- Biomedical Research (2016) Volume 27, Issue 3
Chemical constituents of Tripterygium wilfordii Hook F and proapoptotic molecular mechanism of burselignan on SMMC-7721 cells.
Bin Zhang, Tiankui Qiao*, Caixia GaoDepartment of Oncology, Jinshan Hospital, Fudan University, Shanghai 201508, PR. China
- Corresponding Author:
- Tanikui Qiao
Department of Oncology
Jinshan Hospital, Fudan University, PR. China
Accepted Date: March 24, 2016
Abstract
To study the chemical constituents in leaves of Tripterygium wilfordii Hook F, and to explore the proapoptotic action of burselignan on SMMC-7721 cells and its molecular mechanism. Various chromatographic approaches were employed for separating chemical constituents, and compounds were structurally elucidated based on physicochemical properties and spectral data. Antiproliferative activity of burselignan on SMMC-7721 cells was detected by MTT assay, and burselignan's effects on Bcl-2, Bax and Survivin protein expressions were analyzed by Western blotting. Five compounds were isolated and identified, namely lyoniresinol, isolariciresinol, burselignan, dibutyl phthalate and daucosterol. Burselignan inhibited the proliferation of SMMC-7721 cells. Western blotting showed that burselignan could increase Bax expression, and decrease Bcl-2 and Survivin expression. Burselignan can significantly inhibit the proliferation of SMMC-7721 cells, and induce their apoptosis by regulating the expression of Bax, Bcl-2 and Survivin proteins.
Keywords
Tripterygium wilfordii Hook F leaf, Chemical constituents, Apoptosis, SMMC-7721
Introduction
Tripterygium wilfordii Hook F is a plant in the genus Tripterygium of the family Celastraceae, which is mainly grown in China's Zhejiang, Anhui, Jiangxi, Hunan, Yunnan and other places [1]. Bitter and strongly toxic, the plant has winddispelling, dampness-eliminating, blood-activating, collateraldredging, swelling-relieving, analgesic, insecticidal and detoxifying functions. Tripterygium wilfordii Hook F is prominently effective in curing rheumatoid arthritis, lupus erythematosus and other autoimmune diseases. According to existing studies, Tripterygium wilfordii Hook F mainly contains sesquiterpenoids (alkaloids), diterpenoids, triterpenoids, as well as a small amount of lignans and phenolic compounds [2-4].
Diterpenoids in Tripterygium wilfordii Hook F are abietane diterpenoids with α,β-unsaturated five-membered lactone ring and tri-oxygen structure, which have a variety of activities and are used for psoriasis, diabetes and rheumatoid arthritis [5-7]. Apoptosis, as an essential biological phenomenon, plays a vital role in maintaining body's homeostasis. Apoptosis is not only present in the normal tissues, but is also widespread in tumor tissues. Through research on apoptosis and its mechanism, researchers have found that tumor genesis, development and drug resistance are closely associated with apoptosis [8]. Tumor cell-specific induction of apoptosis has become one of the targets for oncotherapy research and a hallmark of evaluating tumor therapeutic efficacy, which can provide a reliable basis for the study of tumor treatments [9]. It has been reported that Tripterygium wilfordii can induce tumor cell apoptosis [10,11]; however, its mechanism of action is unclear yet. Therefore, we first studied the chemical constituents of Tripterygium wilfordii Hook F, and then examined whether monomer constituents could induce apoptosis in human hepatoma cells in vitro, and identified changes in some apoptosis-related factors, in order to explore the tumor cell apoptosis-inducing molecular mechanism of monomer compounds in Tripterygium wilfordii Hook F.
Materials and Methods
Instruments, reagents and drug
Agilent 1200 series LC/MSD Trap-SL system; INOVA-500 and MECURY-400 NMR spectrometers (with TMS as internal standard); TLC silica gel GF254 and column chromatography silica gel (200~300 mesh), purchased from Qingdao Haiyang Chemical Plant; D101 macroporous resin, purchased from Nanjing Shuguang Reagent Company; Sephadex LH-20 dextran gel, purchased from Pharmacia; Agilent 1200 HPLC system. Tripterygium wilfordii Hook F leaves were collected from Yunnan, which were identified by professor Tang Jian of Yunnan University of TCM as the dried leaves of Tripterygium wilfordii Hook F and preserved in the Herbarium of Yunnan University of TCM with a specimen number of 20150611A.
