Research Article - Biomedical Research (2017) Volume 28, Issue 18
Changes of lower-limb trabecular bone density after sciatic nerve transection in immature rats
Hyun-Yoon Ko1, Jae Hyeok Chang2*, Yong Beom Shin2, Myung Jun Shin2, Yong-Il Shin1, Chang Hyung Lee1, Soo Yeon Kim1 and Ji Won Hong2
1Department of Rehabilitation Medicine, Pusan National University Yangsan Hospital, Yangsan, Gyeong-nam, Republic of Korea
2Department of Rehabilitation Medicine, Pusan National University Hospital, Busan, Republic of Korea
- *Corresponding Author:
- Jae Hyeok Chang
Department of Rehabilitation Medicine
Pusan National University Hospital
Republic of Korea
Accepted on August 28, 2017
Abstract
Purpose: The changes in the bone density of immature Sprague-Dawley (SD) rats whose sciatic nerve was cut were investigated to understand the patterns of bone density deterioration and to establish the rationale for preventive managements.
Materials and Methods: Eighteen six-day-old SD rats were randomly divided into the right sciatic neurectomy and sham-operation group. The trabecular bone densities at the 3rd lumbar spine (L3), L4, and L5, bilateral proximal and distal femoral bones, and proximal and distal tibia at weeks 4, 8, and 12 were measured through a CT scan.
Results: In both the surgery and control groups, statistically significant changes in body weight were observed at weeks 4, 8, and 12. The trabecular bone density of the surgery group at week 4 significantly decreased in the right proximal and distal tibia; and at week 8, a significant decrease was observed in the right tibia and the distal femur. At week 12, no statistically significant difference in the trabecular bone density was found between the groups.
Conclusions: In immature SD rats, the reduction of trabecular BMD caused by sciatic neurectomy began in the 4th week after surgery, and proceeded from the distal part to the proximal part. The earlier reduction in the trabecular BMD of the tibia is explained by the thickness of the femur and the quadriceps femoris muscle, which are not affected by a sciatic nerve lesion. Further studies on cortical bone changes and bone metabolism markers may be needed to provide data on bone density deterioration caused by the neural damage during the growing period.
Keywords
Bone density, Sciatic nerve, Sprague-Dawley rats, Osteoporosis, Immobilization.
Introduction
Bone mass increases during childhood and adolescence, peaks in young adulthood (peak bone mass), and thereafter, starts decreasing [1]. Bone growth lasts throughout life, but is mostly completed between ages 10 and 18 [2]. Patients whose bone mass does not reach its peak during childhood because of nerve-injury-induced osteoporosis are at permanent risk of fractures [3]. However, there are insufficient studies on the bone growth and density of children with immobilization-induced osteoporosis such as brachial plexus injury, traumatic peripheral nerve injury, or cerebral palsy. Immobilization due to nerve injury affects the bone growth and the bone strength of humans and animals. Furthermore, there are no treatment guidelines for immobilized osteoporosis in bone growing ages.
Sciatic neurectomy is an effective modeling method of disuse osteoporosis. Unloading through hind limb immobilization resulted from neurectomy can also induce bone loss [4]. The bone mass reduction is caused by an initial decrease in the osteoclastic bone resorption [5]. This suggests that disuse-induced osteoporosis would lead to more severe loss of the main trabecular structures. Upon repeated loadings and unloadings, the distribution of loads in immobilized bones would be inhomogeneous because of loss of the adaptation to the loading direction [6]. Neurectomy decreases bone formation in the tibiae of mice four weeks after surgery [7]. In the immobilization model, the bulk of the bone loss occurs in the hind limbs, because they are the sites of the greatest mechanical loading [8].
Few studies have been conducted on the decrease in the bone density due to lower-limb immobilization in growing infants, and no guidelines have been established. In animal models with bone density deterioration, the changes in their bone density are easily observed in their hind limbs, which bear most of their weight, after they undergo sciatic neurectomy to induce lower-limb immobilization. In this study, sciatic neurectomy was performed on growing Spargue-Dawley (SD) rats to investigate the changes in their trabecular bone density.
