Research Article - Journal of Gastroenterology and Digestive Diseases (2017) Volume 2, Issue 3
Changes in resting metabolic rate and orexigenic hormones after laparoscopic gastric band surgery
- *Corresponding Author:
- Deborah L Chua, MD
Department of Medicine New York University School of Medicine 123 William St., 15th Floor New York NY 10038 USA
Tel: 212-227-3688
E-mail: deborah.chua@nyumc.org
Accepted date: November 15, 2017
Citation: Oscar Mitchell JL, Weinshel E, Ren-Fielding C, et al. Changes in resting metabolic rate and orexigenic hormones after laparoscopic gastric band surgery. J Gastroenterol Dig Dis. 2017;2(3):33-39.
Abstract
Introduction: Knowledge regarding the effects of laparoscopic adjustable gastric banding (LAGB) on resting metabolic rate (RMR) and the hormonal milieu is limited. Our study aimed to determine how LAGB impacts RMR and hormonal response to a meal. Methods: Twenty-five patients were enrolled. Patients were seen before and two months after surgery. At each visit, RMR was measured and blood was collected fasting and after drinking Ensure to measure hormonal levels including glucagon-like peptide 1, gastric inhibitory polypeptide, peptide YY, ghrelin, pancreatic polypeptide, leptin, insulin and amylin. Results: Of the 25 patients, 17 completed the study, 1 completed RMR only, and 7 dropped out. Mean weight loss was 12.3 kg ± 4.9 and mean percentage of excess BMI loss was 22.6% ± 9.9. Median RMR before and after LAGB decreased significantly. Decrease in RMR correlated with amount of weight loss. Median fasting leptin and ghrelin levels decreased pre- and post-LAGB, but ghrelin did not reach significance. No differences were seen in hormonal response to a meal. Conclusion: Weight loss after LAGB resulted in decreased RMR, fasting leptin levels and possibly lowered ghrelin. Further studies are needed to delineate the long-term effects of LAGB on metabolic rate and the hormonal milieu.
Keywords
Bariatric surgery, Gastric banding, Resting metabolic rate, Ghrelin, Leptin.
Introduction
Obesity is a growing epidemic in the United States with significant medical and economic consequences. In 2011-2014, 36.5% of adults were obese, which is defined as having a body mass index (BMI) of 30 or greater [1]. Obesity is a major risk factor for cardiovascular disease, stroke and type 2 diabetes mellitus, as well as certain types of cancers [2].
Bariatric operations lead to rapid and sustained loss of excess body weight (EBW) and laparoscopic adjustable gastric bands (LAGB) have been reported to lead to EBW loss of 42% at 1 year and 55.2% at 5 years [3]. Since 2002, there has been an increase in the rate of bariatric surgeries performed, although the rate has plateaued since 2009 [4]. The mechanisms by which bariatric surgeries lead to such profound weight loss is incompletely described, as is the extent to which the mechanisms differ between all the metabolic surgery procedures [5]. LAGB has been shown to achieve weight loss through several mechanisms including an overall reduction in total energy intake, increased satiety, decrease in appetite, and alteration of fasting digestive hormone levels [6-9]. While the levels of leptin are generally found to be lower after bariatric procedures [5,8-11], there is considerable variability in the literature regarding the levels of other digestive hormones such as ghrelin after LAGB [7,9,11,12]. There is also limited knowledge regarding the effects of LAGB on the body’s post-prandial hormonal response, resting metabolic rate (RMR) and whether this correlates with changes in a patient’s hormonal milieu.
In our pilot study, we hypothesized that RMR after metabolic surgery, particularly LAGB, would increase due to changes in body composition and increase in lean body mass percentage. We also hypothesized that the hormonal response to a standardized meal would change after metabolic surgery. We suspected that hunger stimulating hormones such as ghrelin would decrease and satiety hormones such as GLP-1 would increase in response to a food bolus after LAGB. We aimed to understand the impact of LAGB on RMR and hormonal response to a meal by measuring RMR and fasting and post-prandial gastrointestinal hormone levels before and 2 months after surgery.
Materials and Methods
Participants
Twenty-five adult subjects aged 18 to 65 with a BMI ≥ 35 who were due to undergo LAGB surgery at a single academic center were prospectively enrolled in the study between November 2013 and September 2014. Subjects with prior metabolic surgery, thyroid disease, lung disease, active smoking, or current use of medications causing weight loss or weight gain were excluded from the study. The Institutional Review Board approved the study and written consent was obtained from all participants. No participant received a stipend for participation in the study.
