Research Article - Biomedical Research (2017) Volume 28, Issue 6
Chemopreventive potential of fish oil against 7, 12-dimethyl benz(a)anthracene and croton oil induced two-stage mouse skin papillomagenesis
Samir Qiblawi1*, Sasikumar Dhanarasu2 and Moh’d Alaraj31Department of Histology, College of Medicine, University of Hail, Saudi Arabia
2Department of Biochemistry, College of Medicine, University of Hail, Saudi Arabia
3Department of Pharmacology, College of Medicine, University of Hail, Saudi Arabia
- *Corresponding Author:
- Samir Qiblawi
Department of Histology
College of Medicine, University of Hail
Saudi Arabia
Accepted date: November 5, 2016
Abstract
Cancer chemoprevention is a novel and recognized approach to inhibit, delay or reverse the process of cancer development by using natural products. Our aim of this study was to examine the chemopreventive potential of fish oil on 7, 12-Dimethylbenz (a) Anthracene (DMBA)-initiated and croton oil-promoted skin papillomagenesis model in mice. Oral treatment of fish oil at a dose of 25 μl per animal resulted in significant reduction of tumor incidence, tumor burden, tumor yield, and the cumulative number of papillomas in DMBA+croton oil+fish oil treated group compared to only DMBA +croton oil administered positive control group. Pre-treatment of fish oil also increased the latency period of tumor development and a significant reduction in the weight and size of the skin papillomas. Furthermore, biochemical assays revealed a significant increase in the hepatic enzymatic and nonenzymatic antioxidants like glutathione-S-transferase, superoxide dismutase, catalase, and reduced glutathione whereas lipid peroxidation was found to be significantly reduced as a result of fish oil treatment. Thus, the results of the present study clearly indicate that fish oil has potent chemopreventive efficacy against two-stage skin carcinogenesis which can be partially attributed to its anti-oxidative and anti-peroxidative effect.
Keywords
Fish oil, Chemoprevention, Papilloma, Skin carcinogenesis, Antioxidant enzymes
Introduction
Cancer is the second leading cause of death in human population after cardiovascular diseases [1]. Amongst all forms of cancer, skin cancer is currently the most common type of cancer and of particular concern because its incidence is increasing at high rate [2]. Recent estimation of the cancer incidence in Saudi Arabia indicates that skin cancer accounted for 3.2% of all newly diagnosed cases in year 2010. It affected 52.7% males and 47.3% females with a male to female ratio of 111:100 [3]. Although solar radiation is the leading cause of human skin cancer, it is also acknowledged that exposure to various xenobiotics such as industrial chemicals, arsenic, pesticides, cigarette smoke and pollutants also contribute to growing incidences of cutaneous neoplasia in humans. Chemoprevention, which is a relatively newer strategy, could be an important armamentarium for skin cancer since it can delay, retard or reverse the process of cancer development [4-6].
Recently, promoting the regular use of whole cardamom in the diet, especially in high-risk groups, may be helpful in significantly reducing cancer incidence and tumor burden [7]. The uses of natural agents present in the common diet and beverages consumed by human population have gained considerable attention. These agents have shown chemopreventive potential against cancer of various organs including skin and antimicrobial activity [8-13]. Many of our routine food items of plant and animal origin have rich source of fat. Diets high in fat have been linked with an increased risk of various types of cancers [14]. Increasing evidence from animal and human studies indicate that the amount and composition of dietary fat, particularly of essential Polyunsaturated Fatty Acids (PUFAs), may modulate development of cancer [15-18]. Dietary fats, especially ω-3 Polyunsaturated Fatty Acids (PUFA) have inhibitory role in carcinogenesis [19].
Fish oil rich in ω-3 Polyunsaturated Fatty Acid (n-3PUFAs) such as Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) has shown to exhibit wide range of biological activities including antimicrobial, antioxidative, anti-inflammatory, anti-arthritic and immuno-modulatory effect [20-24]. Fish oil has also been reported as one of the potential modifiable protective factor against various forms of cancers [25-28]. However the effect of fish oil against skin carcinogenesis is not well studied. In this study, we tried to explore the effect of oral administration of fish oil against DMBA and croton oil mediated two-stage skin carcinogenesis in terms of tumor development. Furthermore, protective effect of fish oil was also examined in terms of modulation of enzymatic and non-enzymatic anti-oxidants as well as the level of lipid-peroxidation in hepatic tissue. Modulations in the activities of these anti-oxidants are typical biomarkers of oxidative stress which are closely linked with the development of skin tumor.
Material and Methods
Animals
Random bred male Swiss albino mice (aged 6-8 weeks; weight: 21.01 ± 0.80) were used for the experiments. The animals were kept in ventilated cages in air-conditioned animal facility at 25°C ± 2°C room temperature with 12 h light/dark cycle. They were provided with Purina chow diet pellets (Grain Silos and Flour Mills Organization, Riyadh, Saudi Arabia) and tap water ad libitum. All of the experimental protocols were approved by the Institutional Animal Care and Use Committee of University of Hail.
