Research Article - Biomedical Research (2017) Volume 28, Issue 6
Chewing ameliorates stress-induced enhancement of anxiety behaviours in an animal model of post-traumatic stress disorder
Shinjiro Miyake1*#, So Koizumi1#, Kenichi Sasaguri2 and Toshitsugu Kawata1
1Department of Oral Science, Orthodontic Science, Graduate School, Kanagawa Dental University, Japan
2Department of Dentistry, Oral and Maxillofacial Surgery, Jichi Medical University, Shimotsuke, Tochigi, Japan
#These authors equally contributed for this work
- *Corresponding Author:
- Shinjiro Miyake
Department of oral science, Orthodontic Science
Graduate School, Kanagawa Dental University, Japan
Accepted date: November 23, 2016
Abstract
Post-Traumatic Stress Disorder (PTSD) is a stress-related mental disorder caused by traumatic experience, with symptoms such as intrusive memories, hyperarousal, and avoidance, which endure for years. Single-Prolonged Stress (SPS) is an animal model proposed for PTSD. Rats exposed to SPS showed enhanced Hypothalamic-Pituitary-Adrenal (HPA) axis inhibition, which has been reliably reproduced in PTSD patients, and increased Glucocorticoid Receptor (GR) expression in the hippocampus. We aimed to elucidate whether chewing improved stress-induced fear responses and anxiety behaviours along with ameliorating feedback inhibition of HPA responses. SPS rats were subjected to restraint stress by immobilization for 2 h before forced swimming and ether anaesthesia exposure. SPS with chewing (SC) group were allowed to chew on a wooden stick during the latter half of the immobilization period, whereas the stress without chewing (ST) group were not allowed to do so. Fear response and anxiety behaviour were significantly increased in the ST group compared with the SC group. Further, we found significantly reduced field Excitatory Postsynaptic Potentials (EPSPs) in the CA1 region of the hippocampus in the ST group compared with control and SC groups. The results suggest that chewing is a behavioural mechanism to cope with PTSD.
Keywords
Post-traumatic stress disorder, Chewing, Stress.
Introduction
It has been reported that decreased masticatory function caused by loss of molars, tooth attrition, or long-term intake of soft foods leads to impaired learning and memory processes and inhibits the negative-feedback response by the down regulation of Glucocorticoid Receptor (GR) protein and mRNA expression in the hippocampus [1-8]. Chewing improves stress-induced suppression of spatial memory along with increased hippocampal GR expression [9]. Post-Traumatic Stress Disorder (PTSD) is a stress-related mental disorder caused by experiencing traumatic events [10] and presents with characteristic symptoms, including intrusive memories (flashback), hyperarousal, and avoidance. The clinical pathophysiology of PTSD in patients involves multiple brain systems, such as disturbances of the autonomic nervous system and the Hypothalamic-Pituitary-Adrenal (HPA) axis [10-13]. The Single-Prolonged Stress (SPS) model produces a core symptom of PTSD, the enhanced fear response to the traumatic cue. This investigative tool is typically used for PTSD studies. A rat model involving SPS has been developed and employed for PTSD research [14,15]. The rats exposed to SPS show enhanced inhibition of the HPA system. The expression level of GR was significantly increased in the hippocampi of SPS rats, which could explain the potentiated feedback inhibition of the HPA response [16].
Chewing has been shown to alter HPA axis function and improve the ability to cope with stress in rodents [9]. The aim of this study was to test the hypothesis that chewing improves stress-induced fear response and anxiety behaviour while ameliorating feedback inhibition of the HPA responses.
Materials and Methods
Single prolonged stress (SPS) paradigm
Male Sprague-Dawley rats aged 10 weeks (Nihon SLC, Shizuoka, Japan) and weighing 300-330 g were used. The rats were group-housed (four/cage) in a room maintained under controlled light conditions (12:12 h light: dark cycle) and temperature (22°C ± 3°C). They had free access to food pellets and tap water. The experimental protocol was reviewed and approved by the Committee on Ethics on Animal Experiments of Kanagawa Dental College. SPS rats were prepared independently for each experiment to prevent carryover effects due to multiple behavioural tests. After the acclimation period, the rats were exposed to SPS followed by 20 m forced swimming (25°C) and exposure to ether anaesthesia [17]. The rats were then returned to their home cage and left undisturbed until the experimental manipulations.