RPMI 1640 medium was purchased from GIBCO BRL; FBS was purchased from Nanjing Mende Biotechnology; MTT powder was purchased from Serva; PI and RNase A were purchased from Sigma; Annexin V apoptosis detection kit was purchased from Bendermed, Germany; Bax polyclonal antibody and Bcl-2 monoclonal antibody were purchased from Nanjing Huaishide Bioengineering Co., Ltd.; Survivin polyclonal antibody, HRP-goat anti-mouse IgG, HRP-goat anti-rabbit IgG and HRP-horse anti-goat IgG were purchased from Santa Cruz; nitrocellulose membrane was purchased from Millitore; acrylamide, sodium dodecyl sulfate and N,N'- methylene bisacrylamide were purchased from Biomol; Tris was purchased Promaga; western blotting chemiluminescence kit and low molecular marker were purchased from Amersham Pharmacia Biotech.
Extraction and separation
20 kg of Tripterygium wilfordii Hook F leaves were crushed, and extracted under heat reflux with 50% ethanol for 2 h three times. After evaporating to a small volume, the aqueous extract was defatted with petroleum ether, and then extracted with chloroform. The resulting chloroform extract was subjected to silica gel column chromatography (cyclohexane-ethyl acetate), further subjected to silica gel column chromatography, and gradient eluted with chloroform-methanol system to give fractions. Afterwards, the fractions were chromatographed on silica gel column, eluted with cyclohexane-acetone system, and separated through Sephadex LH-20 (with methanol-water as the mobile phase) and preparative liquid chromatography (with methanol-water as the mobile phase) to give five compounds.
Cell lines and cultivation
Human hepatoma SMMC-7721 cells were provided by the Tumor Research Institute of Kunming Medical University, which were cultured in a 5% CO2 incubator at 37°C with saturated humidity in a 10% FBS-containing RPMI 1640 medium adherently in monolayer. Logarithmic phase cells with a density of 0.5×106~1×106/ml were used in the experiment.
MTT assay of drug susceptibility
Logarithmic phase cells were digested, collected, adjusted to a 5 × 104/ml single cell suspension, and seeded into 96-well plates. 24 h later, burselignan prepared with RPMI 1640 medium was added at final concentrations of 25.6 mg/L, 12.8 mg/L, 6.4 mg/L, 3.2 mg/L, 1.6 mg/L, 0.8 mg/L, 0.4 mg/L and 0.2 mg/L, respectively. In addition, a blank control group containing RPMI 1640 medium only and a negative control group that was concentration adjusted only while not given experimental treatment were set up. Each concentration had six replicate wells. After culturing for additional 24, 48 and 72 h, respectively, supernatant was discarded, and each well was added with 50 μl of MTT until the final concentration was 0.42 mg/ml, then mixed well, and incubated in a 37°C, 5% CO2 incubator for 4 h. Finally, supernatant was discarded, bluepurple granules were dissolved in DMSO, and absorbance (A value) of each well was measured at 570 nm with Ascent microplate reader. The experiment was repeated 2 times. IC50 was calculated by linear regression using SPSS statistical analysis software.
Morphological observation of apoptosis
Viable cells in culture flasks were observed under Olympus 70 inverted microscope, and ultrastructurally observed under electron microscope. Cells were double fixed in 2.5% glutaraldehyde and 1% osmic acid, dehydrated with gradient ethanol and acetone, embedded in Epon 812 resin, sliced with an ultramicrotome, and double stained with sodium acetate and lead citrate, followed by observation and photography under TEM.
FACscan detection of cell cycle and apoptosis
Cells were digested with 0.25% trypsin, blown, collected, centrifuged and washed in PBS twice, and then fixed in 70% cold ethanol. After removing ethanol by washing twice with PBS, the cells were added with RNase A (0.1 mg/ml), and allowed to stand at 37°C for 30 min. Afterwards, cells were stained with 1 ml of 50 mg/L PI for 30 min, and analyzed for DNA content and cell cycle with a flow cytometer using Cellquest software.
Quantification of apoptosis by Annexin V-FITC labelling
Cells were digested with 0.25% trypsin, blown, collected, washed once with PBS, and centrifuged at 1000 rpm at 4°C for 10 min. After centrifugation, supernatant was discarded, cells were resuspended with binding buffer, and cell number was adjusted to 5 × 105/ml. 195 μl of the cell suspension was added with 5 μl of Annexin V-FITC, mixed at room temperature, and incubated for 10 min. Next, cells were washed with 200 μl of binding buffer, and centrifuged at 1000 rpm at 4°C. Afterwards, supernatant was discarded, cells were resuspended with 190 μl of binding buffer, added with 10 μl of 20 mg/L PI, and detected by flow cytometry.