Chemicals
DMBA was obtained from Acros Organics, Hanover Park, IL, USA. Croton oil was purchased from Alfa Aesar (Lancashire, United Kingdom). Kits for Superoxide Dismutase (SOD), Catalase (CAT), Glutathione-S-Transferase (GST), Glutathione (GSH), and Lipid Peroxidation (LPO) were obtained from Cayman Chemical Co., Michigan, USA. Fish oil under the brand name Maxepa was purchased from local drug store. It contains 180 mg/ml Eicosapentaenoic Acid (EPA) and 120 mg/ml Docosahexaenoic Acid (DHA). All other chemicals used in the present study were of analytical grade obtained from local supplier.
Experimental design
This study composed of two separate experiments. Experiment I was designed to evaluate the chemopreventive potential of fish oil against skin papillomagenesis in Swiss albino mice. Experiment II aimed to study the chemo modulatory effect of fish oil on the activities of the hepatic detoxification and antioxidants enzymes and the level of lipid-peroxidation.
Experiment I: To evaluate the chemopreventive potential of fish oil: To test the chemopreventive efficacy of fish oil against DMBA and croton oil-induced mouse skin papillomagenesis, forty eight mice were divided equally into four groups of 12 mice each.
Group I, these animals received only topical acetone and were considered as negative control group; Group II, animals from this group were applied with a single topical application of DMBA (100 μg in 50 μL of acetone) on their shaven backs. Two weeks after the initiation, these animals were administered with 0.1 ml of croton oil (1% in 100 μl of acetone) three times a week until the termination of the experiment (up to 16 weeks). These animals serve as positive control.
Group III, animals from this group were treated with daily oral administration of fish oil (25 μl/animal/day), 15 days prior to the initiation by DMBA and continued throughout the experimental protocol (up to 16 weeks). Croton oil was also administered topically to these animals three times a week until the end of the experiment (16 weeks). These animals serve as treatment group; Group IV, animals from this group were given an oral treatment of fish oil (25 μl/animal/day) only for 16 weeks.
The number of skin tumors on each affected mouse was recorded. Skin papillomas were defined as lesions with a diameter˃1 mm that were present for at least two consecutive observations.
Morphological analysis of tumors/papillomas
The chemopreventive response was assessed on the basis of tumor incidence, burden, yield, and cumulative number of papillomas and average latent period that were calculated as follows: tumor incidence, percentage number of animals having tumors; tumor burden, total number of tumors counted per group/number of tumor bearing mice; tumor yield, total number of tumors counted per group/total number of mice in the group; cumulative no of papillomas; total number of tumors that appeared by the end of the experiment; average latent period, was calculated by multiplying the number of tumors appearing each week by the time in weeks after the application of the promoting agent and dividing the sum by the total number of tumors. In addition the weight and size of the each tumor appeared in animals at the termination of experiment were measured.
Experiment II: Modulatory effect of fish oil on antioxidant enzymes and lipid-peroxidation: This experiment was performed in order to investigate the chemo modulatory influence of fish oil on hepatic enzymatic and non-enzymatic antioxidants (GST, SOD, CAT, and GSH) and the level of Lipid Peroxidation (LPO). For the purposes of enzymatic assays animals were sorted into two groups of eight animals each. Group I, animals were kept on a normal diet for 15 days; Group II, animals were treated orally with Fish oil (25 μL/ animal/day) for 15 days. At the end of the experiment mice were sacrificed by cervical dislocation. Liver tissue was obtained and stored at-80°C until analysis.
Estimation of reduced glutathione (GSH) level
The tissues were homogenized in 5-10 ml of cold buffer (50 mM MES, pH 6.0, containing 2 mM EDTA). The homogenate was then centrifuged at 10,000 xg for 15 minutes at 4°C, and the supernatant assayed for GSH levels using the Cayman assay kit (Cayman Cat No:703002), which involved an optimized enzymatic recycling method and GR. The sulfhydryl group of GSH reacts with 5, 5-Ditho-Bis-2-Nitrobenzoic Acid (DTNB) and produces yellow colored 5-Thio-2-Nitrobenzoic Acid (TNB). The rate of TNB production is directly proportional to the concentration of GSH in the sample. The absorbance of TNB at 410 nm was used to estimate the amount of GSH in the sample.
Determination of the glutathione s-transferase (GST) activity
The tissue was homogenized in 2.5 ml of cold buffer (100 mM potassium phosphate and 2 mM EDTA, pH 7.0) and centrifugation at 10,000 xg for 15 min at 4˚C was done. The GST activity was determined by measuring the conjugation of 1-Chloro-2, 4-Dinitrobenzene (CDNB) with reduced GSH using a GST assay kit (Cat No. 703302, Cayman, MI, USA) Conjugation is accompanied by an increase in the absorbance at 340 nm and the rate of increase is directly proportional to the GST activity in the sample [29].