Immobilization and chewing condition during SPS
The Control (CT) rats were not immobilized, but the rats in SPS-without chewing (ST) and SPS-with chewing (SC) groups were immobilized according to a well-established protocol [18,19] before the 20 m forced swimming and exposure to anaesthesia. Restraint stress was induced by securing each rat to a wooden board in the supine position using a leather belt, and all four legs were fixed at a 45° angle to the body midline using adhesive tape. The ST rats were maintained in this position for 2 h, whereas the SC rats were allowed to chew on a wooden stick during the latter half of the immobilization period. Every rat in group SC responded to the wooden stick by chewing on it with a rapid and repetitive sequence of jaw opening and closing movements for at least two-thirds of the total the chewing period.
Dexamethasone (Dex) suppression test (DST) and measurement of plasma corticosterone
One week after SPS, Dex (0.05 mg/kg; Sigma, St. Louis, MO, USA) or vehicle (saline containing 5% ethanol) was administered subcutaneously 2 h prior to the second stress exposure (30 m restrain). Each animal was restrained as described above and blood samples were collected from the tail vein at 0 and 30 m after the beginning of the second restraint period. The concentration of the plasma corticosterone was determined with an enzyme immunoassay kit (Diagnostic System Laboratories Webster, TX, USA).
Behavioural studies
Measurement of spontaneous locomotors activity: Seven days after SPS, the rats were placed at the center of a cubic chamber (650 W × 650 H × 450 D mm). The total distance travelled by the animal in 15 min was measured by an automated analysing system (Topscan, CleverSys, Inc., Reston, VA, USA). All animals were habituated to the testing room for 20 min before the start of the session. The test room was dimly illuminated with indirect white lighting, as rats are nocturnal and their natural exploratory behaviour is hindered in wellilluminated conditions.
Elevated plus maze (EPM): Seven days after SPS, the rat was placed in the center of the EPM facing an open arm and allowed to explore the maze for 5 min under the constant surveillance of a video camera. The EPM consisted of two open and two closed arms (1 m in length) constructed from steady black Perspex and raised approximately 60 cm off the floor. Two of the opposing arms (50 cm × 10 cm) were enclosed by 40 cm high side and end walls (closed arms), whereas the other two arms had no walls (open arms). The degree of aversive behaviour was determined by calculating the ratio of the time spent in the open arms relative to the total time in both arms; excluding the time spend in the central square.
Contextual fear paradigm (CF): The Contextual Fear paradigm (CF) was conducted 7 days after SPS. On the first day, each rat was exposed to the conditioning context (180 s, in the conditioning chamber (325 W × 280 H × 500 D mm) without any stimulation). Immediately after, a footshock (0.8 mA, 4 s) was delivered through a stainless steel grid floor by a shock generator scrambler (SGS-003; Muromachi, Tokyo, Japan). After the footshock, the rat was immediately transferred to its home cage. Twenty-four hours after the initial footshock, the rat was placed in the conditioning chamber where it had previously received the footshock, and the contextual fear response was evaluated by measuring the duration of freezing behaviour during 180 s. Freezing was defined as the total absence of body or head movement except for breathing. Freezing behaviour was recorded using a video recorder, and later scored blindly by the experimenter. Fear was quantified as the amount of time spent freezing.
