Research Article - Journal of Molecular Medicine and Therapy (2017) Journal of Molecular Medicine and Therapy (Special Issue 1-2017)
Characterization of glycine release from mouse hippocampal slices.
- *Corresponding Author:
- Simo S. Oja
Tampere University Medical School, Tampere FI 33014 University of Tampere Finland
E-mail: simo.oja@uta.fi
Accepted date: October 10, 2017
Citation: Oja SS, Oja O S, Saransaari P. Characterization of glycine release from mouse hippocampal slices. Mol Med Ther. 2017;1(1):14-18
Abstract
Glycine release from the mouse hippocampus was studied with a superfusion system in which freely floating slices were exposed to a continuous flow of medium. The slices were exposed to K+ stimulation and ischemic conditions in addition to a great number of synaptic effectors and second messengers. The release was markedly increased by K+ stimulation and under ischemic conditions. Many of the synaptic effectors and second messengers did not regulate the release, but nitric oxide was strongly stimulatory. The release was apparently inhibited by adenosine receptors and inhibition of phosphodiesterases.
Keywords
Glycine, Hippocampus, Ischemia, Nitric oxide, Second messengers, Adenosine receptors.
Abbreviations
AMPA: 2-Amino-3-hydroxy-5- methyl-4-isoxazolepropionate; L-AP4: L-(+)-2-amino- 4-phosphonobutyric acid; 2R,4R-APDC: (2R,4R)- 4-aminopyrrolidine-2,4-dicarboxylate; CGS 21680: 4[2-{[6-amino-9-(N-ethyl-b-D-ribofuranuronamidosyl)- 9H-purin-2-yl]ethyl}benzenepropanoate]; RO-2017724: 4-(3-butoxy-4-methoxyphenyl)methyl-2-imidazolidone; CNS: central nervous system; CTC: 4-(3-chloro- 2-pyridin y l ) -N-[4-(1,1-dimethylethyl)phenyl]-1- piperazinecarboxamide; CHA: N6-cyclohexyladenosine; DPCPX :8-cyclopentyl-1,3-dipropylxanthine; CPPG: (RS)- 2-cyclopropyl-4-phosphonophenylglycine); DHPG: (S)-3,5- dihydroxyphenylglycine; R-PIA: R(-)N6-(2-phenylisopropyl) adenosine; HEPES: N-2-hydroxyethylpiperazine-Nˊ-2- ethanesulfonic acid ; HA: hydroxylamine; NMDA: N-methyl- D-aspartate; SNAP: S-nitroso-N-acetylpenicillamine; NOArg: Nω-nitro-L-arginine; ODQ: 1H-(1,2,4)oxadiazolo(4,3-a) quinoxalin-1-one; PMA: phorbol 12-myristate 13-acetate; PDE: phosphodiesterase; L-SOP: O-phospho-L-serine; SNP: sodium nitroprusside; t-ACTD: trans-(1±)-1-aminocyclopentane-trans- 1,3-dicarboxylate.
Introduction
Extracellular glycine has two important functions in the central nervous system (CNS). First, it was initially thought to be confined to the spinal cord and the brain stem, where it acts as a major inhibitory transmitter, acting through strychnine-sensitive receptors [1,2]. Secondly, it has subsequently been shown to play an important excitatory role as a co-agonist of glutamate at strychnine-insensitive recognition sites in excitatory N-methyl- D-aspartate (NMDA) receptors [3-5]. Both of these functions stress the pivotal role of glycine release from the intracellular sites of brain tissue. The concentration of glycine in the CNS is relatively high and only a fraction of it is involved in the synaptic functions [6,2]. NMDA receptors are abundant throughout the CNS. Glycine binding sites in the CNS may not normally be saturated and glycine can thus regulate the activity of NMDA receptors [7]. Glutamate alone cannot activate these ionotropic receptors. These facts were the impetus for our studies on glycine release from brain slices, in which the experimental conditions can be relatively easily modified by exposing the preparations to incubation media of different compositions. In the present experiments the slices were incubated in the presence and absence of oxygen and exposed to a number of effectors known to impact on synaptic functions.