Cell DNA extraction and agarose gel electrophoresis
DNA fragments were collected referencing to literature [12], electrophoresed on a 1.2% agarose gel (50 V, 2 h), stained with EB, then detected and photographed with an automated electrophoresis gel image analyzer.
Western blot detection
Collected cells were treated with lysate, electrophoresed on SDS-PAGE with protein extract sample, and transferred to a membrane. After blocking, primary antibody was added (at a dilution of 1:250), and membrane was washed three times with PBS and twice with TBS, with each time lasting 10 min. Next, secondary antibody was added (at a dilution of 1:2500), and membrane was washed twice with PBS and twice with TBS, with each time lasting 10 min. Afterwards, membrane was immersed in an appropriate amount of ECL chemiluminescence reagents (solution A: solution B = 1:1) for 1 min, removed, dried at room temperature, wrapped in plastic, and exposed to film for 2~60 min, then washed.
Results
Structure elucidation
Compound 1: C22H28O8 white square crystals. [α]20 D+19.3° (MeOH, c 0.074). ESI-MS m/z 443 [M+Na]+. 1H-NMR (DMSO-d6, 500 MHz) δ: 6.35 (2H, s, H-2,6), 4.88 (1H, d, J=5.0 Hz, H-7), 1.76 (1H, m, H-8), 6.22 (1H, s, H-2’), 2.54 (1H, dd, J=15.0, 4.5 Hz H-7’), 2.33 (1H, dd, J=15.0, 12.0 Hz, H-7’), 1.45 ( 1H, m, H-8’), 3.14~3.52 (4H, m, H-9,9’), 3.66 (6H, s, OMe-3, OMe-5), 3.79 (3H, s, OMe-3c), 3.34 (3H, s, OMe-5c), 8.01, 8.16 (2H, s, Ar-OH), 4.63, 4.36 (2H, t, - CH2OH); 13C-NMR (DMSO-d6, 125 MHz) δ: 138.0 (C-1), 106.2 (C-2), 146.9 (C-3), 133.7 (C-4), 147.4 (C-5), 104.7 (C-6), 40.9 (C-7), 46.4 (C-8), 62.1 (C-9), 128.8 (C-1’), 106.7 (C-2’), 146.7 (C-3’), 137.6 (C-4’), 145.7 (C-5’), 125.1 (C-6’), 32.4 (C-7’), 39.9 (C-8’), 63.6 (C-9’), 56.9 (OMe-3), 56.6 (OMe-5), 56.2 (OMe-3’), 59.1 (OMe-5’). The above data were consistent with that reported in the literature [13], so compound 1 was identified as (+)-lyoniresinol.
Compound 2: C20H24O6 pink powder. [α]20 D+46.7° (MeOH, c 0.063). ESI-MS m/z 383 [M+Na]+, ESI-MS m/z 359 [M-H]-. 1H-NMR (DMSO-d6, 500MHz) δ: 6.78 (1H, d, J=115 Hz, H-2), 6.77 (1H, d, J=8 Hz, H-5), 6.52 (1H, dd, J=8, 1.5 Hz, H-6), 3.66 (1H, d, J=9.5 Hz, H-7), 1.88 (1H, m, H-8), 6.62 (1H, s, H-2’), 6.08 (1H, s, H-5’), 2.68~2.59 (2H, m, H-7’), 1.62 (1H, m, H-8’), 3.33 (2H, m, H-9), 3.24 (1H, m, H-9’), 3.54 (1H, m, H-9’), 3.66 (3H, s, OMe-3), 3.56 (3H, s, OMe-3’), 8.43, 8.74 (2H, s, Ar-OH), 4.78, 4.34 (2H, t, -CH2OH); 13CNMR (DMSO-d6, 125 MHz) δ: 133.7 (C-1), 114.2 (C-2), 146.3 (C-3), 145.6 (C-4), 115.4 (C-5), 121.1 (C-6), 46.6 (C-7), 45.9 (C-8), 59.2 (C-9), 127.4 (C-1’), 111.5 (C-2’), 147.3 (C-3’), 144.7 (C-4’), 116.1 (C-5’), 137.1 (C-6’), 32.1 (C-7’), 37.9 (C-8’), 63.4 (C-9’), 55.5 (OMe-3), 55.6 (OMe-3’). The above data were consistent with that reported in the literature [14], so compound 2 was identified as (+)-isolariciresinol.