Electrophysiology
We anesthetized the rats at 7 days after SPS, decapitated them, quickly removed their brains, and chilled them in Artificial Cerebrospinal Fluid (ACSF). We dissected the hippocampi, embedded them in agar blocks for slicing, cut transverse sections (450 μm in thickness) with a vibrating tissue slicer (Dosaka, Kyoto, Japan), and transferred them to a holding chamber at room temperature (25°C). We allowed the slices to recover for at least 60 min and then transferred them to an immersion-type recording chamber perfused at 1 ml/min with ACSF containing 0.1 mM picrotoxin (Sigma-Aldrich, Tokyo, Japan) at room temperature. To prevent epileptiform discharges in pyramidal neurons, we made a cut at the border between the CA1 and CA3 areas. A glass pipette filled with 3 M NaCl (sodium chloride) and positioned in the stratum radiatum of the CA1 area recorded the field Excitatory Postsynaptic Potential (fEPSP). Bipolar stainless-steel electrodes (World Precision Instruments, Sarasota, FL, USA) placed in the stratum radiatum on opposite sides of the recording pipette stimulated the Schaffer collateral branches. We adjusted the intensity of fEPSP in the baseline period to around 30% of the maximal response and then recorded stable baselines fEPSP activity by applying a 40 μs voltage pulse at the determined intensity every 30 s for at least 10 min. Tetanic stimulation (1 s, 100 Hz), applied nine times at intervals of 90 s, induced Long-Term Potentiation (LTP). All signals were filtered at 2 kHz using a low-pass Bessel filter and digitized at 5 kHz using a MultiClamp 700A interface running pCLAMP software (Axon Instruments, Union City, CA, USA). We measured the initial slopes of the fEPSP and normalized them to the average of the baseline values. The average size of the slopes of the fEPSPs recorded between 30 and 40 min after the end of the tetanic stimulation provided the basis for our statistical comparisons.
Statistical analysis
The plasma corticosterone levels were compared using t-tests. All other comparisons were done using one-way Analysis of Variance (ANOVA), followed by post hoc Tukey-Kramer test. All statistical analyses were conducted using Statcel software, version III.
Results
Plasma corticosterone levels in the SPS model rats
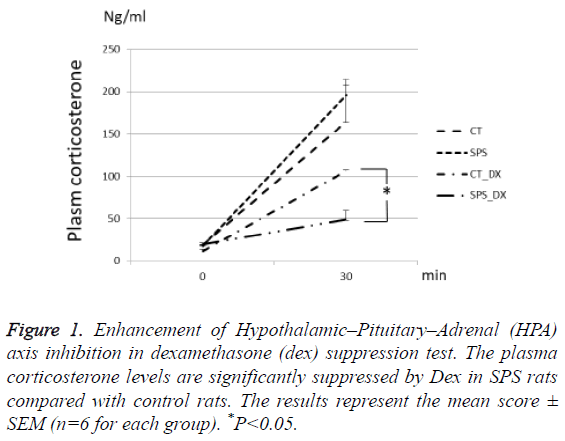
One week after the exposure to SPS, the rats were given Dex or vehicle 2 h prior to the second stress of 30 m restraining. Dex-induced inhibition of the plasma corticosterone increase was significantly potentiated in the SPS rats compared with the non-stressed rats (P=0.002, n=6 for each group). The others groups showed no significant differences (Figure 1).
Figure 1: Enhancement of Hypothalamic–Pituitary–Adrenal (HPA) axis inhibition in dexamethasone (dex) suppression test. The plasma corticosterone levels are significantly suppressed by Dex in SPS rats compared with control rats. The results represent the mean score ± SEM (n=6 for each group). *P<0.05.
Spontaneous locomotor activity
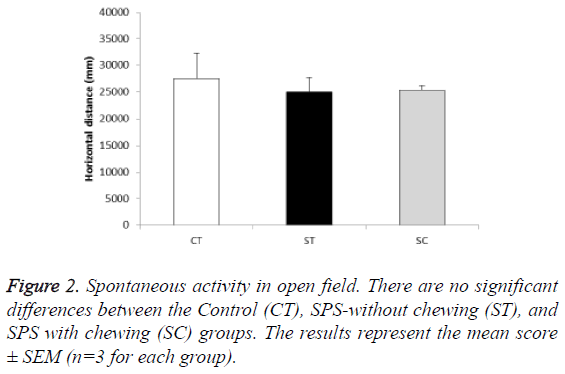
In the open field test for 15 min, the baseline locomotor activity was comparable among the groups (F (2, 6)=0.165, P>0.05; post hoc: P>0.05 for CT (27,534 mm, n=3), ST (25,036 mm, n=3), and SC groups (25,453 mm, n=3)) (Figure 2).
