- Biomedical Research (2014) Volume 25, Issue 4
Changes of characteristics of neural stem cells in a neonatal rat model with hypoxic-ischemic encephalopathy.
Xiaojuan Yin1,#, Lei Dong2,#, Yu Wang1,#, Wei Wei1, Yannan Chai1 and Zhichun Feng1,*1Affiliated Bayi Children’s Hospital, Beijing Military Region General Hospital, No 5, Nan Mencang, Dongcheng District, Beijing 100700, P.R.China
2Clinical Laboratory Center, PLA Air Force General Hospital, 30 Fucheng Road, Haidian District, Beijing 100142, P.R.China
#These authors contributed equally
- *Corresponding Author:
- Zhichun Feng
Affiliated Bayi Children’s Hospital
Beijing Military Region General Hospital
No 5, Nan Mencang, Dongcheng District
Beijing 100700, P.R.China
Accepted April 02 2014
Abstract
This study aimed to investigate changes of characteristics of neural stem cells (NSCs) in a neonatal rat with hypoxic-ischemic brain damage (HIBD) and provide effective method and time window for the treatment of hypoxic-ischemic encephalopathy (HIE). A total of 231 7-day-old neonatal SD rats were randomly divided into control, hypoxic and HIBD groups (n=77 per group). Each group was randomly divided into 7 subgroups according to the time of sacrifice. Immunohistochemistry analysis was performed to confirm the expression of Nestin and HE staining was performed to detect the pathological change of HIBD. NSCs were mainly distributed in hippocampus, ependyma of lateral ventricle, subventricular zone (SVZ), striatum and cortex in three groups, while NSCs in HIBD group presented a regional distribution. As compared with control and HIBD groups, the number of NSCs was higher in hypoxia group at the same time point from day 3 (P<0.05). There was no difference in the number of NSCs in control and HIBD group at the same time of sacrifice (P>0.05) except day 3. The similar tendency of the number of NSCs was observed in 3 groups. There was no difference in NSCs number at the same time of sacrifice among 3 groups within 3 days expect hypoxia group. After 3 days, there was a trend of decreased number of NSCs in 3 groups with extending time (P<0.05). NSCs existed in brain tissue with pathological changes in the rats with HIBD all the way, and NSCs could benefit from hypoxia in a certain time.
Keywords
brain damage; animal model; Nestin
Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) and neonatal hypoxic-ischemic brain damage (HIBD) that results from asphyxia in the perinatal period, both have a poor longterm outcome and are major causes of disability of nervous system in childhood. HIE is not only a common disease among perinatal diseases, but also an important reason which can result in long-term lingering effects of nervous system including abnormal behavior, severe epilepsy, mental retardation, cerebral palsy and others. To date, the specific pathophysiology of HIE caused by perinatal hypoxia and the effective strategies for the treatment of HIE are poorly understood [1]. Although advances have been made, there is still no consensus for the treatment of HIE [2,3]. Therefore, HIE has become a major problem that seriously affects the quality of life of children worldwide. Though the effective therapies for HIE are absent, the study about the pathologic mechanism of HIE remains a basic way for seeking effective treatment strategies of HIE. Studies have showed that one of the reasons that result in permanent HIBD is that neural stem cells (NSCs) located in SVZ are sensitive to hypoxia/ ischemia, thus, causing neural cells necrosis and apoptosis one after another [4]. So far, NSCs from specific brain areas or developed from progenitors of different sources are of therapeutic potential for neurodegenerative diseases. The treatment strategies involve (i) transplantation of exogenous NSCs; (ii) pharmacological modulations of endogenous NSCs; and (iii) modulation of endogenous NSCs via the transplantation of exogenous NSCs. There has been a consensus about the therapeutic potential of transplanted NSCs [5], while less is known about its mechanism. In this present study, neonatal rat model with HIBD was established to observe the changes of characteristics of neural stem cells. Our study may provide an effective method and the time of therapeutic window for the clinical application of NSCs in treatment of HIE.
Material and Methods
Animals and grouping
A total of 231 Sprague-Dawley (SD) neonate rats aged 7 days and weighing 10-12g (specific pathogen free) were purchased from the Center for Laboratory Animals of Acad emy of Military Medical Sciences (Beijing, China). All the study protocol about experimental animals was approved by the Animal Care and Use Committee of Beijing Military Region General Hospital. The rats were randomly assigned into 3 groups: control, hypoxic and HIBD groups (n=77 per group). According to the time of sacrifice, each group was divided into 7 subgroups (3h, 6h, 1d, 3d, 7d, 14d and 21d, n=11 per subgroup).
Main reagents
Rabbit anti-rat Nestin polyclonal antibody was purchased from Chemicon (Temecula, CA, USA). A 3, 3'- diaminobenzidine (DAB) visualization kit was purchased from Zhongshan Biotech Co (Beijing, China). Some other domestic reagents (analytically pure) were used in this study.