Compound 3: C20H24O6 pink powder. [α]20 D-107.2° (MeOH, c 0.036). ESI-MS m/z 383 [M+Na]+, 399 [M+K]+. 1H-NMR (DMSO-d6, 500 MHz) δ: 6.65 (1H, d, J=1.5 Hz, H-2), 6.62 (1H, d, J=8 Hz, H-5), 6.31 (1H, dd, J=8, 1.5 Hz, H-6), 4.12 (1H, d, J=4.5 Hz, H-7), 1.88 (1H, m, H-8), 6.55 (1H, s, H-2’), 6.33 (1H, s, H-5’), 2.78 (1H, dd, J=16.4, 5.5 Hz, H-7’), 2.55 (1H, dd, J=16.5, 10 Hz, H-7’), 1.80 (1H, m, H-8c), 3.40~3.25 (2H, m, H-9), 3.02 (1H, t, H-9’), 3.42 (1H, m, H-9’), 3.73 (3H, s, OMe-3), 3.81 (3H, s, OMe-3’), 8.62, 8.69 (2H, s, Ar-OH), 4.56~4.29 (2H, br, -OH); 13C-NMR (DMSO-d6, 125 MHz) δ: 134.6 (C-1), 114.2 (C-2), 146.7 (C-3), 145.1 (C-4), 116.2 (C-5), 122.6 (C-6), 44.4 (C-7), 44.1 (C-8), 61.6 (C-9), 127.5 (C-1’), 111.7 (C-2’), 146.1 (C-3’), 144.4 (C-4’), 116.5 (C-5’), 131.7 (C-6’), 31.3 (C-7’), 34.2 (C-8’), 63.1 (C-9’), 55.5 (OMe-3), 55.6 (OMe-3’). The above data were consistent with that reported in the literature [15], so compound 3 was identified as burselignan.
Compound 4: C16H22O4 colorless oil. ESI-MS m/z 279 [M +H]+, 301 [M+Na]+. 1H-NMR (CDCl3, 400 MHz) δ: 7.78 (2H, dd, J=5.6, 3.2 Hz, H-3,6), 7.45 (2H, dd, J=5.6, 3.6Hz, H-4,5), 4.32 (4H, t, J=6.4 Hz, H-2’,2’’), 1.69 (4H, quint, H-3’, 3’’), 1.52 (4H, sext, H-4’,4’’), 0.94 (6H, t, J=7.6 Hz, H-5’, 5’’); 13CNMR (CDCl3, 100 MHz) δ: 167.9 (COO-), 132.6 (C-1,2), 131.9 (C-3,6), 127.9 (C-4,5), 65.2 (C-2’,2’’), 30.8 (C-3’, 3’’), 20.1 (C-4’, 4’’), 13.9 (C-5’, 5’’). 1H-NMR data of the compound were consistent with that reported in the literature [16], so compound 4 was identified as dibutyl phthalate.
Compound 5: white powder. Hardly soluble in methanol, chloroform. After TLC and comparison with daucosterol standard, compound 5 was identified as daucosterol.
MTT assay of drug susceptibility
SMMC-7721 cell inhibitory effect of burselignan exhibited time- and concentration-dependence. The effect was weak at 24 and 48 h, with inhibition rates ranging between 20%~60%, while prominent at 72 h, with inhibition rate of 30~90%. IC50 at 72 h was calculated to be 1.4 mg/L. In subsequent experiments, 1.4 mg/L was regarded as the concentration parameter.