Determination of the catalase activity
Tissue samples were homogenized in cold potassium phosphate buffer, pH 7.0, containing 1 mM EDTA. The mixture was then centrifuged at 10,000x g for 15 minutes at 4°C, and the supernatant assayed for CAT activity using the Cayman assay kit (Cayman Cat. No: 707002). This kit utilizes the peroxidatic function of CAT for determination of enzyme activity. The method is based on the reaction of the enzyme with methanol in the presence of an optimal concentration of H2O2. The formaldehyde produced is measured spectrophotometrically at 540 nm with 4-amino-3-hydrazino- 5-mercapto-1, 2, 4-trizazole as the chromagen.
Determination of the superoxide dismutase activity
The tissues were homogenized in 20 mM HEPES buffer (1 mM EGTA, 210 mM mannitol, and 70 mM sucrose/g tissue), pH 7.2. The homogenate was then centrifuged at 1,500x g for 5 minutes at 4°C, and the supernatant assayed for SOD activity using the Cayman assay kit (Cayman Cat. No 706002). This kit utilizes a tetrazolium salt for the detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. Absorbance was measured at 532 nm. One unit of SOD was defined as the amount of enzyme needed to produce 50% dismutation of superoxide radical.
Determination of lipid peroxidation
Lipid peroxidation was analysed by the formation of Malondialdehyde (MDA) by using Cayman assay kit (Cayman Cat. No 10009055) according to the manufacturer’s protocol. The liver tissue was homogenized in RIPA buffer (50 mM Tris- Cl, pH 8.0, 150 mM NaCl, 1% NP-40, 5 mM EDTA) and centrifuged at 1,600 xg for 10 min at 4°C. Supernatant (0.1 ml) was then mixed with a sodium dodecyl sulphate solution (0.1 ml) and color reagent (4 ml) and the mixture was heated to 100°C for 1 h and then rapidly cooled. Absorbance was measured at 532 nm [30].
Statistical analysis
One way ANOVA followed by student t test was performed. P values<0.05 was considered significant. The values are expressed as means ± SEM.
Results
Effect of fish oil against DMBA and croton oil induced skin papillomagenesis
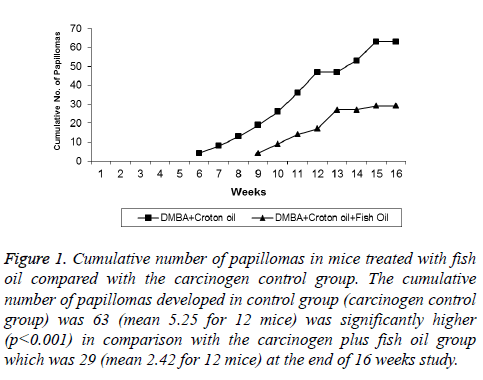
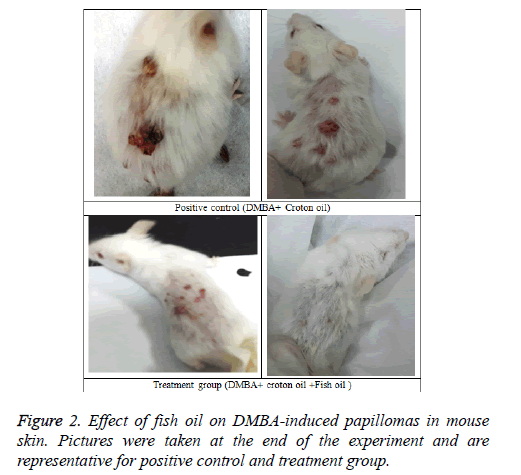
Tables 1 and 2 indicate the chemoprotective effect of fish oil treatment on DMBA-initiated and croton oil promoted twostage skin carcinogenesis. No apparent toxicity of fish oil was observed in terms of change in body weights or gross morphological examination of major organ systems. In group II animals (positive control) papillomas started appearing from week six onwards, and the incidence reached 100% by the time of termination of the experiment (i.e. week 16) (Figures 1 and 2). The cumulative number of papillomas in these animals was 63. The average number of papillomas per mouse (tumor yield) was found to be 5.25 ± 0.36 (Table 1), while the average tumor weight was 175 ± 6.44 mg (Table 2).
| Group | Total no. of papillomas | Tumor incidence | Tumor burden | Tumor yield | Average latency period (weeks) |
|---|---|---|---|---|---|
| Group I control (Vehicle only) | − | − | − | − | − |
| Group II DMBA+Croton oil | 63 | 100% (12/12) | 5.25 ± 0.36 | 5.25 ± 0.33 | 7.95 ± 0.20 |
| Group III DMBA+Croton oil+Fish oil (25 µL/Animal /day) | 29 | 66.66%* (8/12) | 3.625 ± 0.29* | 2.42 ± 0.52** | 11.34 ± 0.42** |
| Group IV Fish oil (25 µL/Animal /day) | − | − | − | − | − |
| Values are expressed as mean ± standard error of 12 animals. *P<0.01 and **P<0.001, significant changes compared with the control (Group II). |
|||||
Table 1: Chemopreventive effect of fish oil on DMBA-initiated and croton oil promoted skin carcinogenesis.