Based on a previous study, I hypothesized that bone loss due to disuse would progress faster in the distal tibia than in the proximal tibia [9]. This study was conducted to investigate the effects on the long bone of rats in the growing phase with sciatic neurectomy-induced osteoporosis. To confirm the growing phase, the changes in the trabecular bone mass in sixday- old SD rats were measured.
Methods
Materials
The protocols for the animal experimentation were previously approved by the Animal Research Committee of Pusan National University, and all subsequent animal experiments adhered to the ‘Guidelines for Animal Experimentation’ of the University. Eighteen six-day-old growing SD rats (Dooyeol Biotech, Korea) were randomly divided into the right sciatic neurectomy group (neurectomized group) and the sham operation group (sham group). After the SD rats were anesthetized with isoflurane, a dorsolateral incision was made on their right hips, through which the right sciatic nerve was exposed, and a 0.5 cm section of the sciatic nerve was performed. For the sham group, the entire right sciatic nerve was exposed but not transected in the sham group. No antibiotics were administrated. All the SD rats were housed under diurnal lighting conditions and fed with food and tap water ad libitum. Their post-operative care included housing them in same gender pairs.
Experiment design
After the surgery, the trabecular Bone Mineral Density (BMD) of one SD rat each from the sciatic neurectomy group and the sham group were examined. At four weeks, eight weeks, and 12 w, their BMD was examined via micro-Computed Tomography (micro-CT; Inveon MM Gantry-STD CT camera, Siemens Medical Solutions, USA). A micro-computed tomograph (micro-CT; 70 kV, 114 μA, 1,000 projections per 180°, 261 ms integration time) with a 15 μm isotropic resolution was made in the proximal and distal femora and the tibiae and lumbar vertebrae. The prospective use of the micro- CT, a non-invasive method that can quantify well the trabecular bone structure, [10] for in vivo longitudinal measurements was examined. The micro-CT data were analysed using IAW version 1.5.0.28, IRW version 2.2, and the reconstruction software COBRA version 6.6.39.
The proximal and distal femora, the proximal and distal tibiae, and the 3rd, 4th, and 5th lumbar vertebrae were measured because there was no consensus in previous studies and some differences may exist between the proximal and distal long bones. The sequential Basso-Beattie-Bresnahan locomotor rating scale (BBB) was also used to evaluate the functional status of the right-sciatic-neurectomized SD rat.
Densitometric analysis
Before each series of measurements, a tissue calibration scan was performed with the calibration phantom with the coefficient of variation of 0.0047. The animal data were gathered immediately after the data on the CT density calibration phantom were acquired (Figure 1).
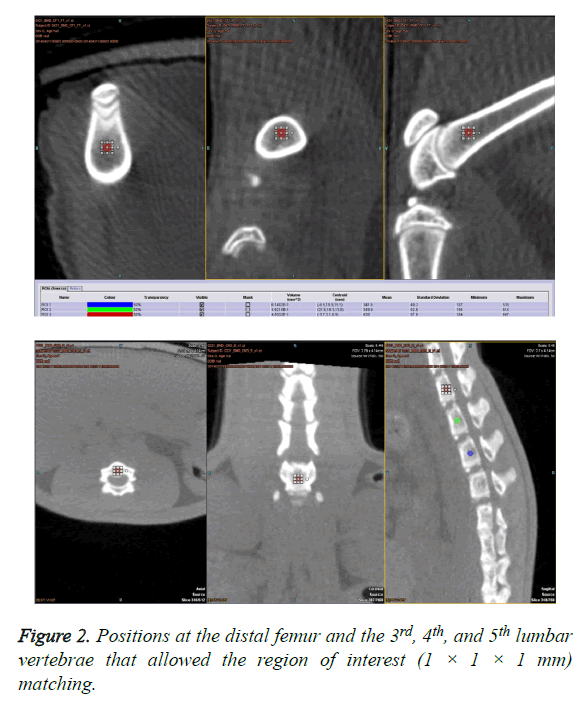
The positions were set as much as possible at the proximal femur, distal femur, proximal tibia, distal tibia, and third lumbar spine (L3), L4, and L5 to allow for the best and the same Region of Interest (ROI) matching (1 × 1 × 1 mm) (Figure 2).