Morphological observation of apoptosis
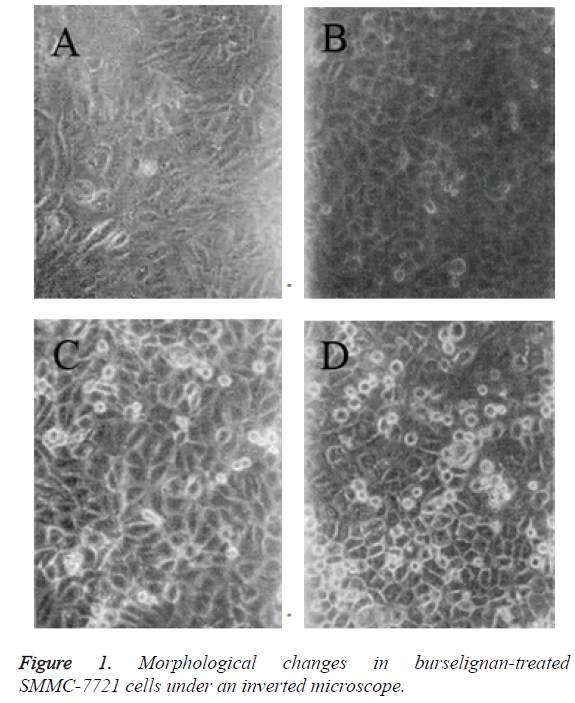
Inverted microscopic observation: Compared with the normal control cells, SMMC-7721 cells treated with 1.4 mg/L burselignan for 24 h and 48 h presented partial rounding, diminished volume, surface budding of a few cells, with buds either included in the juxtamembrane or protruded extracellular surface. Cells became irregularly shaped, and formed apoptotic bodies after bud detachment. Apoptotic bodies were circular or oval, within which highly condensed chromatins were visible (Figures 1A-1C). After treating for 72 h, increase in the number of apoptotic SMMC-7721 cells was observed. A small part of cells were detached, and floating in the culture medium. There were also a few cells which increased in volume, presenting swelling, rupture and necrosis-like morphology (Figure 1D).
TEM observation: Compared with the normal control cells, SMMC-7721 cells treated with 1.4 mg/L burselignan for 24 h and 48 h presented chromatin aggregation near nuclear membrane edge in crescent shape, as well as cytoplasm condensation within apoptotic cells. After treating for 72 h, apoptotic SMMC-7721 cells and their apoptotic bodies were observed. Several condensed chromatin clumps in the cytoplasm had no nuclear envelope; dilated endoplasmic reticula were arranged along exposed chromatin clumps; and within apoptotic bodies being formed, detachment of nuclear enveloped chromatin clumps from apoptotic cells was visible.
FACscan detection results of cell cycle and apoptosis
SMMC-7721 cells treated with 1.4 mg/L burselignan for 24, 48, 72 h, as well as blank control cells at corresponding time points were collected separately for flow cytometry. The results showed that the sub-G1 and G0 peaks lower than G1 phase DNA content were the apoptotic cells, apoptotic bodies, and necrotic cells and debris. With prolonged action of burselignan on SMMC-7721 cells, DNA contents of sub-G0 and G1 apoptotic peaks increased gradually, with 24, 48 and 72 h apoptosis rates of 7.2%, 9.7% and 27.7%, respectively. G2 and M phase cell percentages were 35.7%, 52.2% and 47.3%, while those for blank control cells at corresponding phases were 15.2%, 12.4% and 16.7%, respectively.
Annexin V-FITC labeling quantification results
Flow cytometry combined with Annexin V-FITC labeling results showed that proportions of apoptotic SMMC-7721 cells were low after treating for 24 and 48 h with 1.4 mg/L burselignan, which were 5.3% and 6.1%, respectively. At 72 h, proportion of apoptotic cells increased to 22.1%. This further confirmed the strong apoptosis-inducing effect of burselignan on SMMC-7721 cells.
DNA agarose gel electrophoresis results
When SMMC-7721 cells were treated with 1.4 mg/L burselignan for 72 h, apoptosis-specific ladders appeared as the apoptotic cell DNAs were degraded into regular fragments. At 120 h, most cells were necrotic. The necrotic cells appeared as a continuous membrane-like band because of the irregular degradation of their DNAs, while the genomic DNA bands of normal viable cells stayed in the vicinity of loading well due to high molecular weight and short migration distance.