| Group | Tumor Size (mm) | Tumor weight (mg) | ||
|---|---|---|---|---|
| <2 | 2-5 | 6-9 | ||
| Group I: Control (Vehicle only) | - | - | - | - |
| Group II: DMBA+croton oil | 31.00 ± 0.12 | 19.00 ± 0.9 | 13.0 ± 0.49 | 175 ± 6.44 |
| Group III: DMBA+croton oil+Fish oil | 19.00 ± 0.24** | 7.00±0.45** | 3 ± 0.61** | 36.75 ± 2.28** |
| Group IV: Fish oil (25 µL/Animal/day) | - | - | - | - |
| Values are expressed as mean ± standard error of 12 animals. **Significant difference between the experimental and control groups at P<0.001. |
||||
Table 2: Tumor size and weight in DMBA induced and croton oil promoted skin tumorigenesis in Swiss albino mice with and without fish oil treatment.
Figure 1: Cumulative number of papillomas in mice treated with fish oil compared with the carcinogen control group. The cumulative number of papillomas developed in control group (carcinogen control group) was 63 (mean 5.25 for 12 mice) was significantly higher (p<0.001) in comparison with the carcinogen plus fish oil group which was 29 (mean 2.42 for 12 mice) at the end of 16 weeks study.
Mice of group III (treatment group), which were given a continuous treatment of Fish oil orally at pre, peri, and post-initiation phase (i.e. 2 weeks prior to DMBA application until the end of week 16) showed a significant reduction (P<0.001) in the incidence of tumors (66.66%) compared with the group II animals (positive control) (Table 1). At the termination of the experiment at 16 weeks, compared with a total of 63 tumors in positive control group of animals (group II), only 29 tumors in fish oil-treated group (group III) were recorded. Compared with the positive control group (group II), such decrease in the total number of tumor in the fish oil-treated group corresponds to 54% inhibition. The average number of tumors per mice (Tumor yield) (2.42 ± 0.52), and the average number of tumors per tumor bearing mice (Tumor burden) (3.625 ± 0.29) were also found to be significantly lower (P<0.001) compared to group II animals (Table 1). In group III animals, tumor appearance was also considerably delayed until week 9.
Furthermore, these mice also showed significant decreases in tumor size and weight (P<0.001) compared with the positive control group (Group II) (Table 2). The average tumor weight in the fish oil-treated group of mice was (36.75 ± 2.28) mg. Fish oil also prolonged the average latency period (i.e. time lag between the application of the promoter and the appearance of 50% of tumors) of tumor by approximately 3.4 weeks (42.6%) compared to Group II animals (Table 1). None of the mice from the group I (i.e. acetone alone) or group IV (fish oil alone) developed tumors.
Effect of fish oil on hepatic anti-oxidants enzymes and lipid peroxidation
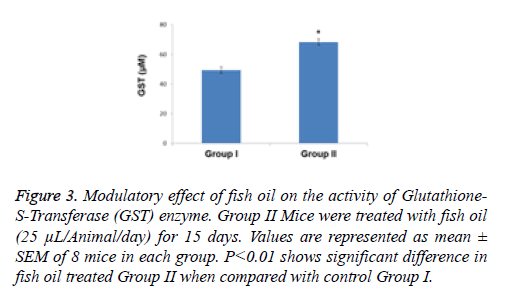
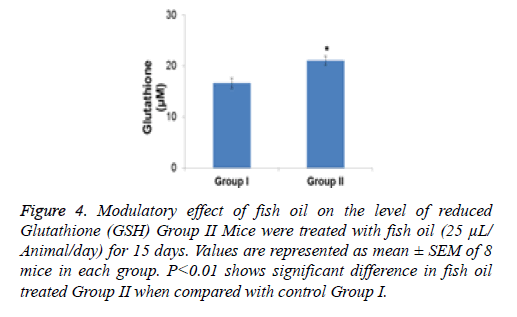
The effect of 2 weeks of dietary feeding of fish oil was evaluated on the activities of detoxification and antioxidant enzymes in mouse liver. The specific activity of GST exhibited a significant (P<0.01) increase compared with the control group by 1.38-fold as a result of treatment with fish oil (Figure 3). The content of GSH was significantly (p<0.001) enhanced by 1.26 fold in the group of animals supplemented with fish oil compared with the control group (Figure 4).
Figure 3: Modulatory effect of fish oil on the activity of Glutathione- S-Transferase (GST) enzyme. Group II Mice were treated with fish oil (25 μL/Animal/day) for 15 days. Values are represented as mean ± SEM of 8 mice in each group. P<0.01 shows significant difference in fish oil treated Group II when compared with control Group I.
Figure 4: Modulatory effect of fish oil on the level of reduced Glutathione (GSH) Group II Mice were treated with fish oil (25 μL/ Animal/day) for 15 days. Values are represented as mean ± SEM of 8 mice in each group. P<0.01 shows significant difference in fish oil treated Group II when compared with control Group I.