Statistical analysis
Multiple comparisons of the group data were performed through a two-factor analysis with the intervention (sham operation vs. neurectomy) and the time after the surgery (four weeks, eight weeks, and 12 w). All the statistical analyses were performed using SPSS 18 software (SPSS Inc., Chicago, IL, USA). A Mann-Whitney U test was used to evaluate the difference between the experimental group and the control. A significance level of p<0.05 was set.
A Friedman test was conducted to evaluate whether or not there were differences in the time-sequentially measured trabecular BMD. A posthoc analysis was also performed to analyse the difference according to the time. In addition, the Wilcoxon signed-rank test was conducted to analyse differences across the three times. Bonferroni correction was used to correct the type I errors, and the statistical significance level was set at p=0.017.
Results
General observations
Weight changes were observed during the 12 w experimental period in the sham-operated rats. There was no difference between the body weight of the unilateral sciatic neurectomized rats and that of the sham-operated rats throughout the study. The BBB scores of the right sciatic neurectomized rats were all 3 points.
Changes in the trabecular BMD
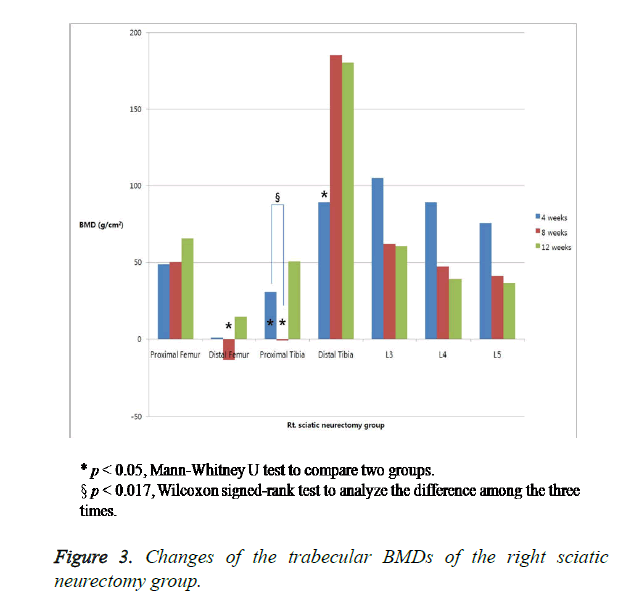
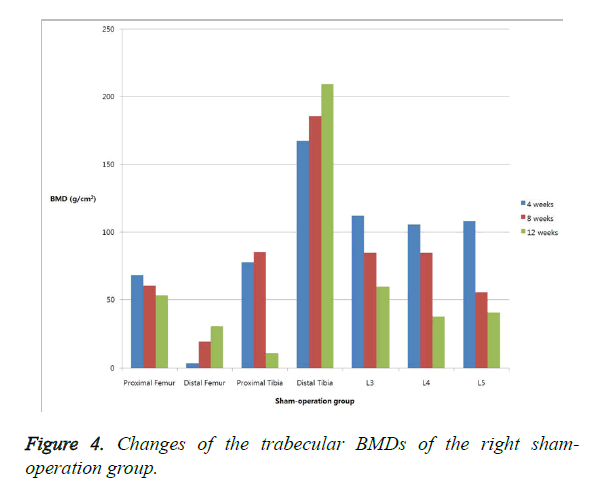
The changes in the trabecular BMD are summarized in Table 1. In the right proximal and distal tibiae, the BMD was significantly lower in the neurectomized rats than in the sham-operated rats four weeks after the surgery. Eight weeks after the surgery, the BMD was significantly lower in the neurectomized rats in the right proximal tibiae and the distal femora. Twelve weeks after the surgery, the BMD showed no significant changes in the two groups. No significant difference in the BMD of the lumbar vertebrae was observed between the neurectomized rats and the sham-operated rats at any time (Figures 3 and 4).
