in children: altered glucose and insulin dynamics and reversal by a somatostatin agonist. J Pediatr. 1999;135(2):162-8.
- Hatipoğlu N, Kurtoglu S. Micropenis: Etiology, Diagnosis and Treatment Approaches. J Clin Res Pediatr Endocrinol. 2013;5(4):217-23.
- Koral K, Geffner ME, Curran JG. Trans-sphenoidal and sphenoethmoidal encephalocele: report of two cases and review of the literature. J Australas Radiol. 2000;44(2):220-4.
- Spray CH, Mckiernan P, Waldron KE, et al. Investigation and outcome of neonatal hepatitis in infants with hypopituitarism. Acta Paediatr. 2000;89(8):951-4.
- Phillips Plt, Brodsky MC. Congenital optic nerve abnormalities in pediatric ophthalmology and strabismus 2nd edition, edited by wright KW. Springer Verlag Inc. 2003;918-22.
- Stanley T. Diagnosis of Growth Hormone Deficiency in Childhood. Curr Opin Endocrinol Diabetes Obes. 2012;19(1):47-52.
- GH Research Society. Consensus Guidelines for the Diagnosis and Treatment of Growth Hormone (GH) Deficiency in Childhood and Adolescence: Summary Statement of the GH Research Society. J Clin Endocrinol Metab. 2000;85(11):3990-3.
- Richmond EJ, Rogol AD. Growth hormone deficiency in children. Pituitary. 2008;11:115.
- Al-Jurayyan NAM. Central Adrenal Insufficiency in Children in a Major Teaching Hospital Riyadh, Saudi Arabia. (Ponte) Int Sci Res J. 2016;72(10):271-6.
- Grossman AB. The Diagnosis and Management of Central Hypoadrenalin. J Clin Endocrinol Metab. 2010;95(11):4855-63.
- Loh JA, Verbalis JG. Diabetes insipidus as a complication after pituitary surgery. Nat Clin Pract Endocrinol Metab. 2007;3(6):489-94.
- JK Devin. Hypopituitarism and central diabetes insipidus: perioperative diagnosis and management. 2012;23(4):679-89.
- Lania A, Persani L, Beck-Peccoz P. Central hypothyroidism. Pituitary. 2008;11(2):181-6.
- Al-Jurayyan NA, Al Issa SD, Al Nemri AM, et al. The spectrum of 46XY disorders of sex development in a University centre in Saudi Arabia. J Pediatr Endocrinol Metab. 2015;28(9-10):1123-7.
- Karavitaki N, Wass JA. Craniopharingioma. Endocrinol Metab Clin North Am. 2008;37(1):173-93.
- Darzy KH, Shalet SM. Hypopituitarism as a Consequence of Brain Tumors and Radiotherapy. Pituitary. 2005;8(3-4):203-11.
- Pietila S, Makipernaa A, Koivisto AM, et al. Growth impairment and gonadal axis abnormalities are common in survivors of paediatric brain tumours. Acta Paediatr. 2017;106(10):1684-8.
- Wachter D, Gondermann N, Oertel MF, et al. Pituitary insufficiency after operation of supratentorial intra- and extra axial tumors outside of the sellar–parasellar region. 2011;34(4):509-16.
- Benvenga S, Campenni A, Ruggeri RM, et al. Hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85(4):1353-61.
- Aimaretti G, Ambrosio MR, Di Somma C, et al. Traumatic brain injury and subarachnoid haemorrhage are conditions at high risk for hypopituitarism: Screening study at 3 months after the brain injury. Clin Endocrinol (Oxf). 2004;61(3):320-6.
- Kokshoorn NE, Smit JW, Nieuwlaat WA, et al. Low prevalence of hypopituitarism after traumatic brain injury: A multicenter study. Eur J Endocrinol. 2011;165(2):225-31.
- Bondanelli M, Ambrosio MR, Zatelli MC, et al. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152(5):679-91.
- Ghigo E, Masel B, Aimaretti G, et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19(9):711-24.
- Kurtulmus N, Mert M, Tanakol R, et al. The pituitary gland in patients with Langerhans cell histiocytosis: a clinical and radiological evaluation. Endocrine. 2015;48(3):949-53.
- Galvan ST, Vilaseca AP, Alevras TM, et al. Endocrine changes in histiocytosis of the hypothalamic pituitary axis. J Endocrinol Nut. 2015;62(2):72-5.
- Schaefer S, Boegershausen N, Meyer S, et al. Hypothalamic-pituitary insufficiency following infectious diseases of the central nervous system. Eur J Endocrinol. 2008;158(1):3-9.
- Tanriverdi F, Alp E, Demiraslan H, et al. Investigation of pituitary functions in patients with acute meningitis: a pilot study. J Endocrinol Invest. 2008;31(6):489-91.
- Jones M, Drut R, Valencia M, et al. Empty Sella syndrome, panhypopituitarism and diabetes insipidus. Pediatr Pathol. 2005;24(3):191-204.
- Radha RP, Maheshwari R, Karthik Reddy TS, et al. A Study of prevalence of endocrine abnormalities in primary empty sella. Indian J Endocrinol Metab. 2013;17(1):S125-6.