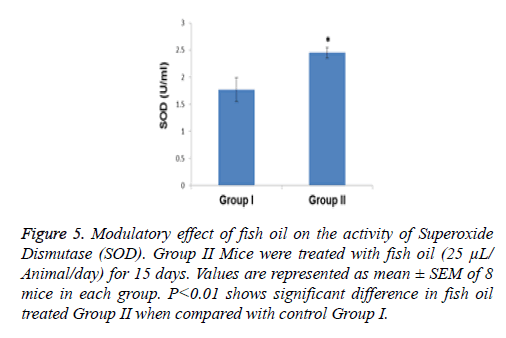
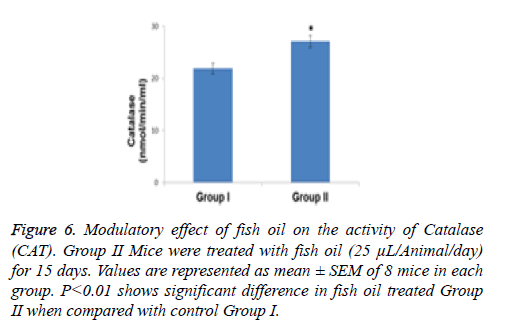
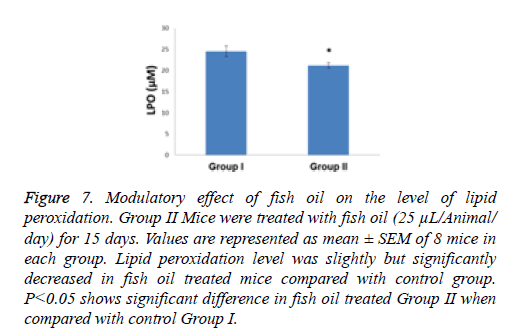
Compared with the control group the specific activity of SOD showed a significant increase (p<0.01) of 1.38- fold in the group of animals treated with the above mentioned dietary modulator (Figure 5). Similarly, the specific activity of CAT was significantly enhanced by 1.23-fold (p<0.001) in the group of animals treated with fish oil (Figure 6). The level of lipid peroxidation was slightly but significantly (p<0.05) decreased due to treatment with fish oil compared to the group I animals (control group) (Figure 7).
Figure 5: Modulatory effect of fish oil on the activity of Superoxide Dismutase (SOD). Group II Mice were treated with fish oil (25 μL/ Animal/day) for 15 days. Values are represented as mean ± SEM of 8 mice in each group. P<0.01 shows significant difference in fish oil treated Group II when compared with control Group I.
Figure 6: Modulatory effect of fish oil on the activity of Catalase (CAT). Group II Mice were treated with fish oil (25 μL/Animal/day) for 15 days. Values are represented as mean ± SEM of 8 mice in each group. P<0.01 shows significant difference in fish oil treated Group II when compared with control Group I.
Figure 7: Modulatory effect of fish oil on the level of lipid peroxidation. Group II Mice were treated with fish oil (25 μL/Animal/ day) for 15 days. Values are represented as mean ± SEM of 8 mice in each group. Lipid peroxidation level was slightly but significantly decreased in fish oil treated mice compared with control group. P<0.05 shows significant difference in fish oil treated Group II when compared with control Group I.
Discussion
Cancer chemoprevention aims to halt or reverse the development and progression of precancerous cells through dietary or pharmacological supplementation of antioxidants during the time period between tumor initiation and malignancy [31].
The present investigation has clearly shown that the oral administration of fish oil could exert a strong chemopreventive effect against DMBA-initiated and croton oil promoted two stage skin carcinogenesis in Swiss albino mice, as evidenced not only by the significantly reduced tumor incidence but also by the reduced tumor burden and tumor yield. Fish oil treatment also resulted in significantly longer tumor latent periods and lower papillomas cumulative number. The tumor appearance was delayed by approximately 3.4 weeks when oral treatment of fish oil was given continuously at the initiation and promotional stages of carcinogenesis.
Many in vivo and in vitro experiments also indicate chemopreventive potential of fish oil various models of carcinogenesis [25-28,32,33]. Mernitz et al. [27] found that fish oil supplementation was able to decrease lung tumor prevalence by 78% and 80% compared to groups receiving soybean oil and corn oil supplementation, respectively in A/J Mouse. This inhibition on lung tumor prevalence was associated with increased expressions of cell cycle inhibitor p21Cip1 and lipoxygenase isoform 15-LOX in the lungs. In another study, Sarotra et al. reported that fish oil has a dose and time-dependent chemopreventive effect in colon cancer mediated through oxidative stress and apoptosis [28]. Lou et al. found that high fat fish oil (HFFO) diet has beneficial effect against UVB-induced skin carcinogenesis and these effects may be associated with an inhibition on UVB-induced inflammatory response [32]. Protective effect of fish oil was also observed against prostate cancer mouse model [33].