| Weeks | Right sciatic neurectomy group | Sham-operation group | |||||
|---|---|---|---|---|---|---|---|
| 4 | 8 | 12 | 4 | 8 | 12 | ||
| Weight (g) (SD) | 109.55 (29.37) | 274.01§ (70.64) | 351.50§ (109.68) | 112.52 (19.66) | 291.11§ (56.79) | 380.36§ (94.16) | |
| Proximal femur | Rt. (SD) | 48.62 (16.50) | 50.05 (28.36) | 65.7 (30.99) | 68.07 (21.02) | 60.33 (23.23) | 52.94 (37.66) |
| Lt. (SD) | 58.05 (17.97) | 43.77 (29.51) | 60.64§ (30.83) | 61.66 (14.76) | 54.19 (34.21) | 45.31 (45.30) | |
| Distal femur | Rt. (SD) | 0.77 (10.62) | -333.19 | 14.45 (51.53) | 3.73 (13.69) | 19.28 (44.64) | 30.22 (79.70) |
| Lt. (SD) | 6.46 (15.77) | 6.65 (35.72) | 45.22 (82.42) | 0.54 (10.16) | 2.77 (24.70) | 11.17 (53.07) | |
| Proximal tibia | Rt. (SD) | 30.67* (27.39) | -1.03*§ (30.36) | 50.52 (96.21) | 77.48 (41.53) | 84.99 (44.17) | 10.96 (77.40) |
| Lt. (SD) | 61.19 (22.94) | 23.58 (36.34) | 32.6 (99.81) | 55.1 (39.63) | 63.9 (26.50) | 28.98 (85.74) | |
| Distal tibia | Rt. (SD) | 89.16* (32.52) | 185.10§ (40.64) | 180.37 (42.00) | 167.36 (61.42) | 185.70 (44.31) | 209.28 (110.08) |
| Lt. (SD) | 127.35 (35.78) | 174.67 (60.97) | 228.29 (130.80) | 176.03 (67.23) | 169.73 (46.59) | 261.19 (113.08) | |
| L3 | 104.98 (36.73) | 61.97 (41.80) | 60.47 (67.93) | 111.8 (53.95) | 84.32 (41.17) | 59.47 (72.03) | |
| L4 | 89.24 (25.44) | 47.05 (45.52) | 39.02 (63.01) | 105.33 (44.37) | 84.43 (31.93) | 37.23 (49.35) | |
| L5 | 75.63 (26.83) | 41.28 (39.43) | 36.22 (61.86) | 101.81 (63.47) | 55.25 (32.53) | 40.46 (59.26) | |
| BMD: Bone Mineral Density; SD: Standard Deviation. *p<0.05, Mann-Whitney U test to compare two groups. §p<0.017, Wilcoxon signed-rank test to analyse the difference among the three times. | |||||||
Table 1. Changes in weight and the trabecular BMDs of two groups.
Sham group analysis
The body weights of the female and male rats at w 8 and 12 significantly differed. At w 8, the trabecular BMD of the left proximal femur, distal femur, and both-sides distal tibia showed statistically significant differences. At w 12, differences between the groups were observed in all the areas, except in the right distal tibia and L5 (Table 2).
| Weeks | Right sciatic neurectomy group | Sham-operation group | |||||
|---|---|---|---|---|---|---|---|
| 4 | 8 | 12 | 4 | 8 | 12 | ||
| Weight (g) (SD) | 111.8 (20.80) | 333.14* (18.30) | 456.18* (14.21) | 113.43 (21.26) | 238.58* (39.11) | 258.58* (42.60) | |
| Proximal femur | Rt. (SD) | 54.98 (15.99) | 50.63 (18.30) | 27.33* (4.36) | 84.44 (13.95) | 72.45 (25.30) | 84.96* (36.01) |
| Lt. (SD) | 61.37 (19.26) | 36.85* (34.83) | 17.09* (25.59) | 62.03 (9.27) | 75.87* (19.38) | 80.58* (40.16) | |
| Distal femur | Rt. (SD) | -2.06 (15.55) | -4.16 (15.57) | -20.51* (43.76) | 10.97 (7.21) | 48.58 (54.14) | 93.62* (68.81) |
| Lt. (SD) | -3.6 (11.07) | -12.99* (12.82) | -30.69* (21.05) | 5.72 (6.90) | 22.47* (21.83) | 63.48* (18.74) | |
| Proximal tibia | Rt. (SD) | 64.06 (46.17) | 51.12* (22.55) | -47.65* (40.59) | 94.24 (32.91) | 127.33*(14.91) | 84.23* (29.96) |
| Lt. (SD) | 41.36 (24.94) | 42.89* (7.97) | -36.78* (27.48) | 72.27 (51.48) | 90.18* (11.45) | 11.18* (48.78) | |
| Distal tibia | Rt. (SD) | 157.94 (61.18) | 173.66 (43.78) | 209.32 (110.53) | 179.13 (68.83) | 200.74 (38.98) | 209.24 (126.57) |
| Lt. (SD) | 157.82 (65.21) | 175.4 (57.37) | 312.34* (120.51) | 198.8 (71.68) | 162.65 (35.78) | 197.25* (70.17) | |
| L3 | 110.14 (49.36) | 61.4 (36.63) | 9.76* (45.80) | 113.87 (67.10) | 112.96 (27.57) | 121.61* (42.08) | |
| L4 | 100.49 (39.56) | 77.46 (43.18) | -1.82* (20.62) | 11.39 (55.45) | 93.16 (6.99) | 86.05 (14.44) | |
