- Biomedical Research (2010) Volume 21, Issue 3
Chemical analysis of stones and its significance in urolithiasis
Pushpa Durgawale*, Anissa Shariff, Anup Hendre, Sangita Patil, Ajit SontakkeDepartment of Biochemistry, Krishna Institute of Medical Sciences and Research Centre, Karad, India.
- Corresponding Author:
- Pushpa P. Durgawale
Department of Biochemistry
Krishna Institute of Medical Sciences and Research Centre
Karad. 415 110 Maharashtra. India.
Accepted date: February 18 2010
Abstract
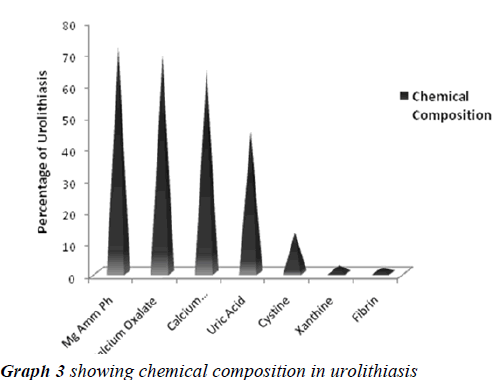
The search of literature showed Urolithiasis as a multifactorial recurrent disease, distributed worldwide in urban, rural , non industrial and industrial regions with different chemical composition of analyzed stones in context to various etiological and risk factors. The present study was aimed to qualitatively analyze the uroliths obtained by surgical intervention at Krishna hospital Karad, a South West region in Maharashtra (India), to evaluate the predominant constituent present in them and report its significance. The study reported, urolithiasis was more suffered by individuals between the age group of 30 to 60 years with more predominance in males than females. The chemical analysis of uroliths showed that all the assessed stones were of mixed heterogeneous type, Magnesium Ammonium Phosphate (71.2%) was predominant constituent followed by Calcium Oxalate (68.8%), Calcium Carbonate (64.0%), Urate (44.8%), Cystine (12.8%), Xanthine (2.4%) and Fibrin (1.6%). The study concludes simple qualitative laboratory based method for assessing chemical composition of various uroliths allowed a reliable diagnosis of stone contents whose data may be useful in advising the people of this region for taking preventive measures for reducing the risk of prevalence and recurrence of urolithiasis in them.
Keywords
Urolithiasis, uroliths, renal stones.
Introduction
Urolithiasis, the process of forming stones in the kidney, bladder and/or urethra is a complex phenomenon yet not clearly understood, this cannot be contributed to any single factor, and may be due to metabolic disturbances, infections, hormonal influences, dietary conditions and habits, poor fluid intake which concentrates and decreases the urine volume, immobilization or lesions or obstructions in the bladder or kidney or increased excretion of stone forming components such as calcium, magnesium, oxalate, carbonate, phosphate, urate, xanthine, cystine, etc.
Renal stones, one of the most painful urologic disorders, have beset humans for centuries. Researchers have found evidence of kidney stone in 7,000 year old Egyptian mummy [1]. Each year, worldwide people make almost 3 million visits to health care providers and more than half a million patients go to emergency room with urolithiasis [2]. Epidemiological studies indicate many factors like age, sex, industrialization, socioeconomic status, diet and environment, influences urolithiasis [3]. The prevalence of renal stone formation is approximately 2-3% in the general population [4]. Alarming high incidence of urolithiasis with varied chemical composition of calculi has been reported from different regions of India [5-11]. Endemic bladder calculi are common in developing countries like Eastern Europe, South-East Asia, and India and in the Middle East, where dietary protein is derived from plant sources. Upper urinary calculi associated with urease producing bacterial infections occur in England and Europe. Kidney stones are not usually fatal although some primary conditions that produce kidney stones can lead to death from problems associated with primary disease or complications of renal failure. Infected stones may lead to urosepsis and death [12].
There was however no data on the chemical composition of renal calculi from the population of Western Maharashtra, thus the present pilot study was undertaken to qualitatively analyze the renal stones with the relationship between the sex, age group and location of uroliths in patients of urolithiasis and gather some information in this regard. A knowledge of chemical composition of renal stones may be of great importance both as a guide for the clinical management and also for better understanding of physicochemical principles underlying the formation of calculi that may help to give advice and suggestions for the people and patients to carry out preventive measures in reducing the risk of prevalence and recurrence of urolithiasis in this region respectively.