Chemical carcinogens can bind to DNA and result in mutagenic events that contribute to malignant transformation [34]. Because Fish oil treatment was started before the initiation phase of carcinogenesis, the effect of Fish oil could be considered a “blocking” effect on DMBA induced Skin carcinogenesis and the possible mode of action would be directed toward the role of fish oil treatment in modulating the metabolism of the carcinogen. Chen et al. reported that the blocking agents that inhibit chemical carcinogenesis could exert their preventive effect by several mechanisms, including enhancing detoxification of carcinogens, inhibiting cytochrome P450-mediated activation of carcinogens, scavenging free radicals and trapping the carcinogens and preventing their interaction with DNA [35].
DMBA, due to its pro-carcinogenic nature, is metabolized by phase I enzymes such as cytochrome P450 to its ultimate carcinogenic metabolite, dihydrodiol epoxide, which binds to and damage DNA, contributing to mutation and carcinogenesis [36]. Oxidative stress caused by Reactive Oxygen Species (ROS) that are excessively generated during the metabolic activation of DMBA also contributes to the pathogenesis of cancer [37].
It has been believed that chemopreventive agents exert their anti-carcinogenic effect by counteracting the reactive oxygen species-induced oxidative tissue damage and improving antioxidant defense mechanism of the host [38]. The present experimental investigation reported a significant enhancement in the activities of non-enzymatic as well as enzymatic antioxidants in the liver of mice. GSH is an important non-protein thiol antioxidant that plays a significant role in protecting cells against oxidative stress (GSH redox cycle), and also directly detoxifies ROS and neutralizes reactive intermediate species generated from exposure to xenobiotics, including chemical carcinogens [39]. In the present study, the elevated levels of GSH induced by fish oil in the murine liver would probably help in the elimination of free radicals generated during carcinogen metabolism resulting in inhibition of skin carcinogenesis.
GSTs are a critical phase II enzymes that play a vital role in inducing the detoxification of xenobiotics including carcinogens, and induction of GSTs is a property found in many naturally occurring chemopreventive agents, ameliorating toxicity and carcinogenicity, especially during the initiation phase [40]. The specific activity of hepatic GST exhibited an increase in the group of animals treated with fish oil. From a mechanistic perspective, the main function of GST is to catalyse the conjugation of electrophilic xenobiotic carcinogens to the endogenous nucleophile [41] and divert ultimate carcinogens from reacting with critical cellular molecules [42] resulting in avoidance of initiation of cancer. Our results are further supported by an earlier investigation, which reported that genes for Glutathione Transferases (GSTs) were up regulated in mice fed with fish oil supplemented diets [43].
SOD and CAT enzymes are important antioxidant enzymes of the antioxidant defence system that protect against oxidative stress and are effective in detoxifying superoxide radicals and hydrogen peroxide, respectively. They act as anti-carcinogens and inhibitors at initiation and promotion stages of the carcinogenesis process [44]. Lowered activities of SOD and CAT were reported in various forms of cancers [45]. Hence, the elevation of the specific activities of both SOD and CAT in fish oil-treated animals might have provided the cells protection against ROS and peroxide molecules, leading to chemoprevention against skin carcinogenesis. In recent study, it has been found that supplementation of fish oil capsules, containing EPA: DHA in the ratio of 1.5:1, in breast cancer patients undergoing chemotherapy, significantly improved their serum antioxidant levels as well as quality of life parameters [24].
Lipid peroxidation, a well-known marker of oxidative stress, is increased during the carcinogenic process and Malondialdehyde (MDA), was observed to be the most mutagenic and carcinogenic product of lipid peroxidation [46]. Although there was slight reduction in the level of lipid peroxidation of fish oil treated mice but was found statistically significant when compared with the control group. Inhibition of lipid peroxidation is suggestive of the antioxidant property of fish oil that has an ability to scavenge free radicals. This inhibitory effect of fish oil on lipid peroxide formation has also been reported by other researchers [47].
As mentioned above, it seems that the primary mechanism of chemoprevention by fish oil is the enhancement of the antioxidant defense system, which could lower the oxidative damage produced by reactive oxygen species. Since fish oil significantly induces the specific activity of GSH, GST, SOD and CAT, we speculate that it can intervene in the formation of active carcinogen metabolites as well as the processes involved in tumor promotion. The decrease in the level of lipid peroxidation in the group of animals treated with fish oil was correlated with the elevation of antioxidant status of animals, indicating its important role in reducing oxidative stress that in turn made the cells resistant to cancer induction. This result was found paralleled with the observed reduction in the incidence of skin papillomas. A similar observation was also noted with proanthocyanidins from grape seeds on the inhibition of skin photocarcinogenesis concomitant with reduction in lipid peroxidation [48].
Saw et al. found that anti-oxidative and anti-inflammatory potential of Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) found in fish oil was responsible for protection against prostate carcinogenesis in TRAMP mice model [33].
The present findings have clearly demonstrated the cancer preventive potential of fish oil against DMBA-initiated and croton oil-promoted skin tumorogenesis in the murine model system. The results are also suggestive of the ability of fish oil to enhance the antioxidant status of animals, which might have provided to chemoprevention of papillomagenesis. In addition to the aforementioned mechanisms, we also anticipate that an anti-inflammatory potential as well as an immunomodulatory action of fish oil might have contributed to its overall efficacy against skin chemical carcinogenesis that requires further study.