| L5 | 99.35 (67.52) | 52.69 (28.41) | 14.38 (63.03) | 104.9 (68.13) | 58.45 (41.47) | 73.06 (39.93) | |
| BMD: Bone Mineral Density; SD: Standard Deviation. *p<0.05, Mann-Whitney U test to compare two groups. *p<0.05, Mann-Whitney U test to compare two groups. | |||||||
Table 2. Changes in weight and the trabecular BMDs of Sharm-operation group.
Discussion
BMD loss after peripheral nerve injury in childhood and adolescence is not well-documented in clinical studies. Moreover, not only osteoporosis but also growth retardation may occur after immobilization due to nerve injury. Patients whose bone mass does not reach its peak during childhood because of nerve-injury-induced osteoporosis are at permanent risk of fractures. However, there have been no established guidelines for evaluating and treating immobilized osteoporosis in children. In present study, the bone quality and density in growing animal models is to be determined.
In a clinical situation, it was demonstrated that the BMD of the femur and tibia significantly decreased in patients with spinal cord injury [11-13]. The previous study showed that the decrease was more significant in the trabecular BMD than in the cortical bone in six-weeks-old growing rats [14]. The study showed that 28-40% bone mass was lost in the epiphyses of the femur and tibia, but only 24-29% in the diaphyses. The faster loss in the metaphyses could have been due to the larger surface area of the trabecular bone than of the cortical bone [14]. BMDs at the metaphyses in both the proximal and distal femora and the tibiae were sequentially analysed in this study.
At 10 months of age, the bone growth rate in the proximal tibial epiphyses is less than 3 μm/d. Among the test rats, their growth stopped at age 15 months. If experimentation starts at around 10 months of age, which is the peak bone mass age for rats, the longitudinal bone growth adjacent to the epiphyseal plate of the tibia will be less than 1 mm [15,16]. Although rats become sexually mature at age 2.5 months, their skeleton is considered mature after age 10 months [16]. The earliest statistically significant bone loss in the proximal and distal tibial metaphyses for this model is seen 14-30 d after the start of immobilization [17].
The reason why six-day-old rats were used is because the rats can be separated from their mothers four days after birth and the stress is low. I also judged that the minimum period of time to undergo surgery is six days.
The age of a four-week-old rat is equivalent to that of an eightyear- old human; of an eight-week-old rat, to a 14 y old human; of a 12 w old rat, to a 16 y old human; of a six-month-old rat, to a 20 y old human; and of a 20-month-old rat, to a 50 y old human [17-19]. The peak bone mass in a rat is reached at 10 months [17-20]. This study was carried out for only 12 w because such age in rats is comparable to that of a 16 y old human. This meets our goal of measuring changes in the BMD during the growing phase.
In previous studies on the bone mass and density in children, no significant difference was observed between genders from their birth to when they were six years of age [19]. After age 6, the bone mass of the male infants grew at a constant ratio and then abruptly increased after adolescence, whereas that of the female infants abruptly increased before adolescence. During adolescence, the bone size of males becomes larger than that of females due to mainly the males’ thickened cortical bone. Accordingly, males’ BMD (gm/cm2) is greater than females’ BMD, but the bone density (gm/cm3) per unit volume measured using quantitative computed tomography has been known not to show a significant difference.