Materials and Methods
The study included one hundred and twenty five uroliths obtained by surgical intervention of Urolithiasis patients, clinically and radiographically diagnosed at Krishna Hospital and research centre Karad a South-West region of Maharashtra (India), during a period of 2006-2008.The data of Urolithiasis cases was collected with the help of structured questionnaire proforma.The stones obtained from Surgery department to clinical Biochemistry laboratory were washed with distilled water to remove the debris, dried completely and weighed. The stones were cut and crushed, the powdered form was qualitatively ana lyzed for their chemical composition adopting standard methods [13] using chemicals of Analytical reagent grade. The research project was approved and permitted by the ethical committee of institute.
Results
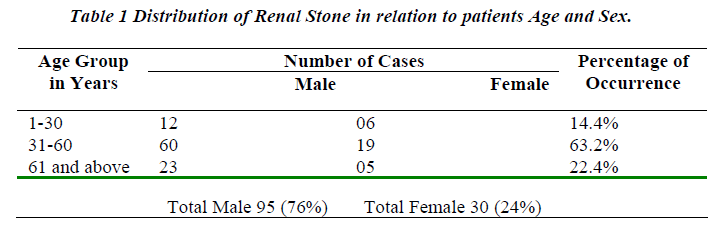
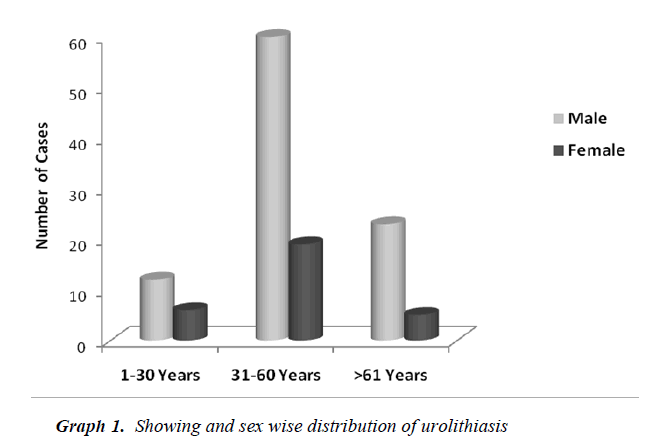
Table No. 1 depicts the urolithiasis cases classified and grouped according to age, sex and percentage of occurrence. High percentage of urolithiasis was reported in males than females of age group between 31-60 years.
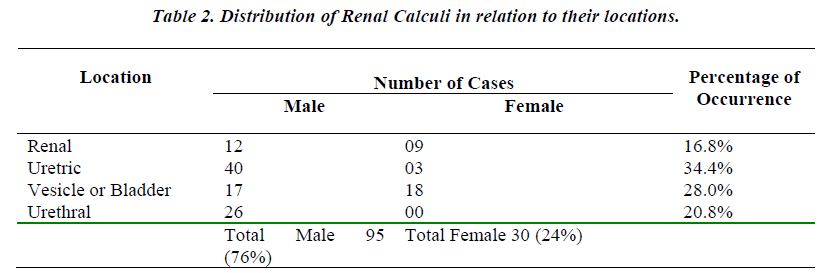
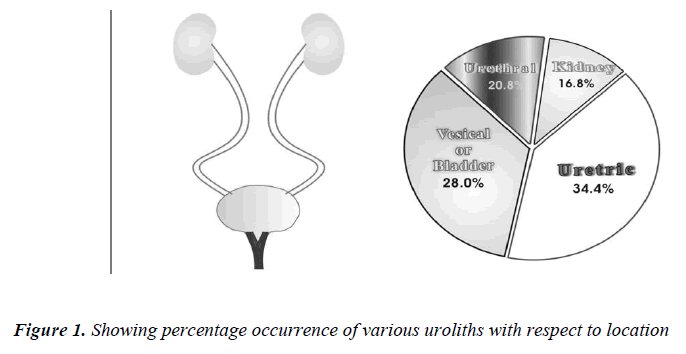
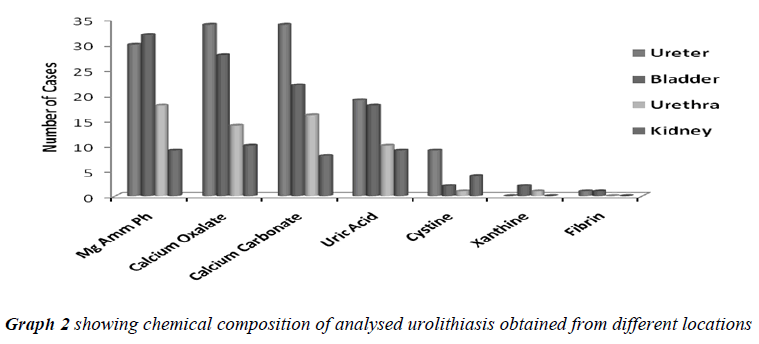
When the uroliths were grouped according to their locations in the urinary tract, the result of analysis showed a high incidence of stones in ureter followed in the order by bladder, urethra and kidney (Table No. 2).