Western blotting results
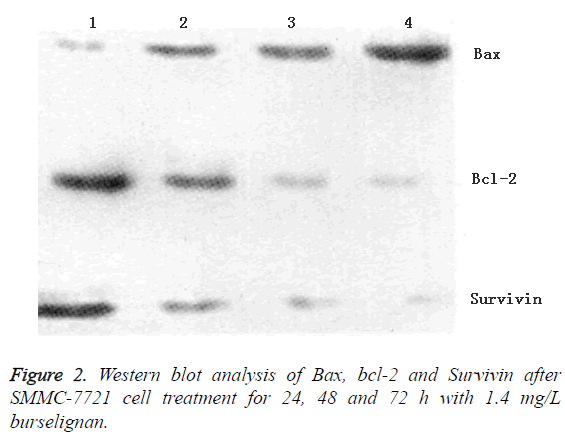
SMMC-7721 cells treated for 24, 48 and 72 h with 1.4 mg/L burselignan were collected, as well as blank control cells, all of which were extracted for total proteins to detect the expressions of apoptosis-related proteins bcl-2, Bax and Survivin. The results showed that over time, Bax expression gradually increased, while bcl-2 and Survivin expressions decreased gradually (Figure 2).
Discussion
Apoptosis is an initiative cell death process regulated by genes, which is influenced and constrained by external factors as well. With the deepening of research on tumor molecular biology, it has been recognized that tumor occurrence and development are not only associated with the proliferation and differentiation abnormalities of tumor cells, but are also associated with abnormal regulation of apoptosis. Therefore, induction of tumor cell apoptosis has become a novel therapeutic direction.
According to the results of this study, burselignan-treated human hepatoma SMMC-7721 cells exhibited marked apoptotic morphology. Flow cytometry showed appearance of sub-diploid apoptotic peak before G1 phase, which was associated with G2 or M phase arrest. Gel electrophoresis revealed ladder pattern of DNA nucleosomal fragmentation. Annexin V-FITC labeling quantification of apoptotic cells also demonstrated strong apoptosis-inducing effect of burselignan on hepatoma SMMC-7721 cells.
Apoptosis is a complex biological process involved by multiple genes, where bcl-2 family plays an important role [17]. Members of bcl-2 family have a dual function, of which bcl-2, bcl-XL, etc. inhibit apoptosis, while Bax, bcl-XS, etc. promote apoptosis [18]. Bcl-2 gene encodes a protein with relative molecular mass of about 26,000. High bcl-2 protein expression can protect cells from death, so bcl-2 is believed to be an apoptosis-related gene having a significant anti-apoptotic effect. Bcl-2 can block the final pathway of apoptosis induction, which plays a cytoprotective role by preventing damaged DNA from being translated into signals of genes involved in apoptosis or by blocking the activity of these gene products. Composed of 192 amino acids, Bax gene encodes a protein with relative molecular mass of 21,000, which inhibits bcl-2 or bcl-XL activity by forming homodimers with itself or heterodimers with bcl-2, bcl-XL, and thereby plays a proapoptotic role. Structural characteristics and interactions between Bax and bcl-2 proteins closely link the two within cells, that is, regulation formed by bcl-2 or Bax heterodimers is a vital aspect of apoptosis regulation [19]. Bcl-2 and Bax protein levels are directly related to the apoptosis regulation; increasing bcl-2 inhibits apoptosis, whereas increasing Bax promotes apoptosis. Our study found that after treating SMMC-7721 cells with burselignan, bcl-2 protein expression decreased, while Bax protein expression increased. This indicates that burselignan may induce SMMC-7721 cell apoptosis by altering the regulatory equilibrium of bcl-2 and Bax heterodimers.
Conclusion
Our study also found decreased protein expression of antiapoptotic gene Survivin after treatment of SMMC-7721 cells with burselignan. Survivin, as a recently discovered antiapoptotic protein, belongs to the Inhibition of Apoptosis Protein (IAP) family, which is by far the most effective antiapoptotic protein. Survivin gene encodes a cytoplasmic protein comprising 142 amino acids with relative molecular mass of approximately 16,200. Survivin has a conserved Baculoviral IAP Repeat (BIR) domain at the N-terminal, which is the main motif playing a direct role in inhibiting the activation or catalytic reaction of apoptotic executors caspase-9, caspase-3, caspase-6 and caspase-7; meanwhile, Survivin lacks Ring zinc finger at the C-terminal, which helps enhance its antiapoptotic function [20]. Furthermore, Survivin expression is positively correlated with bcl-2; both Survivin and bcl-2 genes are regulated by GC-rich promoters without TATA box; either transcription or activation can enhance cell proliferation, so the two can synergistically exert antiapoptotic effect [21]. Apoptosis-inducing mechanism of burselignan may be through inhibition of caspase-3 and caspase-7 activities in the apoptosis pathway downstream by inhibiting the expression of antiapoptotic protein Survivin.