Effect of the chewing in SPS model on the EPM
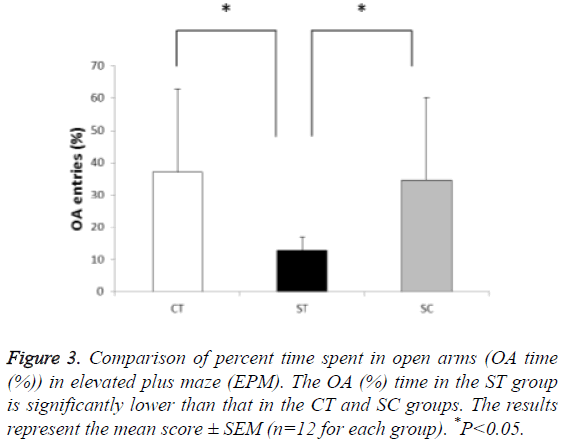
In the percent time spent in the open arms, the time spent by the ST group (12.9%, n=12) was significantly lower than that of the CT (37.1%, n=12) and SC groups (34.5%, n=12) (F (2, 33)=4.764, P<0.05; post hoc: P<0.05 for ST group vs. CT group and ST group vs. SC group)). The CT and SC groups showed no significant differences in the percent time spent (Figure 3).
Effect of the chewing in SPS model on the contextual fear response
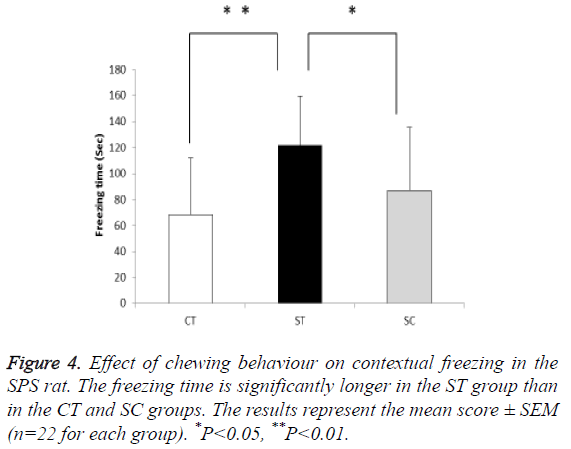
Followed by a one-week undisturbed period after SPS, the ST group (121.7 s, n=22) exhibited significant increase in the contextual freezing response compared with the CT (68.1 s, n=22) and SC (87.0 s, n=22) groups (F (2, 63)=8.532, P<0.01; post hoc: P<0.05 for ST group vs. CT group, P<0.01 for ST group vs. SC group)). There was no significant difference in the duration of freezing behaviour between the CT and SC groups (Figure 4).
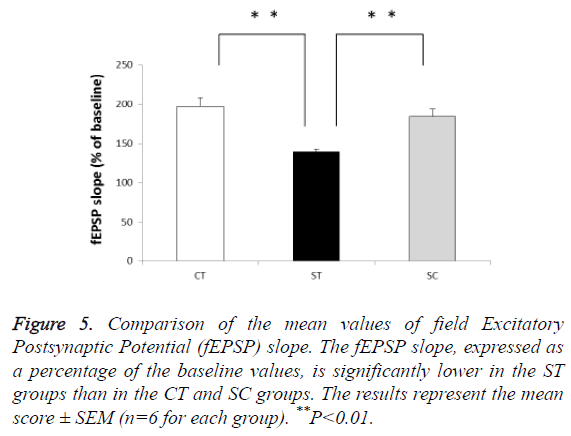
Effect of the chewing in SPS model on hippocampal CA1 LTP
The ST group showed potentiation impairment of fEPSP slopes compared with the SC (139% of baseline, n=6) and CT (197% of baseline, n=6) groups (F (2, 15)=62.5, P<0.01; post hoc: P<0.01 for ST group vs. CT group and ST group vs. SC group)). There was no significant difference between the CT (197% of baseline, n=6) and SC (184% of baseline, n=6) groups (Figure 5).
Figure 5: Comparison of the mean values of field Excitatory Postsynaptic Potential (fEPSP) slope. The fEPSP slope, expressed as a percentage of the baseline values, is significantly lower in the ST groups than in the CT and SC groups. The results represent the mean score ± SEM (n=6 for each group). **P<0.01.