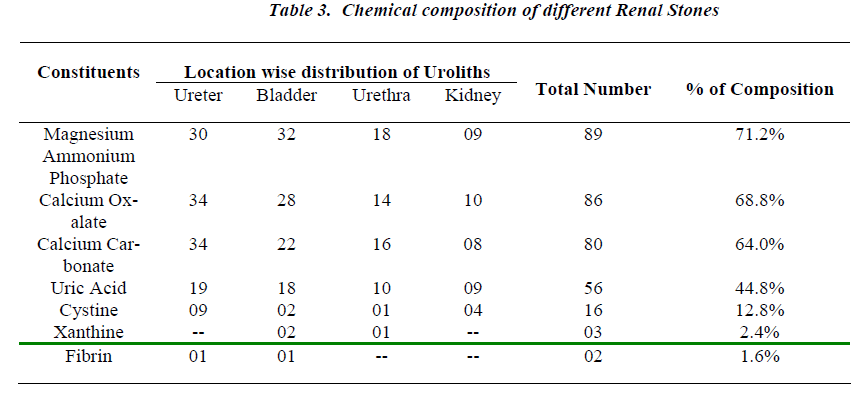
All the analyzed uroliths were of heterogenous mixed type, each was chemically different which is clearly depicted in Table No.3.
Discussion
Urolithiasis is calculus formation in any part of the urinary system, occurring in both sexes. The present study reported the weights of analyzed uroliths between 10 gms and 170 gms, predominantly suffered by males, located mostly in ureter, with mixed heterogenous chemical composition. The calculi causing urinary obstructions occurred more commonly in males may be due to smaller diameter and increased length of urethra. Calculi results from the precipitation of stone constituents might be due to supersaturation in urine. The presence of mixed stones suggests a multi factorial etiology. Magnesium ammonium phosphate (71.2%) was predominant constituent which predicts the presence of bacteriuria and urinary tract infections caused by urea splitting bacteria (Proteus sp, Kledsiella sp). The calculi must be treated as infected foreign body and removed in their entirety. The presence of calcium oxalate (68.8%) and calcium carbonate (64.0%) were more next common constituent in all the analyzed stones. For calcium calculi, risk factors may vary by population. The risk factors may be hypercalciuria, hyperparathyroidism, hypocitruria, renal tubular acidosis. Hyperoxaluria can be primarily caused by excess ingestion of oxalate containing foods (rhubarb, spinach, cocoa, nuts, pepper, beet and tea) or the excess oxalate absorption due to various enteric diseases (bacterial overgrowth syndromes, chronic pancreatitis or biliary disease) or ileojejunal surgery [14]. Other risk factors may include taking high doses of vitamin C and mild hyperuricosuria [15]. Our study also reported the stones chemically composed of urate which may be due to hyper uricosuria a disorder of uric acid metabolism; Gout, excess intake of vitamin D, urinary tract infections and blockage of urinary tract. The uric acid content of body may also increase due to increased non vegetarian diet causing risk of hyperuricosuria and further formation of urate stones. Cystine, xanthine and fibrin stones were rarely found in analyzed stones that may be associated components of stones.