Establishment of hypoxic-ischemic brain-damage (HIBD) rat model
Neonatal hypoxic-ischemic brain damage model was established as the previous methods [6]. Briefly, the left carotid artery of 7-day-old postnatal SD rat was isolated and ligated doubly. After 2 hours recovery with their mothers, offsprings were placed into a transparent plastic container which was ventilated with a constant flow of humidified mixture of 8% oxygen and 92% nitrogen for 2.5 hr.
Sample collection
At the different time points, 3 groups of rats were sacrificed under anesthesia via inhalation of anhydrous diethyl ether. The heart and aorta were exposed. Blood in the circulation was removed by perfusion with 20ml/kg cold normal saline via an aortic cannula. The brain was collected, the macroscopic features of which were recorded and was placed in a freezing microtome at -20°C for 2h. Frozen tissue sections (8μm) were placed on slides previously treated with polylysine, fixed with acetone for 20min and stored at -20°C on standby.
HE staining
The frozen sections were treated successively with xylene, ethanol at different concentrations (100%, 95%, 80% and 70%), distilled water (2 min), Harris hematoxylin (1 min), 10% ethanol in acid (several seconds), flowing water (1 min), ethanol at different concentrations (75%, 85% and 95%; 2 min for each), 1% eosin (1 min), 95% ethanol (several seconds), 100% ethanol (twice; 10 min for each) and xylene (twice; 10 min for each). Then, these sections were mounted and observed under the light microscope, and the representative photographs were captured.
Detection of Nestin expression in neural stem cells
The routine SABC method was used for immunohistochemical analysis and visualization was done with DAB. Pathological examination was done under a light microscope. The cells with yellow-brown granules in the cytoplasm were regarded as Nestin positive cells. The number of positive cells and total cells were counted at a magnification of ×400. A total of 100 grids were included in a field and 5 sections were examined in each group. A total of 10 fields were randomly selected for examination and thus 50 fields were employed for the detection of the positive cells. Then, the number of Nestin positive cells was calculated in each group.
Statistical analysis
All data were shown as mean ± standard deviation (SD) for normally distributed continuous variables, and statistical analysis was performed with SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Analysis of variance was performed to determine comparisons among different groups. Differences with P < 0.05 were considered to be statistically significant.
Results
Behavior of rats with HIBD
After ligation of left common carotid artery and hypoxia for 10 min, rats appeared dysphoria; hypoxia for 15-20 min caused cyanosis and deep rapid breathing; hypoxia for 20-30 min led to unstable standing and dragging step of right hind limb during creeping; hypoxia for 35-60 min significantly reduced the activity; hypoxia for more than 1 h caused lethargy and irritability in 90% of rats with HIBD. At 1 h after post-hypoxic re-oxygenation, rats circled towards left side. Abnormal behaviors were not observed among rats receiving hypoxia alone.
Pathological examination of brain in the rat with HIBD
At 3 h after ischemia/hypoxia, focal karyopyknosis of neurons was observed in the left cortex and striatum; the lesions in the striatum were enlarged and there were some small lesions presented in the hippocampus and thalamus at 6 h; massive necrosis in the cortex, striatum, hippocampus and thalamus and degradation or absence of cells were observed at 24 h; at day 3 after hypoxia/ischemia, proliferation of glial cells around the lesions was observed while a great deal of pyknotic nuclei and nuclear debris at the center of lesions were also noted; only a few pyknotic nuclei and proliferative glial cells increased at day 7; A great loss of neurons was observed in the lesions and glial scars presented in cortex, striatum, hippocampus and thalamus at days 14 and 21. In control group, the brain had clear structural layers and cells presented clear borderline, and the nucleus were located at the center of the cells.
NSCs morphological changes of left hemisphere at different time of sacrifice
NSCs were mainly distributed in hippocampus, ependyma of lateral ventricle, SVZ, striatum and cortex in control group. NSCs of hippocampus were mainly located in molecular layer, cone cell layer and endoparticle cell layer. The number of NSCs was high within 3 days, while it was the least at day 21. NSCs could be observed in ependyma of lateral ventricle at different time points, while fewer NSCs were observed in striatum and cortex. There was no difference in the distribtion of NSCs in hypoxia and HIBD groups compared with control group, while NSCs in HIBD group presented regional distribution. At 3h, obvious lesion was not found in the lesion side of brain. At 6h, the extent of tissue necrosis was enlarged and the number of NSCs decreased in necrotic area, while the number of NSCs in karyomitosis increased, particularly in ependym and SVZ. At days 1 and 3, though a large number of tissue necrosis was observed in necrotic area, NSCs and neurosphere still could be found. At day 7, more necrosis and atrophy of brain tissues were observed with proliferation of surrounding tissues, while neurosphere and residual NSCs still existed in necrotic area. At day 14, necrosis and atrophy further aggravated, even hollow, resulting in the distribution range of NSCs more narrowed, however, the proliferation of NSCs still could be found in necrotic area. At day 21, along with the regional distribution of further reduction, most NSCs existed in other nerve cells with a single form, while a few neurospheres with proliferation of NSCs still could be observed.