In this study, the trabecular bone density was separately measured per unit volume, unlike in previous clinical studies. In further studies to analyse the growing rat BMD, an intervention may be needed at w 4, when the rats may already be separated by gender, to continue the analysis to upto w 12. The trabecular BMD was measured in various areas because no study had targeted five-week-old immature rats, and there has been no consensus on the level of decrease in the bone density.
The present study showed that the tibiae lost bone mass at a slightly greater rate than the femora. In the metaphyses, the relative loss in the bone mass was greater in the distal and proximal tibia. A previous study showed that the femora lost bone mass at a greater rate than the tibiae. The earlier reduction in the bone mass of the tibia is explained by the thickness of the femur and the quadriceps femoris muscle, which are not affected by a sciatic nerve lesion. In particular, the trabecular BMD decrease in the distal tibia was greater than that in the proximal tibia between w 4 and 8. The distal femoral changes after the tibia BMD decrease corresponded to the authors’ hypothesis that the bone density decrease started from the distal to the proximal tibia. The bone density at w 12 tended to decrease, but no statistically significant difference was observed. The BMD difference was significant in the early stage of the neural damage. Afterwards, the difference became insignificant because the bone resorption increased due to the neural effects, then the bone turnover rate decreased due to the mechanical effects.
The trabecular BMDs in the lumbar vertebrae also decreased in both groups because the trabecular bone portion decreased and the cortical bone portion increased. Although the trabecular BMD in the L3, L4, and L5 vertebrae decreased in both the sham group and the neurectomized group, the differences were statistically not significant. The decrease can be attributed to the thickening of the cortical bone as the rats grew.
The right and left bone density of the proximal tibia tended to decrease, albeit insignificantly, between w 4 and 8. The trabecular BMD changes in the female rats were greater than those in the male rats. Differences in the distal and proximal tibia were observed at w 4 and 8; and at w 12, a significant difference was observed as well in the distal femur. In the meantime, no statistically significant difference in the BMD was observed in the male rats during the measurement period, presumably due to the active functioning of the sex hormones during the growing phase.
BMD is the current gold standard measurement of osteoporosis and the widely available paramount predictor of fracture. It is seen as capable of explaining 50-80% variances in bone strength [14]. However, it does not indicate structural changes and trabecular micro-architecture because there is a substantial overlap of those who have and have not experienced skeletal fracture. Thus, examining the micro-architecture is important in assessing the changes in the bone quality after neurectomy. In this study, because three-dimensional volumetric BMDs were analysed, the micro-architecture is suspected of being fed into the volumetric BMD. The micro-architecture might be reflected in this study because the 3-dimensional volumetric BMD instead of the 2-dimensional BMD was evaluated. The limitation of the study is that it did not measure the cortical bone mass, because the cortex of six-day-old rats is too thin. Because of the femur location, its cross-sectional area is difficult to calculate, and it cannot be fully extended parallel to the plate of the CT table.
In this study, the bone density was measured without gender separation at w 4 and 8 because the gender could be differentiated 2.5 months after birth. At w 10 and afterwards, it was confirmed that there were nine male and nine female rats. The sample size was too small for the results to be analysed based on gender separation.
In summary, there was a significant decrease in the BMD in the hind limbs after immobilization, and the neurectomized rats also had a deteriorated trabecular BMD in the distal femora and the proximal and distal tibiae. The change in the BMD in the lumbar vertebrae was relatively minor compared with that in the tibiae. Therefore, the changes in the BMD of the immature rats went from distal to proximal. These findings will allow better interpretation of the pathogenesis of osteoporosis after immobilization, and will help with the management and prevention of osteoporosis in immature rats. This is the first study on bone density deterioration due to lower-limb immobilization after the appearance of trabecular bone density changes and peripheral nerve damages in six-day-old immature SD rats. Further studies on cortical bone changes and bone metabolism markers may be needed to provide data on bone density deterioration caused by neural damage during the growing period.
Conclusion
The reduction of trabecular BMD caused by sciatic neurectomy began in the 4th w after surgery, and proceeded from the distal part to the proximal part. The earlier reduction in the trabecular BMD of the tibia is explained by the thickness of the femur and the quadriceps femoris muscle, which are not affected by a sciatic nerve lesion. The observation of BMD of rat up to 12 w is equivalent to seeing changes in BMD from childhood to adolescence of human.