The hippocampus is the brain region particularly sensitive to lack of oxygen and glucose. Most of the excitatory synaptic transmission in the hippocampus is mediated by the ionotropic glutamate receptors of the 2-amino-3-hydroxy-5-methyl-4- isoxazolepropionate (AMPA) and NMDA type. Transient hypoglycemia has been shown to potentiate the evoked NMDA receptor-dependent synaptic responses and even more so those governed by AMPA receptors [8]. Energy deprivation leads to neuronal cell death, caused primarily by excitotoxicity due to excessive glutamate release [9,10]. We sought now to characterize the mechanisms of release of preloaded [3H] glycine in slices prepared from the mouse hippocampus from young adult (3-month-old) and developing (3-day-old) mice in a superfusion system. The possible involvement of second messengers in the release and its regulation by adenosinergic compounds were also addressed together with a number of agents known to affect synaptic functions and transmitter release.
Experimental Procedure
Materials
Young adult (3-month-old) and 7-day-old NMRI mice (Orion, Espoo, Finland) of both sexes were used in the experiments. All efforts were made to minimize both the suffering and the number of animals used. The experiments conformed to the European Community Directive for ethical use of experimental animals (2010/63/EU) and were approved by the National Animal Experiment Board of Finland. [3H]Glycine (specific radioactivity 1.67 PBq/mol) was obtained from Amersham International, Bristol, UK.). N6-Cyclohexyladenosine (CHA), R(-)N6-(2-phenylisopropyl)adenosine (R-PIA), S-nitroso- N-acetylpenicillamine (SNAP), and quinacrine were from Sigma (St.Louis, MO). L-(+)-2-Amino-4-phosphonobutyric acid (L-AP4), 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ), genistein, zaprinast, tamoxifen, phorbol 12-myristate 13-acetate (PMA), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), 4-(3-chloro-2-pyridinyl)-N-[4-(1,1-dimethylethyl) phenyl]-1-piperazinecarboxamide (CTC), 4[2-{[6-amino- 9-(N-ethyl-b-D-ribofuranuronamidosyl)-9H-purin-2-yl] ethyl}benzenepropanoate] (CGS 21680), trans-(1±)-1- aminocyclopentane-trans-1,3-dicarboxylate (t-ACTD), (S)-3,5- dihydroxyphenylglycine (DHPG), (2R,4R)-4-aminopyrrolidine- 2,4-dicarboxylate (2R,4R-APDC), L(+)-2-amino-4- phosphonobutyrate (L-AP4), O-phospho-L-serine (L-SOP), 4-(3-butoxy-4-methoxyphenyl)methyl-2-imidazolidone (RO- 2017724), and (RS)-2-cyclopropyl-4-phosphonophenylglycine) (CPPG) were from Tocris Bioscience (Bristol, UK). Other reagents were from common commercial sources.
Methods
Slices 0.4 mm thick weighing 5-6 mg were manually prepared with a tissue slicer of Stadie-Riggs type from the hippocampi of the mice. The slices were immediately transferred to 5 ml of oxygenated medium and incubated with 0.01 mM [3H]glycine (50 MBq/l) at 37°C for 15 min under agitation. The standard medium contained (in mmol/l) NaCl 127, KCl 5, CaCl2 0.8, MgSO4 1.3, Na2HPO4 1.3, N-2-hydroxyethylpiperazine-Nˊ-2- ethanesulfonic acid (HEPES) 15, NaOH 11 and D-glucose 10 (pH 7.4). The slices were then transferred into 0.25 ml cups and superfused with the above medium (unless otherwise specified) at a rate of 0.25 ml/min for 50 min in a system in which freely floating shaken slices were kept under a continuous flow of oxygen in order to preserve their viability [11]. Most of the drugs tested were added to medium at the beginning of superfusions. Some were added at 30 min when so indicated. The drug concentrations were chosen as being according to our experience effective [12]. The superfusion medium was pooled during the first 20 min and thereafter 2-min fractions (0.5 ml) were directly collected into small scintillation vials with a fraction collector. After superfusion the slices were weighed, homogenized in icecold 5% (w/v) trichloracetic acid solution, and centrifuged, and the clear supernatants were used for scintillation counting. The effluent samples were subjected to the same analyses.