References
- China National Traditional Chinese Medicine Corporation. Compendium of Chinese Medicinal Material Resources. Beijing: Science Press, 1994.
- Ma ZQ, Li YJ, Wu LP, Zhang X. Isolation and insecticidal activity of sesquiterpenes alkaloids from Tripterygiumwilfordii Hook F. Ind Crops Prod 2014; 52: 642-648.
- Tamaki T, Kawamura A, Komatsu Y, Kawamura H, Maruyama H, Morota T. Phenolic nortriterpenedemethylzeylasteral: a new immunosuppressive component of TripterygiumWilfordii Hook F. Transplant Proc1996; 28: 1379-1380.
- Tao XL, Lipsky PE. Triptolide, an active component of Tripterygiumwilfordii Hook F inhibits induction of IL-2 gene expression by limiting activity of transcription factors AP-1 and NFAT. Arthritis Rheumatol 1998; 41: S196.
- Wu C, Jin HZ, Shu D, Li F, He CX, Qiao J, Yu XL, Zhang Y, He YB, Liu TJ. Efficacy and Safety of Tripterygiumwilfordii Hook F Versus Acitretin in Moderate to Severe Psoriasis Vulgaris: A Randomized Clinical Trial. Chin Med J (Engl) 2015; 128: 443-449.
- Ma RX, Xu Y, Jiang W, Zhang W. Combination of Tripterygiumwilfordii Hook F. and angiotensin receptor blocker synergistically reduces excretion of urinary podocytes in patients with type 2 diabetic kidney disease. BiotechnolBiotechnol Equip 2015; 29: 139-146.
- Lv QW, Zhang W, Shi Q, Zheng WJ, Li X, Chen H, Wu QJ. Extended report: Comparison of Tripterygiumwilfordii Hook F. with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann RheumDis 2015; 74: 1078-1086.
- Roberts JR, Allison DC, Donehower RC, Rowinsky EK. Development of polyploidization in taxol-resistant human leukemia cells in vitro. Cancer Res 1990; 50: 710-716.
- Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994; 73: 3108.
- Xiong J, Wang H, Guo GM, Wang SZ, He LQ, Chen HF, Wu J. Male Germ Cell Apoptosis and Epigenetic Histone Modification Induced by Tripterygiumwilfordiis Hook F. PLoS ONE 2011; 6: e20751.
- Gu M, Zhou HF, Xue B, Niu DB, He QH, Wang XM. Effect of Chinese herb Tripterygiumwilfordii Hook F monomer triptolide on apoptosis of PC12 cells induced by Abeta1-42. Sheng Li XueBao 2004; 56: 73-78.
- Herrmann M, Lorenz HM, Voll R, Griinke M, Woith W, Kalden JR. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res 1994; 22: 5506-5507.
- Liu LS, Huang HL, Liu RH, Ren G, Shao F, Ye YH, Lin T. A new lyoniresinol derivative from Smilax microphylla. Nat Product Commun 2013; 8: 113-114.
- Zhang F, Han LF, Pan GX, Peng S, Andre N. A new phenolic amide glycoside from Cimicifugadahurica. Yao XueXueBao 2013; 48: 1281-1285.
- Cao X, Li C, Yang J, Wei B, Luo Y, Zhang D. Study on chemical constituents from leaves of Tripterygiumwilfordiis. J Chinese MateriaMedica 2011; 36: 1028-1031.
- Lars Ö, Anders LH, Folke F. Studies of water in organic solvents using NMR and partition techniques-II Di-isopropyl ether, dibutyl phthalate and chloroform. J Inorg Nuclear Chem 1972; 34: 2605-2616.
- Reed JC. Double identity for proteins of the Bcl-2 family. NATURE 1997; 387: 773-776.
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science 1998; 281: 1322-1326.
- Marone M, Ferrandina G, Macchia G, Mozzetti S, Pasqua A, Benedetti-Panici P, Mancuso S, Scambia G. Bcl-2, Bax, Bcl-x(L) and Bcl-x(S). Expression in Neoplastic and Normal Endometrium. Oncology 2000; 58: 161-168.
- Li FZ, Ambrosini G. Control of apoptosis and mitotic spindle checkpoint by survivin. NATURE 1998; 396: 580-584.
- Ambrosini G, Adida C, Sirugo G, Altieri DC. Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J BiolChem 1998; 273: 11177-11182.