Discussion
In this study, we provide evidence that chewing improves stress-enhanced fear response. We first performed DST to confirm that the SPS paradigm caused enhanced feedback inhibition of the HPA axis, which was previously shown by the suppression of plasma ACTH through cortisol administration immediately before stress exposure [17]. One week after exposure to the stress, the rats were given Dex or vehicle 2 h prior to the second stress. Dex-induced inhibition of plasma corticosterone increase was significantly potentiated in the SPS rats, suggesting that during the several days after SPS exposure, alterations in the HPA response were established.
A rat model involving SPS has been developed and employed for PTSD research [14,15]. In the present experiments, the ST group was exposed to restraint stress for 2 h without chewing, and the SC group was exposed to restraint stress for 2 h with chewing. In a previous study using a similar condition, restraint stress for 2 h significantly increased ACTH and corticosterone levels in stressed rats, but chewing for latter half of the 2 h stress period significantly decreased both these level [20]. In addition, our previous research, also using similar condition, showed that 2 h restraint stress significantly impaired hippocampus-dependent special memory but chewing for latter half of the 2 h stress period significantly reduced the stress-impairment of hippocampal function [9]. To examine the possibility that SPS influenced the locomotor activity in the fear response, we evaluated the distance travelled by rats from different groups in the open field test for 15 min. It showed no significant effect of SPS and chewing. This indicated that SPS did not affect spontaneous locomotor activity. We found that chewing ameliorated the SPS-induced fear responses in behaviour tests, further the SPS-induced suppression of LTP in CA1 hippocampal neurons was improved by chewing. Several recent studies have suggested that chewing of a wooden stick during restraint stress rescues the stress-impaired hippocampal function [21,22]. Restraint stress induces the expression of corticotrophin-releasing hormone in the paraventricular nucleus of the rat hypothalamus [23], whereas chewing during restraint stress significantly suppresses this enhanced corticotrophin-releasing hormone expression [18]. Together with our data, these findings suggest that stress produces increased fear response via HPA axis dysregulation, but chewing prevents these deficits. Although previous researchers have shown that increased GR protein levels in the hippocampus are associated with increased negative-feedback sensitivity to glucocorticoids and the subsequent dampened neuroendocrine response to stress [24-29], this study demonstrates that chewing ameliorates the increased fear condition by the inhibition of the HPA axis. We emphasize that the masticatory activation plays an effective role in the maintenance of negative feedback of HPA under severe stress conditions. However, there is no consensus regarding the relevant neuronal and humoral pathways from the oral cavity to the hippocampus. The neuronal pathways by which mastication directly and indirectly influences the HPA axis should be clarified in future studies.
Conclusion
The present results support our hypothesis that chewing is a behavioural skill that helps to cope with stress.
Declaration of Conflicting Interests
The authors declare no potential conflict of interest
References
- Ichihashi Y, Arakawa Y, Iinuma M, Tamura Y, Kubo KY. Occlusal disharmony attenuates glucocorticoid negative feedback in aged SAMP8 mice. NeurosciLett 2007; 427: 71-76.
- Kubo KY, Iwaku F, Watanabe K, Fujita M, Onozuka M. Molarless-induced changes of spines in hippocampal region of SAMP8 mice. Brain Res 2005; 1057: 191-195.
- Onozuka M, Watanabe K, Mirbod SM. Reduced mastication stimulates impairment of spatial memory and degeneration of hippocampal neurons in age SAMP8 mice. Brain Res 1999; 826: 148-153.
- Onozuka M, Watanabe K, Nagasaki S. Impairment of spatial memory and changes in astroglial responsiveness following loss of molar teeth in aged SAMP8 mice. Behav Brain Res 2000; 108: 145-155.
- Onozuka M, Watanabe K, Fujita M, Tonosaki K, Saito S. Evidence for involvement of glucocorticoid response in the hippocampal changes in aged molarless SAMP8 mice. Behav Brain Res 2002; 131: 125-129.