P.P. Singh et.al in their 196 stone analysis in Manipur showed calcium and oxalate were present in all the stones. Phosphate was present in 194 and urate was present in 146 stones [16]. Urinary stone composition in North East Thailand from 120 adults and 22 children reported , calcium oxalate was the main component of both adult (49%) and childhood (44%) stones and were usually found in the upper urinary tract. Magnesium ammonium phosphate and urate were the stones found mainly in the upper urinary tracts [17]. Urinary biochemical profile of patients with ureteric calculi in Jodhpur (North-West India) also reported predominance of calcium, magnesium and oxalates in their stone analysis study [18]. Spectrum of pediatric urolithiasis in Western India (Mumbai) reported 94.8% stones were of mixed types and only 5.2% were pure. The common constituents in their analyzed stone were calcium (98.7%), oxalate (87.0%), phosphate (84.4%) and urate (76.6%) [19].
Chemical composition of urinary calculi in Maiduguri, Nigeria showed a male preponderance with female (12:1). The calculi occurred more in the upper part of urinary tract (70.9%) than the lower part of the tract (29.1%). Calcium containing stones constituted the majority (76.9%), urate (16.3%), struvite constitutes (4.3%), xanthine (1.7%) and cystine (0.9%) [20]. Gault M H and Chafe in Canadian population found phosphate stones were on average heavier and relatively more common in women, had an earlier age peak frequency in women than oxalate stones. In contrast oxalate stones were much common of lighter weight and became more frequent with time [21]. The types of stones formed depend mainly on the composition of urine, which in turn reflects the type of diet consumed in the areas. The stone problem in the tropics was compounded by low urine volumes of poor drinking [22].
Pediatric urolithiasis in Pakistanis showed the pattern of calculus disease changed from a predominantly lower tract site in the mid 1980s to the upper tract in the mid 1990s [23]. Stone composition, urinary risk factors and dietary analysis suggest that diet, dehydration and nutritional habits and urinary tract infections are the main causative factors of stone disease. Fluid intake and epidemiology of urolithiasis suggests a sufficient intake of fluid is one of the most important preventive measures for stone formation and stone recurrence [24]. Bacteriology of urinary tract stones reported that up to half of renal stones and associated urine specimens have been positive on culture and up to 50% of such stones contain Magnesium Ammonium Phosphate [25]. Urinary tract infections caused by urease producing Gram negative organisms, mainly produced stones constituting Magnesium Ammonium Phosphate, Carbonate apatite and Mono ammonium urate, an alkaline urine is most favourable to their formation [26].
Studies in the Durban area, with its high incidence of kidney and bladder stones, indicate that Calcium Oxalate is the overwhelmingly dominant (80%) followed by urate; the occurrence of phosphate stones, especially those containing Struvite were low [27].The study from Saudi Arabia reported hypercalciuria and hyperuricosuria showed correlation with family history of stones and obesity with risk factors for urolithiasis in both genders [28]. Urolithiasis in Okinawa,Japan accounted 81.6% for calcium oxalate stones while 15.8% for urate stones[29]. Frequency of renal stones was highest in Berlin and lowest inA Moscow; lesser distribution of phosphate lithiasis in Berlin than in Kirghizia and particularly in Moscow. Prevalence of struvite stones from Moscow was linked with the vital activity of Proteus and E .coli [30].
Many earlier studies from different geographical areas of world, reported recurrence of Urolithiasis with male predominance had multi etiological and risk factors as, urinary stasis, urinary tract infections, excess calorie intake, high consumption of non-veg diet, fruits and vegetables rich in oxalates, chocolates, tea and alcohol, smoking, low intake of fluids, [31-42], which could be prevented by shift to a nutritionally balanced, common sense diet containing sufficient amounts of fluids,1200 mg of calcium per day, reduced amount of flesh protein, salt, increase diet rich in alkali [43-45].
The data of chemical composition of assessed renal stones from the cases of urolithiasis in population of Karad city and nearby areas of south west region of Maharashtra reported more predominance of Struvite infection stones, the study suggested the factors for formation of stones in this area were more followed after urinary tract infections which made urine alkaline there by precipitating the phosphates. The Calcium calculi formation may be due to altered eating and drinking habits which promoted it in these individuals. The collected information in this regard may help to look through the risk factors for calculi formation in this area which might enable the health care providers to plan preventive measures in order to reduce the high incidence of urolithiasis in this area and further advice to the suffered patients to prevent the recurrence of stone formation in them.