In experiments designed as ‘’ischemia’’ the slices were incubated in glucose-free medium bubbled with N2 gas [13]. The different effectors were added to the medium at the beginning of superfusions. Desaturation curves of labeled glycine from the slices were plotted as a function of time on the basis of the radioactivities remaining in the slices after superfusion and recovered in the collected superfusate fractions [11]. After equilibration of the experiments the efflux rate constants of glycine for the time interval of 32-40 min (k2) were computed as negative slopes for the regression lines of the logarithm of radioactivity remaining in the slices vs. superfusion time. During the elapsed time of 32 min of superfusion the release of neurotransmitters from our slices, including glycine, reaches a stabile rate [12,14-16]. At 30 min a new medium containing 50 mM K+ was applied to replace the preceding medium in superfusion chambers in a number of experiments when depolarization effects were tested. The chemical identity of the radioactivity released from the slices was analyzed in several experiments of different nature by thin layer chromatography. From 85 to 93 percent radioactivity was found to be authentic labeled glycine. The release assessed thus represents glycine not its metabolites.
The significance of the results was assessed by two-way analysis of variance (ANOVA) using an SPSS statistic, version 17.0 computer program. The analyses were made by grouping the results according to the experimental conditions and the nature of the effectors studied. When significant effects were detected, the post hoc Bonferroni test was applied to bring out differences between the sample means. They were considered significant when the calculated p values were less than 0.05 or 0.01.
Results
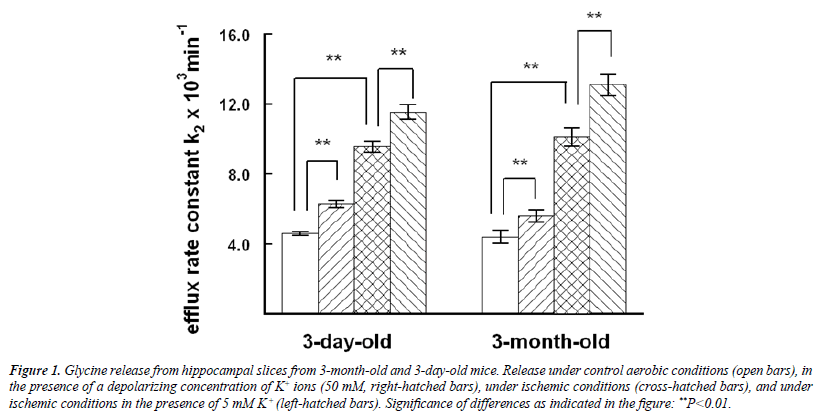
Glycine release from hippocampal slices was of the same order in 3-day-old and 3-month-old mice under standard aerobic conditions (Figure 1). In both age groups stimulation by 50 mM K+ significantly potentiated the release under aerobic and ischemic conditions, ischemia itself being an even stronger effector. The ANOVA showed the marked differences between the experimental groups (F=7.601, d, f.=3, P=0.000). For example, in the Bonferroni test in 3-month-old mice the significance of the difference between the standard control and K+ stimulation was P=0.008, and between the standard control and ischemia P=0.000, and the effect of K+ stimulation in ischemia P=0.001. Only a fraction of labeled glycine was realeased from the slices during the experiments. More than 90 per cent was recovered in the slices in all experiments at the end of superfusion.
Figure 1: Glycine release from hippocampal slices from 3-month-old and 3-day-old mice. Release under control aerobic conditions (open bars), in the presence of a depolarizing concentration of K+ ions (50 mM, right-hatched bars), under ischemic conditions (cross-hatched bars), and under ischemic conditions in the presence of 5 mM K+ (left-hatched bars). Significance of differences as indicated in the figure: **P<0.01.
In the next set of experiments the effects of nitric oxide generators were studied (Table 1). In standard medium statistical analysis revealed significant effects on glycine release (F=15.775, d.f.=3, P=0.000). The Bonferroni test showed that they resulted from 1 mM S-nitroso-N-acetylpenicillamine (SNAP), which markedly enhanced the release (P=0.001). Exposure to 50 mM K+ significantly potentiated the release in the presence of 5 mM hydroxylamine (HA) (P=0.000), 1 mM sodium nitroprusside (SNP) (P=0.002) and 1 mM SNAP (P=0.000). The blocker of NO synthase 1 mM Nω-nitro-L-arginine (NO-Arg) significantly reduced the K+-stimulated release in standard medium (P=0.000) and in the presence of 1 mM SNP (P=0.001) but not with 5 mM HA (P=0.242) or 1 mM SNAP (P=0.427).