- Tsutsui K, Kaku M, Motokawa M, Tohma Y, Kawata T. Influences of reduced masticatory sensory input from soft-diet feeding upon spatial memory/learning ability in mice. Biomed Res 2007; 28: 1-7.
- Watanabe K, Tonosaki K, Kawase T, Karasawa N, Nagatsu I. Evidence for involvement of dysfunctional teeth in the senile process in the hippocampus of SAMP8 mice. ExpGerontol 2001; 36: 283-295.
- Watanabe K, Ozono S, Nishiyama K, Saito S, Tonosaki K. The molarless condition in aged SAMP8 mice attenuates hippocampal Fos induction linked to water maze performance. Behav Brain Res 2002; 128: 19-25.
- Miyake S, Yoshikawa G, Yamada K. Chewing ameliorates stress-induced suppression of spatial memory by increasing glucocorticoid receptor expression in the hippocampus. Brain Res 2012; 1446: 34-39.
- American Psychiatric Association (APA). Diagnostic and statistical manual of mental disorders (DSM-IV). (4th edn.) 1994.
- Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Arch Gen Psychiatry 1993; 50: 295-305.
- Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry 2001; 62: 41-46.
- Yamasue H, Kasai K, Iwanami A. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. ProcNatlAcadSci USA 2003; 100: 9039-9043.
- Iwamoto Y, Morinobu S, Takahashi T. Single prolonged stress increases contextual freezing and the expression of glycine transporter 1 and vesicle-associated membrane protein 2 mRNA in the hippocampus of rats. ProgNeuropsychopharmacolBiol Psychiatry 2007; 31: 642-651.
- Khan S, Liberzon I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology (Berl) 2004; 172: 225-229.
- Liberzon I, Lopez JF, Flagel SB. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol 1999; 11: 11-17.
- Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology 1997; 22: 443-453.
- Hori N, Yuyama N, Tamura K. Biting suppresses stress-induced expression of Corticotropin-Releasing Factor (CRF) in the rat hypothalamus. J Dent Res 2004; 83: 124-128.
- Hori N, Lee MC, Sasaguri K, Ishii H, Kamei M. Suppression of stress-induced nNOS expression in the rat hypothalamus by biting. J Dent Res 2005; 84: 624-628.
- Lee T, Saruta J, Sasaguri K. Allowing animals to bite reverses the effects of immobilization stress on hippocampal neurotrophin expression. Brain Res 2008; 1195: 43-49.
- Ono Y, Kataoka T, Miyake S, Cheng SJ, Tachibana A. Chewing ameliorates stress-induced suppression of hippocampal long-term potentiation. Neuroscience 2008; 154: 1352-1359.
- Ono Y, Kataoka T, Miyake S. Chewing rescues stress-suppressed hippocampal long-term potentiation via activation of histamine H1 receptor. Neurosci Res 2009; 64: 385-390.
- Givalois L, Naert G, Rage F. A single brain-derived neurotrophic factor injection modifies hypothalamo-pituitary-adrenocortical axis activity in adult male rats. Mol Cell Neurosci 2004; 27: 280-295.
- Meaney MJ, Aitken DH, Bodnoff SR. Early postnatal handling alters glucocorticoid receptor concentrations in selected brain regions. BehavNeurosci 1985; 99: 765-770.
- Meaney MJ, Aitken DH, Viau V. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology 1989; 50: 597-604.
- Meaney MJ, Aitken DH, Sharma S. Basal ACTH, corticosterone and corticosterone-binding globulin levels over the diurnal cycle, and age-related changes in hippocampal type I and type II corticosteroid receptor binding capacity in young and aged, handled and nonhandled rats. Neuroendocrinology 1992; 55: 204-213.
- Meaney MJ, Diorio J, Francis D. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. DevNeurosci 1996; 18: 49-72.
- Donnell D, Larocque S, Seckl JR. Postnatal handling alters glucocorticoid, but not mineralocorticoid messenger RNA expression in the hippocampus of adult rats. Mol Brain Res 1994; 26: 242-248.
- Stamatakis A, Pondiki S, Kitraki E, Diamantopoulou A, Panagiotaropoulos T. Effect of neonatal handling on adult rat spatial learning and memory following acute stress. Stress 2008; 11: 148-159.