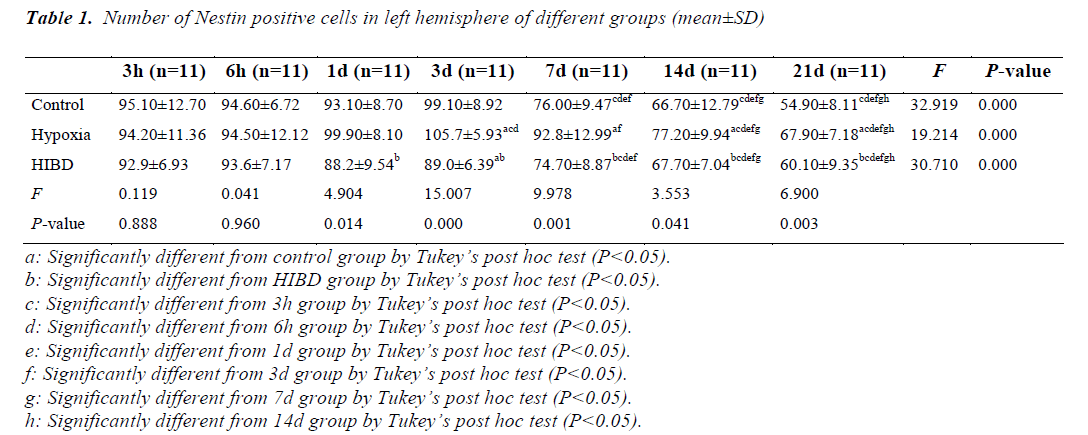
Comparison of the number of NSCs in left hemisphere among different time of sacrifice
As compared with control and HIBD group, the number of NSCs was higher in hypoxia group at the same time point from day 3 (P<0.05). There was no difference in the number of NSCs between control and HIBD group at the same time of sacrifice (P>0.05) except day 3. The similar change tendency of the number of NSCs was observed in 3 groups. There was no difference in NSCs number at the same time of sacrifice among 3 groups within 3 days expect hypoxia group. After 3 days, there was a trend of decreased number of NSCs with extending time in 3 groups (P<0.05) (Table 1).
Discussion
NSCs, commonly existing in nervous system of embryo and adults, can form nerve cell, astrocyte and oligodendrocyte, which may have an active role in the repair of brain tissue damage. Therefore, NSCs have been considered to be of potential value for the treatment of nervous system degenerative disease and HIE. At present, there have two paths for nervous system degenerative disease therapy via NSCs as follows: one method is to directly activate NSCs of brain, inducing endogenous NSCs self repair and differentiation, but it is still limited to mammals experimental research stage; another method is to transplant NSCs into brain tissues [7-10].
In this study, NSCs existed in brain tissue all the way in 3 groups at different time of sacrifice. We found that the number of NSCs was higher in hypoxia group compared with control and HIBD groups. The reason may relate to the proliferation of ststic or inactive NSCs caused by hypoxia. In addition, the dual function of hypoxia/ischemia may aggravate the damage of NSCs. The proliferation of NSCs can be activated in early time of hypoxia-ischemia, which probably has several reasons as follows: NSCs possibly have specfic mechanism of resistance to death; the required time of activation of ion channel and protein expression related to hypoxia- ischemia in NSCs is shorter compared with other cells [11-13]. However, the tolerance of NSCs to hypoxiaischemia is limited to the time and degree of hypoxiaischemia. Study suggested that 8% oxygen could have an upregulation role to the proliferation of NSCs [14]. Hypoxia can be aggravated with ischemia followed by necrosis and liquefaction of brain tissues. Though the proliferation of NSCs in lesion area and surrounding tissues was still on, NSCs died off in lesion area and differentiation of NSCs in surrounding tissues was accelerated after a period of time, consequently, lesion tissues were repaired with glial scar and hollow. In this study, after the proliferation of NSCs to a certain extent, it presented a decreasing trend and the turning time points of 3 groups were almost at day 3, which indicated that age could play a leading role in this change.