| Agent | Standard medium | + 50 mM K+ | + 50 mM K+, NO-Arg |
|---|---|---|---|
| - | 4.39 ± 0.19 (12) | 5.60 ± 0.30 (14) ⃰ ⃰ | 3.32 ± 0.33 (4) ⃰ ⃰ |
| HA | 5.78 ± 0.46 (4) | 13.90 ± 1.26 (4) ⃰ ⃰ | 10.42 ± 1.00 (4) ⃰ |
| SNP | 5.41 ± 0.33 (4) | 8.93 ± 0.06 (3) ⃰ ⃰ | 6.51 ± 0.32 (4) ⃰ ⃰ |
| SNAP | 7.79 ± 0.40 (3) | 11.27 ± 0.82 (4) ⃰ ⃰ | 10.17 ± 1.29 (4) |
Table 1: Effects of NO-generating agents on glycine release from hippocampal slices from 3-month-old mice.
In the third set of experiments the effects of adenosinergic agents and some effectors in the second messenger systems were tested (Table 2). There were significant differences among the effects in standard medium (F=14.571, d.f.=8, P=0.000). Among them, the phosphodiesterase (PDE) inhibitor 0.1 mM zaprinast (P=0.027), selective for the subtypes PDE5, PDE6, PDE9 and PDE11, the histamine N-methyltransferase inhibitor 0.1 mM quinacrine (P=0.000), and the competitive nonselective phosphodiesterase inhibitor and nonselective adenosine receptor antagonist 1 mM 3-isobutyl-1-methylxanthine (IBMX) (P=0.000) inhibited the release. The potent group II/III mGlu receptor antagonist 1 mM (RS)-α-cyclopropyl-4-phosphonophenylglycine (CPPG) was also inhibitory in the presence of 0.1 mM O-phospho-Lserine (L-SOP), the group III metabotropic glutamate receptor agonist. L-SOP itself did not significantly affect the release. The selective adenosine A1 receptor agonists 10 mM N6- cyclohexyladenosine (CHA) (P=0.141) and 0.1 mM R(-)N6-(2- phenylisopropyl)adenosine (R-PIA) (P=0.276), and the potent activator of protein kinase C 0.01 mM phorbol 12-myristate Five mM HA, 1 mM SNP and 1 mM SNAP were added at the beginning of superfusion, 50 mM K+ and 1 mM NO-Arg at 30 min. The efflux rate constants (x 103/min) ± SEM are given for the superfusion period of 30- 40 min. Number of indepedent experiments in parenthesis. Signficance of the effects of K+ and NO-Arg: ⃰P<0.05, ⃰ ⃰P<0.01. Abbreviations: HA=hydroxylamine, SNP=sodium nitroprusside, SNAP=S-nitroso-Nacetylpenicillamine, and NO-Arg=Nω-nitro-L-arginine.
| Agent | Standard | 50 mM K+ | Ischemia |
|---|---|---|---|
| - | 4.39 ± 0.19 (12) | 5.60 ± 0.30 (14) | 10.11 ± 0.53 (8) |
| CHA | 3.44 ± 0.30 (3) | 5.01 ± 0.44 (3) | 10.39 ± 1.18 (3) |
| R-PIA | 3.61 ± 0.25 (4) | 4.95 ± 0.37 (4) | 11.08 ± 0.46 (3) |
| PMA | 3.93 ± 0.35 (8) | 6.79 ± 0.28 (3) | 9.16 ± 1.00 (3) |
| L-SOP | 4.23 ± 0.29 (3) | 4.74 ± 0.37 (4) | 10.26 ± 0.45 (4) |
| L-SOP+CPPG | 2.54 ± 0.08 (4) | 3.08 ± 0.19 (4) | |
| Zaprinast | 3.38 ± 0.43 (4) | ||
| Quinacrine | 2.01 ± 0.38 (3) | ||
| IBMX | 2.56 ± 0.10 (3) |
Table 2: Effects of adenosinergic agents and some compounds involved in the second messengers on glycine release from slices from hippocal slices from 3-month-old mice.