Acknowledgment
We acknowledge the vice chancellor of Krishna Institute of Medical Sciences University, Karad for providing the financial support to carry out this research work in the Department of Biochemistry in collaboration with Surgery Department. We thank the doctors and technical staff of K.I.M.S Surgery Department for their guidance and support throughout the research period.
References
- Prien E L, Frondal C. Studies in urolithiasis. The composition of urinary calculi. J Urol 1947; 57: 549-556.
- Boyce W H, Garvey F K , Straw H G. Incidence of urinary calculi among patients in General Hospitals, 1948 to1952. J Am Med Assoc 1956; 161: 1437-1442.
- Herring L C. Studies on urinary calculi. J Urol 1962; 88: 545-562.
- Anderson DA. A survey of incidence of urolithiasis in Norway from 1853 to 1960. J Oslo. City Hospital 1966; 16: 101-147.
- Newcomb C, Ranganathan S. The composition of urinary calculi. Indian J Med Sci 1930; 17:1037.
- Parikh H S, Shah R C .Chemical composition of urinary calculi. Indian J Med Sci 1960; 14: 401-405.
- Rao B K,Gupta H N,Rangnekar C V. Chemical composition of urinary calculi J Indian Med Assoc 1964; 13: 469-473.
- Thind SK, Nath R. Chemical analysis of urinary calculi in Chandigarh area. Indian J Med Res 1969; 57: 1790.
- Kabra SG, Gaur SB, Sharma SS, Patni MK, Benerji P. Urolithiasis incidence of urinary calculi in South- Eastern Rajasthan- Report of 1144 cases. Indian J Surg 1972; 34: 309.
- Ansari MS, Gupta NP, Hemal AK, Dogra PN, Seth A, Aron M, Singh TP. Spectrum of stone composition: Structural analysis of 1050 upper urinary tract calculi from Northern India. Int J Urol 2005; 12: 12-16.
- Malhotra KK, Ahuja MS, Singh SM and Baphna BC. Correlative study of urinary calculus disease. Ind J Med Sci 1968; 23: 380.
- Halstead SB, Valyasevi A, Umpaivit P. Studies on bladder stone disease in Thailand V. Dietary habits and disease prevalence. Am J Clin Nutr 1967; 20: 1352.
- Hodgkinson A.A combined qualitative and quantitative procedure for the chemical analysis of urinary calculi. J Clinical Pathol 1975; 24:147-151.
- Frank M, De Vries A. Prevention of Urolithiasis. Education to adequate fluid intake in a new town situated in Judean Dessert Mountains. Arch. Environmtl Health 1966; 13: 625.
- Fleish H. Some new concepts in the pathogenesis and treatment of urolithiasis. Urol Int 1965; 19: 382.
- Singh P P, Singh L B, Prasad S N and Singh M G. Urolithiasis in Manipur ( North Eastern region of India ). Incidence and chemical composition of stones. Am J Clin Nutr 1978; 31: 1519-1525.
- Prasogwatana V, Sriboonlue, Suntarapa S. Urinary stone composition in North East Thailand. British J Urol 1983; 55: 353-355.
- Singh P P, Pendse A K, Rathore V and Dashora PK. Urinary biochemical profile of patients with ureteric calculi in Jodhpur region(North West India). Urological Research 1988; 16:105-110.
- Atul M S, Shrikant K, Punekar S V, Billimoria F R, Bapat S D and Deshmukh S S. Spectrum of pediatric urolithiasis in western India. Indian Journal of pediatrics 1991; 58:543-549.
- Mshelia DS, Gali DM, Naaya UH, Habu SA. Chemical composition of urinary calculi in Maiduguri, Nigeria. African J Med and Med Sci 2005; 34: 185-188.
- Gault M H, Chafe L. Relationship of frequency, age, stone weight and composition in 15,624 stones: Comparison of results for 1980 to 1983 and 1995 to 1998. J Urol 2000; 164: 302-07.
- Robertson W.G. Renal stones in the tropics. Semin Nephrol 2003; 23: 77-87.