Conclusion
In conclusion, this study revealed that fish oil could be considered as dietary chemopreventive agent with respect to its ability to enhance carcinogen detoxification and its blocking/ suppressing effect on initiation and promotion stages of chemically induced skin carcinogenesis, which provide an effective dietary approach towards controlling the cancer incidence. Therefore, our findings might have significance and suggest that the regular intake of fish oil is likely to reduce the risk of skin cancer in human population.
Acknowledgement
This study was financially supported by Deanship of Scientific Research, University of Hail, Saudi Arabia. Project No. (CM5 2013).
References
- Jajroudi M, Baniasadi T, Kamkar L, Arbabi F, Sanei M. Prediction of survival in thyroid cancer using data mining technique. Technol Cancer Res Treat 2014; 13: 353-359.
- Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol 2010; 146: 279-282.
- Haya S, Al Eid. Cancer incidence report in Saudi Arabia. Saudi Cancer Registry Ministry of Health, Kingdom Of Saudi Arabia 2010.
- Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science 1997; 278: 1073-1077.
- Mukhtar H. Chemoprevention: making it a success story for controlling human cancer. Cancer Lett 2012; 326: 123-127.
- Steward WP, Brown K. Cancer chemoprevention: a rapidly evolving field. Br J Cancer 2013; 109: 1-7.
- Samir Q, Sasikumar D, Moez Al IF. Chemopreventive effect of cardamom (elettaria cardamomum) against benzo(a)pyrene-induced fore stomach papillomagenesis in Swiss albino mice. J Environ Pathol Tox 2015; 34: 95-104.
- Lahiri-Chatterjee M, Katiyar SK, Mohan RR, Agarwal R. A flavonoid antioxidant, silymarin, affords exceptionally high protection against tumour promotion in the SENCAR mouse skin tumorigenesis model. Cancer Res 1999; 59: 622-632.
- Lamson DW, Brignall MS. Natural agents in the prevention of cancer, part two: preclinical data and chemoprevention for common cancers. Altern Med Rev 2001; 6: 167-187.
- Banerjee S, Panda CK, Das S. Clove (Syzgium aromaticum L.), a potential chemopreventive agent for lung cancer. Carcinogenesis 2006; 27: 1645-1654.
- Qiblawi S, Al-Hazimi A, Al-Mogbel M, Hossain A, Bagchi D. Chemopreventive effects of cardamom (Elettaria cardamomum L.) on chemically induced skin carcinogenesis in Swiss albino mice. J Med Food 2012; 15: 576-580.
- Sadik NA. Chemopreventive efficacy of green tea drinking against 1, 2-dimethyl hydrazine-induced rat colon carcinogenesis. Cell Biochem Funct 2013; 31: 196-207.
- Sasikumar D, Mathi S, Nayef KAAS. Evaluating the pharmacological dose (oral ld50) and antibacterial activity of leaf extracts of Mentha piperita Linn. Grown in Kingdom of Saudi Arabia: A pilot study for nephrotoxicity. Int J Pharmacol 2016; 12; 195-200.
- Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr 1999; 70: 560-569.
- Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 1981; 66: 1191-1308.
- Reddy BS, Maeura Y. Tumor promotion by dietary fat in azoxymethane induced Colon carcinogenesis in female F344 rats: influence of amount and source of dietary fat. J Natl Cancer Inst 1984; 72: 745-750.
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 2002; 56: 365-379.
- Mansara PM, Ketkar M, Deshpande RA, Chaudhary A, Shinde K, Kaul-Ghankeakr R. Improved antioxidant status by omega-3 fatty acid supplementation in breast cancer patients undergoing chemotherapy: a case series. J Med Case Rep 2015; 9: 148.
- Manna S, Chakraborty T, Ghosh B, Chatterjee M, Panda A, Srivastava S, Rana A, Chatterjee M. Dietary fish oil associated with increased apoptosis and modulatory expression of Bax and Bcl-2 during 7, 12- dimethylbenz (a) anthracene-induced mammary carcinogenesis in rats. Prosta Prostaglandins Leukot Essent Fatty Acids 2008; 79: 5-14.
- Kris-Etherton PM, Grieger JA, Etherton TD. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot Essent Fatty Acids 2009; 81: 99-104.
- Saw CL, Huang Y, Kong AN. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: docosahexaenoic acid or eicosapentaenoic acid. Biochem Pharmacol 2010; 79: 421-430.
- Chitra RS, Radhakrishnan CK. Antibacterial activities of polyunsaturated fatty acid extracts from Sardinella longiceps and Sardinella fimbriata. Indian J Geo-Marine Sciences 2011; 40: 710-716.
- Monk JM, Kim W, Callaway E, Turk HF, Foreman JE. Immunomodulatory action of dietary fish oil and targeted deletion of intestinal epithelial cell PPARd in inflammation-induced colon carcinogenesis. Am J Physiol Gastrointest Liver Physiol 2012; 302: 153-167.