All agents were added at the beginning of superfusion, except for 50 mM K+ and 1 mM CPPG at 30 min. The other concentrations: CHA 10 mM, R-PIA 0.1 mM, PMA 10 nM, L-SOP 0.1 mM, zaprinast 0.1 mM, quinacrine 0.1 mM and IBMX 1 mM. The efflux rate constants (x 103/ min) ± SEM are given for the superfusion period of 30-40 min. Number of indepedent experiments in parenthesis. Signficance of the effects is described in the text. Abbreviations: CHA=N6-cyclohexyladenosine, R-PIA=R(-)N6-(2-phenylisopropyl)adenosine, PMA=4β-phorbol 12-myristate 13-acetate, L-SOP=O-phospho-L-serine, CPPG=(RS)- 2-cyclopropyl-4-phosphonophenylglycine and IBMX=3-isobutyl-1- methylxanthine.
13-acetate (PMA) (P=1.000) did not affect the release. There were also significant differences in the K+-stimulated release (F=10.431, d.f.=5, P=0.000). The Bonferroni analysis revealed that they resulted almost totally from the inhibitory effect of CPPG in the presence of L-SOP (P=0.000). Ischemia markedly enhanced the release in the presence of the other agents tested, but CHA, R-PIA, PMA and L-SOP had no significant effect under ischemic conditions.
In the fourth set of experiments a study was made of the possible effects of 5 mM alloxan, which generates reactive oxygen species and also has other effects, 0.2 mM 4-(3-butoxy- 4-methoxyphenyl)methyl-2-imidazolidone (RO-2017724), an inhibitor of cyclic nucleotide phosphodiesterase, selective for PDE4, 10 mM tamoxifen, a competitive partial agonist of the estrogen receptors, 1 mM 4-(3-chloro-2-pyridinyl)- N-[4-(1,1-dimethylethyl)phenyl]-1-piperazinecarboxamide (CTC), an effective vanilloid receptor 1 antagonist, 1 mM genistein, the tyrosine kinase inhibitor, 0.01 mM {[6-amino- 9-(N-ethyl-b-D-ribofuranuronamidosyl)-9H-purin-2-yl]ethyl} benzenepropanoate] (CGS 21680), the adenosine receptor A2a agonist, 1 mM 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), an antagonist for A1 adenosine receptors, 0.1 mM L-(+)- 2-amino-4-phosphonobutyric acid (L-AP4), the selective group III metabotropic glutamate receptor agonist, 0.1 mM 1-aminocyclopentane-1,3-dicarboxylic acid (t-ACPD), which acts as an mGluR agonist, 0.1 mM (2R,4R)-4- aminopyrrolidine-2,4-dicarboxylate (2R,4R-APDC), a highly selective and relatively potent group II metabotropic glutamate receptor agonist, 0.1 mM (S)-3,5-dihydroxyphenylglycine (DHPG), a potent agonist of group I metabotropic glutamate receptors mGluR1 and mGluR5, 1 mM 8-cyclopentyl-1,3- dipropylxanthine (DPCPX), a potent and selective adenosine A1 receptor antagonist, and 10 mM 1H-(1,2,4)oxadiazolo(4,3-a) quinoxalin-1-one (ODQ), a highly selective inhibitor of soluble guanylyl cyclase. However, none of them had any significant effects on glycine release (data not shown).
Discussion
Depolarization by K+ stimulation was able to potentiate glycine release in the present experiments, as in our earlier studies with brain stem slices [17]. But the effects of neuroactive compounds appeared to be different. The release was now unaffected by many agents tested. Various cell-damaging conditions have been shown to affect taurine release in the hippocampus, of them ischemia being particularly potent [18] The release of neurotransmitter amino acids from neurons is generally mediated by two mechanisms, one requiring the entry of Ca2+ and involving exocytosis, the other also responding to depolarization but being in nature Ca2+ independent [19] The present evoked release of glycine has been shown by us to represent mainly Ca2 dependent release [18]. The Ca2+ independent release may involve several other mechanisms, including energy, Na+ and Cl- dependent transporters operating in a reverse direction or mediated through Na+ and Cl- channels [12]. In addition, glycine may also be released from astroglial cells via activation of non- NMDA-type glutamatergic ionotropic receptors in the glial cell membrane [20].