We hope that this basic research will be the background for promoting the study of changes in bone mineral density in growing children with peripheral nerve injury.
Funding
This study was supported by Biomedical Research Institute Grant (2013-6), Pusan National University Hospital.
References
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001; 285: 785-795.
- Fujita Y, Watanabe K, Uchikanbori S, Maki K. Effects of risedronate on cortical and trabecular bone of the mandible in glucocorticoid-treated growing rats. Am J Orthod Dentofacial Orthop 2011; 139: 267-277.
- Rabinovich CE. Osteoporosis: a pediatric perspective. Arthritis Rheum 2004; 50: 1023-1025.
- Turner RT, Bell NH. The effects of immobilization on bone histomorphometry in rats. J Bone Miner Res 1986; 1: 399-407.
- Shen V, Liang XG, Birchman R, Wu DD, Healy D, Lindsay R, Dempster DW. Short-term immobilization-induced cancellous bone loss is limited to regions undergoing high turnover and/or modeling in mature rats. Bone 1997; 21: 71-78.
- Van Rietbergen B, Huiskes R, Eckstein F, Rüegsegger P. Trabecular bone tissue strains in the healthy and osteoporotic human femur. J Bone Miner Res 2003; 18: 1781-1788.
- Kodama Y, Dimai HP, Wergedal J, Sheng M, Malpe R, Kutilek S, Beamer W, Donahue LR, Rosen C, Baylink DJ, Farley J. Cortical tibial bone volume in two strains of mice: effects of sciatic neurectomy and genetic regulation of bone response to mechanical loading. Bone 1999; 25: 183-190.
- Frost HM. The regional acceleratory phenomenon: a review. Henry Ford Hosp Med J 1983; 31: 3-9.
- Brouwers JE, Lambers FM, van Rietbergen B, Ito K, Huiskes R. Comparison of bone loss induced by ovariectomy and neurectomy in rats analyzed by in vivo micro-CT. J Orthop Res 2009; 27: 1521-1527.
- Li XJ, Jee WS, Chow SY, Woodbury DM. Adaptation of cancellous bone to aging and immobilization in the rat: a single photon absorptiometry and histomorphometry study. Anat Rec 1990; 227: 12-24.
- Uchii M, Takashima M, Sugiyama T, Kosaka N. Effect of KW-8232, a novel anti-osteoporotic agent, on bone loss in sciatic neurectomized rats. Jpn J Pharmacol 1998; 78: 241-243.
- Muller R, Ruegsegger P. Micro-tomographic imaging for the nondestructive evaluation of trabecular bone architecture. Stud Health Technol Inform 1997; 40: 61-79.
- Warden SJ, Bennell KL, Matthews B, Brown DJ, McMeeken JM, Wark JD. Quantitative ultrasound assessment of acute bone loss following spinal cord injury: a longitudinal pilot study. Osteoporosis Int 2002; 13: 586-592.
- Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C. Osteoporosis after spinal cord injury. J Orthop Res 1992; 10: 371-378.
- Zehnder Y, Luthi M, Michel D, Knecht H, Perrelet R, Neto I, Kraenzlin M, Zäch G, Lippuner K. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporosis Int 2004; 15: 180-189.
- Jiang SD, Jiang LS, Dai LY. Changes in bone mass, bone structure, bone biomechanical properties, and bone metabolism after spinal cord injury: a 6-month longitudinal study in growing rats. Calcif Tissue Int 2007; 80: 167-175.
- Erben RG. Trabecular and endocortical bone surfaces in the rat: modeling or remodeling? Anat Rec 1996; 246: 39-46.
- Jee WS, Yao W. Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact 2001; 1: 193-207.
- Glastre C, Braillon P, David L, Cochat P, Meunier PJ, Delmas PD. Measurement of bone mineral content of the lumbar spine by dual energy x-ray absorptiometry in normal children: correlations with growth parameters. J Clin Endocrinol Metab 1990; 70: 1330-1333.
- Bonjour JP, Theintz G, Buchs B, Slosman D, Rizzoli R. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab 1991; 73: 555-563.