- Mansara PP, Deshpande RA, Vaidya MM, Kaul-Ghanekar R. Differential ratios of omega Fatty Acids (AA/EPA+DHA) modulate growth, lipid peroxidation and expression of tumor regulatory MARBPs in breast cancer cell lines MCF7 and MDA-MB-231. PLoS One 2015; 10: e0136542.
- Hong MY, Bancroft LK, Turner ND, Davidson LA, Murphy ME. Fish oil decreases oxidative DNA damage by enhancing apoptosis in rat colon. Nutr Cancer 2005; 52: 166-175.
- Wu M, Harvey KA, Ruzmetov N, Welch ZR, Sech L. Omega-3 polyunsaturated fatty acids attenuate breast cancer growth through activation of a neutral sphingomyelinase mediated pathway. Int J Cancer 2005; 117: 340-348.
- Mernitz H, Lian F, Smith DE, Meydani SN, Wang XD. Fish oil supplementation inhibits NNK-induced lung carcinogenesis in the A/J mouse. Nutr Cancer 2009; 61: 663-669.
- Sarotra P, Sharma G, Kansal S, Negi AK, Aggarwal R. Chemopreventive effect of different ratios of fish oil and corn oil in experimental colon carcinogenesis. Lipids 2010; 45: 785-798.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974; 249: 7130-7139.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-358.
- Alberts DS, Garcia DJ. An overview of clinical cancer chemoprevention studies with emphasis on positive phase III studies. J Nutr 1995; 125: 692S-697S.
- Lou YR, Peng QY, Li T, Medvecky CM, Lin Y. Effects of high-fat diets rich in either omega-3 or omega-6 fatty acids on UVB-induced skin carcinogenesis in SKH-1 mice. Carcinogenesis 2011; 32: 1078-1084.
- Saw CL, Wu TY, Paredes-Gonzalez X, Khor TO, Pung D. Pharmacodynamics of fish oil: protective effects against prostate cancer in TRAMP mice fed with a high fat western diet. Asian Pac J Cancer Prev 2011; 12: 3331-3334.
- Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol 2005; 206: 73-93.
- Chen C, Kong AN. Dietary cancer-chemopreventive compounds: from signalling and gene expression to pharmacological effects. Trends Pharmacol Sci 2005; 26: 318-326.
- Silvan S, Manoharan S, Baskaran N, Anusuya C, Karthikeyan S, Prabhakar MM. Chemopreventive potential of apigenin in 7, 12-dimethylbenz (a) anthracene induced experimental oral carcinogenesis. Eur J Pharmacol 2011; 670: 571-577.
- Priyadarsini RV, Nagini S. Quercetin suppresses cytochrome P450 mediated ROS Generation and NF? B activation to inhibit the development of 7, 12-dimethylbenz (a) anthracene (DMBA) induced hamster buccal pouch carcinomas. Free Radic Res 2012; 46: 41-49.
- Sharma S, Stutzman JD, Kelloff GJ, Steele VE. Screening of potential chemopreventive agents using biochemical markers of carcinogenesis. Cancer Res 1994; 54: 5848-5855.
- Ketterer B. Glutathione S-transferases and prevention of cellular free radical damage. Free Radic Res 1998; 28: 647-658.
- Faris MA, Takruri HR, Shomaf MS, Bustanji YK. Chemopreventive effect of raw and cooked lentils (Lens culinaris L) and soybeans (Glycine max) against azoxymethane-induced aberrant crypt foci. Nutr Res 2009; 29: 355-362.
- Awasthi YC, Sharma R, Singhal SS. Human glutathione S-transferases. Int J Biochem 1994; 26: 295-308.
- Talalay P, Delong MJ, Prochaska HJ. Molecular mechanism in protection against Carcinogenesis. Cancer Biol Therap New York Plenum Press 1981; 197-216.
- Takahashi M, Tsuboyama-Kasaoka N, Nakatani T, Ishii M, Tsutsumi S. Fish oil feeding alters liver gene expressions to defend against PPARalpha activation and ROS production. Am J Physiol Gastrointest Liver Physiol 2002; 282: 338-348.
- Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicol 2000; 148: 187-197.
- Thanusu J, Kanagarajan V, Nagini S, Gopalakrishnan M. Chemopreventive potential of 3-(2, 6-bis (4-fluorophenyl)-3-methyl piperidin-4-ylideneamino)-2-thioxoimidazolidin-4-one on 7, 12- dimethylbenz (a) anthracene (DMBA) induced hamster buccal pouch carcinogenesis. J Enzyme Inhib Med Chem 2010; 25: 836-843.
- Blair IA. DNA adducts with lipid peroxidation products. J Biol Chem 2008; 283: 15545-15549.
- Erdogan H, Fadillioglu E, Ozgocmen S, Sogut S, Ozyurt B. Effect of fish oil supplementation on plasma oxidant/antioxidant status in rats. Prostaglandins Leukot Essent Fatty Acids 2004; 71: 149-152.
- Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape Seeds prevents photocarcinogenesis in SKH-1 hairless mice: relationship to decreased fat Lipid peroxidation. Carcinogenesis 2003; 24: 1379-1388.