The molecules released from the cells must first traverse through the extracellular spaces to reach their destination in superfusion medium. During this process some of them are recaptured into cells by uptake in spite of the continuous flow of medium in our system. Glycine released into extracellular spaces is also rapidly reuptake intracellularly by Na+, Cl+-dependent glycine transporters [21,22], two forms of which have been distinguished [23]. The release now assessed thus represents “overflow” of glycine escaping from slices. Under ischemia conditions glycine release was markedly enhanced. The K+ stimulation was even then able to foment it to about the same degree as in oxygenated slices. This is a sign that the integrity of the ischemic slices was at least partly preserved under the present experimental conditions.
The hippocampal formation is functionally a feed-forward synaptic chain, in which all three synaptic links in the pathway are excitatory and use glutamate as transmitter [24]. Presynaptic ionotropic glutamate receptors have been shown to regulate neurotransmitter release in the adult hippocampus [25-27]. Of the ionotropic receptors, NMDA receptor activation has been assumed to play a central role in hypoglycemia-induced glutamate release [28]. In slices from the mouse brain stem NMDA application has likewise evoked glycine release [12]. Activation of NMDA receptors allows Ca2+ to enter the cells. NO synthase is a Ca2+ dependent enzyme [29], being activated in the presence of Ca2+ and then producing NO. NO stimulates soluble guanylate cyclase and in this manner foments the production of 3´,5´-cyclic guanosine monophosphate [30]. In keeping with these events, in the present study on the NO donors the enhancement of glycine release induced by SNP was attenuated by the simultaneous presence of NO-Arg, the blocker of endogenous NO synthase. However, the enhancement evoked by HA was not significantly affected in the presence of NO-Arg. HA can be regarded as an intracellular generator of NO, since it penetrates easily into cells and is broken down by a catalasedependent reaction [31]. On the other hand, SNP and SNAP generate NO extracellularly by spontaneous decomposition, being thus more directly associated with the regulation of glycine release.
A number of second messengers and receptors do not seem to be involved with glycine release in the hippocampus. This situation is quite different from that in the mouse brain stem [12]. In the brain stem genistein has inhibited and DHPG and L-SOP enhanced the release. In both brain areas zaprinast, quinacrine and IBMX inhibit glycine release, indicating the involvement of adenosine receptors. The presynaptic adenosine receptors, particularly of the A1 class, are also known generally to regulate the release of other neurotransmitters [32]. The inhibitory effects of zaprinast and IBMX could also result from the inhibition of phosphodiesterases.
Conclusions
Glycine release from the mouse hippocampus is markedly increased by K+ stimulation and under ischemic conditions. The release is apparently inhibited by adenosine receptors and inhibition of phosphodiesterases, but nitric oxide is strongly stimulatory. However, a great number of synaptic effectors and second messengers do not regulate the release. Since the total concentration of glycine is relatively high this finding may indicate that there exist no very effective and selective regulatory mechanisms of glycine release in the hippocampus.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests
Ethical approval
This article does not contain any studies with human participants. The experiments conformed to the European Community Directive for ethical use of experimental animals (2010/63/EU) and were approved by the National Animal Experiment Board of Finland. All efforts were made to reduce the number and suffering of the experimental animals.
Informed consent
The animals are not able to give their informed consent.
References
- Kirsch J. Glycinergic transmission. Cell Tissue Res. 2006;326(2):535-40.
- de Koning TJ, Fuchs SA, Klomp LWJ. Serine, glycine and threonine. In: Handbook of Neurochemistry and Molecular Neurobiology. 3rd edn. New York, Springer. 2007.
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325(6104):529-31.
- Bashir ZI, Tam B, Collingridge GL. Activation of the glycine site in the NMDA receptor is necessary for the induction of LTP. Neurosci Lett. 1990;108(3):261-6.
- Kemp JA, Foster AC, Leeson PD. The glycine site of the NMDA receptor-five years on. Trends Pharmacol Sci. 1993;14(1):20-5.
- Oja SS, Kontro P, Lähdesmäki P. Amino acids as inhibitory neurotransmitters. Prog Pharmacol. 1977;1(3):1-119.
- Bowery NG, Smart TG. GABA and glycine as neurotransmitters: a brief history. Br J Pharmacol. 2006;147(Suppl 1):S109-19.