- Rizvi S A, Naqvi S A, Hussain Z , Hashmi A, Hussain M et al. Pediatric Urolithiasis: developing nation perspectives. J Urol 2002; 168: 1522-5.
- Siener R and Hesse A. Fluid intake and epidemiology of urolithiasis. Europ J Clin Nutr 2003; 57: 47-51.
- Miano R, Germani S, Vespasiani. Stone and urinary tract infections. Urol Int 2007; 79: 32-6.
- Gault M H, Longerich LL, Crane G, Cooper R. Bacteriology of urinary tract stones. J Urol 1995; 153: 1164- 1170.
- Kerr A and Laing M. Mineralogical studies of human urinary calculi from Natal. Environmental Geochemistry and Health 1992; 14: 19-25.
- Abdel-Halim RE. Urolithiasis in adults. Clinical & biochemical aspects. Saudi Med J 2005; 26: 705-13.
- Hossain RZ, Ogawa Y, Hokama S, Morozumi M, Hatano T. Urolithiasis in Okinawa, Japan: A relative high prevalence of uric acid stones. Int J Urol 2003; 10: 411-415.
- Schubert G, Chudnovskaia MV, Brien G, Tynaliev MT, Popovkin NN, Timin AR. The characteristics of the chemical composition and structure of urinary stones and their prevalence in the cities of Moscow, Berlin and of the Kirghiz SSR. Urol Nefrol 1990; 5: 49-54.
- Mbonu O, Attah C, Ikeakor I. Urolithiasis in an African population. Int Urol Nephrol 1984; 16: 291-296.
- AK Pendse and PP Singh. The etiology of Urolithiasis in Udaipur (Western part of India). Urol Res 1986; 14: 59-62.
- J.U. Monu. Pattern of Urolithiasis in Benin City, Nigeria. J Natl Med Assoc 1989; 81: 695-698.
- Nayar D, Kapil U, Dogra PN. Risk factors in Urolithiasis. The Indian Practitioner 1997; 50: 209-14
- Siener R, Ebert D, Nicolay C, Hesse A. Dietary risk factors for hyperoxaluria in calcium oxalate stone formers. Kidney Int 2003; 63: 1037-43.
- Daudon M, Dore JC, Junger P, Lacour B. Changes in stone composition according to age and gender of patients: A multivariate epidemiological approach. Urol Res 2004; 32: 241-7.
- Daudon M. Epidemiology of nephrolithiasis in France. Ann Urol 2005; 39: 209-31.
- Siener R, Schade N, Nicolay C, von Unruh GE, Hesse A. The efficacy of dietary intervention on urinary risk factors for stone formation in recurrent calcium oxalate stone patients. J Urol 2005; 173: 1601-5.
- Djelloul Z, Djelloul A, Bedjaoui A, Kaid-Omar Z, Attar A, Daudon M, Addou A. Urinary stones in Western Algeria: Study of the composition of 1354 urinary stones in relation to their anatomical site and the age and gender of the patients. Prog Urol 2006; 16: 328-35.
- T.V.R.K. Rao, S. Bano, M. Das. Epidemiology of Urolithiasis and chemical composition of urinary stones in Purnia Division of Bihar. Indian Journal of Community Medicine 2006; 31: 04-06.
- Penniston KL, Nakada SY. Effect of dietary changes on urinary oxalate excretion and calcium oxalate supersaturation in patients with hyperoxaluric stone formation. Urology 2009; 73: 484-489.
- Stitchantrakul W, Kochakarn W, Ruangraksa C, Domrongkitchaiporn S. Urinary risk factors for recurrent calcium stone formation in Thai stone formers.JbMed Assoc Thai 2007; 90: 688-98.
- Hess B. Pathophysiology, diagnosis and conservative therapy in calcium kidney calculi. Ther Umsch 2003; 60: 79-87.
- Sonja Lewandowski and Allen L Rodgers. Idiopathic calcium oxalate Urolithiasis: Risk factors
- and conservative treatment. Science Direct-Clinica Chimica Acta 2004; 345: 17-34.
- Heilberg IP, Schor N. Renal stone dieease: Causes, evaluation & medical treatment. Arq Bras Endocrinol Metabol 2006; 50: 823-831.