The serious damages on children caused by HIE have provoked an intensive search for novel treatment strategies. Though the therapeutic effect of hyperbaric oxygen treatment is not sure, clinical treatment is still keeping trying. In this present study, NSCs existed in lesion area all the way in HIBD group. In addition, the number of NSCs increased in a certain time, while decreased after a period of time. Though the proliferation of NSCs could benefit from hypoxia, there was a time of window. These findings suggest that earlier interruption of NSCs is key point for HIE treatment. Moreover, the method of early use of hyperbaric oxygen for HIE treatment remains to be reacquainted.
At present, most researches [15] emphasize on the therapeutic effect of exogenous NSCs transplantation on HIE, simultaneously neglecting the problems of immunological rejection, reconstruction of the cell loop, integration of function and assessment of prognosis, or emphasize on the therapeutical effect of some interference factors on HIBD at a certain time point, neglecting their dynamic effect on nerve regeneration at different time points and its mechanism. It’s certain that NSCs can be used to treat damage and degenerative disease of nervous system. However, due to the limitations of exogenous NSCs, it may be more promising to induce endogenous NSCs to repair nervous system damage, especially in neonatal HIE, in which attentions should be payed to the time window of inducing endogenous NSCs’ proliferation and differentiation. In this study, the change rules of NSCs were seek out by dynamically observing characteristics of NSCs of rats during the course of HIBD, providing the time window for treating HIE in this way. It should be noted that the observation time in present study was 21 days and could not represent the entire course of HIBD, and therefore, longer observation time of HIBD should be concerned further in the future study.
In this present study, the proliferation of NSCs occurs in the early stages of HIE, while the number of NSCs decreases with the progress of the disease, and eventually NSCs die off. Moreover, hypoxia can promote the proliferation of NSCs in a certain time, which indicates that appropriate time of hyperbaric oxygen application should be employed for HIE treatment.
Acknowledgements
Xiaojuan Yin, Lei Dong and Yu Wang contributed equally to this work. This study was supported by grants from the China Postdoctoral Science Foundation (No. 20070410505).
Conflict of interest
None declared.
References
- Lai MC, Yang SN. Perinatal hypoxic-ischemic encephalopathy. J Biomed Biotechnol 2011; 2011:609813.
- Pimentel-Coelho PM, Mendez-Otero R. Cell therapy for neonatal hypoxic-ischemic encephalopathy. Stem Cells Dev 2010; 19: 299-310.
- Gong M, Bi Y, Jiang W, Zhang Y, Chen L, Hou N, Liu Y, Wei X, Chen J, Li T. Immortalized mesenchymal stem cells: an alternative to primary mesenchymal stem cells in neuronal differentiation and neuroregeneration associated studies. J Biomed Sci 2011; 18: 87.
- Levison SW, Rothstein RP, Romanko MJ, Snyder MJ, Meyers RL, Vannucci SJ. Hypoxia/ischemia depletes the rat perinatal subventricular zone of oligodendrocyte progenitors and neural stem cells. Dev Neurosci 2001; 23: 234-247.
- Gincberg G, Arien-Zakay H, Lazarovici P, Lelkes PI. Neural stem cells: therapeutic potential for neurodege- nerative diseases. Br Med Bull 2012; 104: 7-19.
- Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 1981; 9: 131-141.
- Pincus DW, Goodman RR, Fraser RA, Nedergaard M, Goldman SA. Neural stem and progenitor cells: a strategy for gene therapy and brain repair. Neurosurgery 1998; 42: 858-867; discussion 867-858.
- Cusulin C, Monni E, Ahlenius H, Wood J, Brune JC, Lindvall O, Kokaia Z. Embryonic stem cell-derived neural stem cells fuse with microglia and mature neurons. Stem Cells 2012; 30: 2657-2671.
- Ashton RS, Conway A, Pangarkar C, Bergen J, Lim KI, Shah P, Bissell M, Schaffer DV. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat Neurosci 2012; 15: 1399-1406.
- Xiang Z, Hrabetova S, Moskowitz SI, Casaccia- Bonnefil P, Young SR, Nimmrich VC, Tiedge H, Einheber S, Karnup S, Bianchi R, Bergold PJ. Long- term maintenance of mature hippocampal slices in vitro. J Neurosci Methods 2000; 98: 145-154.
- Hoshida S, Kuzuya T, Fuji H, Yamashita N, Oe H, Hori M, Suzuki K, Taniguchi N, Tada M. Sublethal ischemia alters myocardial antioxidant activity in canine heart. Am J Physiol 1993; 264: H33-39.
- Rordorf G, Koroshetz WJ, Bonventre JV. Heat shock protects cultured neurons from glutamate toxicity. Neuron 1991; 7: 1043-1051.
- Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature 2000; 407: 520-523.
- Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci 2000; 20: 7377-7383.
- Carreira BP, Carvalho CM, Araujo IM. Regulation of injury-induced neurogenesis by nitric oxide. Stem Cells Int 2012; 2012: 895659.