- Quintana P, Alberi S, Hakkoum D, Muller D. Glutamate receptor changes associated with transient anoxia/hypoglycaemia in hippocampal slice cultures. Eur J Neurosci. 2006;23(4):975-83.
- Szatkowski M, Attwell D. Triggering and execution of neuronal death in brain ischemia: two phases of glutamate release by different mechanisms. Trends Neurosci. 1994;17(9):359-65.
- Lee JM, Zipfel GJ, Choi QW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399(6738):A7-14.
- Kontro P, Oja SS. Taurine and GABA release from mouse cerebral cortex slices: potassium stimulation releases more taurine than GABA from developing brain. Devl. Brain Res.1987;465(1-2):277-91.
- Taylor CP, Burke SP. Hippocampal slices: glutamate overflow and cellular damage from ischemia are reduced by sodium-channel blockade. J Neurosci Methods 1995;59(1):121-8.
- Saransaari P, Oja SS. Release of edogenus glutamate, aspartate, GABA and taurine from hippocampal slices from adult and developing mice under cell-damaging conditions. Neurochem Res. 1998;23(4):563-70.
- Saransaari P, Oja SS. Taurine release modified by nitric oxide-generating compounds in the developing and adult mouse hippocampus. Neuroscience. 1999;89(4):1103-11.
- Saransaari P, Oja SS. Mechanisms of glycine release in mouse brain stem slices. Neurochem Res. 2009;34(2):286-94.
- Saransaari P, Oja SS. Adenosine receptor agonists affect taurine relese from mouse brain stem slices in ischemia. Amino Acids. 2010;38(5):1387-93.
- Saransaari P, Oja SS. Glycine release from hippocampal slices in developing and ageing mice: modulation by glutamatergic receptors. Mech Ageing Dev. 1994;76(2-3):113-24.
- Saransaari P, Oja SS. Characteristics of hippocampal glycine release under cell-damaging conditions in developing and adult mice. Neurochem Res. 2001;26(7):845-52.
- Saransaari P, Oja SS. Release of GABA and taurine from brain slices. Prog Neurobiol. 1992;38(5):455-82.
- Snyder SH, Kim PM. d-Amino acids as putative neurotransmitters; focus on d-serine. Neurochem Res. 2000;25(5):553-60.
- Aragon C, Lopez CB. Structure function and regulation of glycine transporters. Eur Pharmacol. 2003;479(1-3):249-62.
- Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporter isoforms: essential regulators of neurotransmission. Trends Biochem Sci. 2005;30(6):325-33.
- Zafra F, Aragon C, Gimenez C. Molecular biology of glycinergic neurotransmission. Mol Neurobiol. 1997;14(3):117-42.
- Frotscher M. Neuronal elements in the hippocampus and their synaptic connections. In: Neurotransmission in the hippocampus. Berlin, Springer-Verlag, 1988; pp: 20-39.
- Pittaluga A, Raiteri M. N-Methyl-D-aspartic acid (NMDA) and non-NMDA receptors regulating hippocampal norepinephrine release. I. Location on axon terminals and pharmacological characterization. J Pharmacol Exp Ther. 1992;260(1):232-7.
- Janáky R, Saransaari P, Oja SS. Release of GABA from rat hippocampal slices: involvement of quisqualate:N-methyl-D-aspartate-gated ionophores and extracellular magnesium. Neuroscience. 1993;53(3):779-85.
- Smirnova T, Stinnakre J, Mallet J. Characterization of a presynaptic glutamate receptor. Science.1993;262(5132):430-3.
- Ichord RN, Johnston MV, Traystman RJ. MK801 decreases glutamate release and oxidative metabolism during hypoglycemic coma in piglets. Brain Res Dev Brain Res. 2001;128(2):139-48.
- Bredt SD, Ferris CD, Snyder SG. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase, identification of flavin and calmodulin binding sites. J Biol Chem. 1992;267(16):10976-81.
- Schuman ER, Madison DV. Nitric oxide and synaptic function. Annu Rev Neurosci. 1994;17:153-83.
- DeMaster EG, Raij L, Archer SL, Weir EK. Hydroxylamine is a vasorelaxant and a possible intermediate in the oxidative conversion of L-arginine to nitric oxide. Biochem Biophys Res Commun. 1989;163(1):527-33.
- Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38(2):107-25.