Data and sample collection
Patients served as their own controls, with blood samples and measurements obtained before LAGB surgery and 2 months post-operatively. We chose 2 months in order to optimize patient retention but also to look at early physiologic changes which have been described after both bariatric surgery and after Very Low Caloric Diet. Patients were instructed to fast for at least 10 hours prior to each visit. Age, gender, body mass index (BMI), weight, height, hip/waist circumference, blood pressure (BP), and medications were routinely recorded at each visit. Hemoglobin A1c (HbA1c) and fasting lipid panel were recorded at the initial visit to obtain baseline levels only and were not drawn at the follow up visit. Baseline testing was performed prior to initiation of pre-operative liquid or restricted diet. RMR was measured using the Med Gem indirect calorimeter following the Med Gem standard protocol. Exercise data was not collected, but patients were instructed to fast, avoid exercise/strenuous activity and caffeine for 4 hours prior to testing and to avoid nicotine for 1 hour prior to testing. Patients were placed in a cool, dark exam room, without any movement or exertion, for at least 10 minutes prior to the measurement. Patients were then asked to breathe into the Med Gem device using a disposable mouthpiece for approximately 7 minutes until the RMR was displayed. RMR was calculated using the Weir equation: RMR=((3.941)(VO2) + (1.106)(VCO2O).
To measure hormonal response, blood samples were collected twice at each visit: Initially after at least ten hours of fasting and then one hour after consuming a standardized liquid meal (Ensure Original: 220 kcal, 6 g fat, 32 g carbohydrate, 15 g sugars, 9 g protein). A butterfly needle was used to draw blood samples. Per standard protocol, plasma was isolated, and a protease inhibitor was utilized to ensure that the plasma hormones were not degraded. In the short term, samples were stored at -20°C. In the long term, samples were stored at -80° C and multiple freeze thaw cycles were avoided.
Plasma assays
A multi-hormone enzyme linked immunoassay panel (Millipore Corp. Billerica, MA, USA) was used to quantify serum active glucagon-like peptide 1 (GLP-1), gastric inhibitory polypeptide (GIP), peptide YY (PYY), ghrelin, pancreatic polypeptide (PP), leptin, insulin and amylin. The accuracy of the panel for amylin, PYY, PP, leptin, insulin, GLP-1, GIP and ghrelin are 81%, 94%, 92%, 78%, 90%, 81%, 91% and 92% respectively [13]. As reported by the manufacturer, the multi-hormone EIA panel has intra- and inter-assay variabilities of 11% and 19%, respectively.
Statistical analysis
Descriptive statistics were generated for analysis of the baseline demographics of the study participants. Continuous variables were reported as mean values ± one standard deviation and categorical variables as frequency distribution. Paired statistical analysis was performed. Wilcoxon signed-rank tests were used to compare median changes for RMR, fasting hormonal levels and hormonal response. Below detectable limit (BDL) was assigned a zero value and included in the analysis. A p-value of <0.05 was considered statistically significant. Spearman rank correlation was used to assess for relationships between percentage of excess BMI loss (%EBMIL, as a measure of band outcome) and fasting hormonal levels and RMR.
Results
Twenty-five subjects were enrolled in the study and 18 completed the study, one of which only completed the RMR analysis. Seven subjects (28%) dropped out from the study (5 patients cancelled their surgery, 2 patients were lost to follow up). In our study population, mean age was 42.5 years ± 9.4 and 72% were female. Mean baseline HbA1c was 6.2 ± 1.2. Mean BMI prior to surgery was 46.3 kg/m2 ± 6.8. Mean weight loss was 12.3 kg ± 4.9, the mean % EBMIL was 22.6% ± 9.9 and mean percentage of total weight loss was 9.7% ± 3.5. The variance in weight loss in the group was 117.51 lb ± 10.84. Subject demographics and clinical data are summarized in Table 1. Three types of gastric bands were used during this period: LAP-BAND AP Standard system, LAP-BAND AP Large system (Allergan Medical, Santa Barbara, CA, USA), and REALIZE-B and C system (Ethicon Endo-surgery, Cincinnati, OH, USA). The choice of band was left to the surgeon’s discretion. The LAGB was performed using anywhere between one and five trocars, with a standard pars flaccida approach. The mean band fill level at 3 months was 5.61 ml ± 1.28.
| Variables | Baseline (n=25)* | Post-operative (n=18) |
|---|---|---|
| Age (years) | 42.5 ± 9.4 | 44.7 ± 9.0 |
| Gender (% female) | 72 | 72 |
| Ethnicity (white/black/Hispanic/other) | 21/2/1/1 | 14/2/1/1 |
| Height (cm) | 168 ± 7.7 | 166 ± 6.1 |
| Weight (kg) | 130 ± 21 | 116 ± 20 |
| BMI (kg/m2) | 46.3 ± 6.8 | 42 ± 7.4 |
| % Excess BMI lost | N/A | 22.6 ± 9.9 |
| Waist Circumference (inches) | 54.6 ± 5.0 | 53.1 ± 5.4 |
| Hip Circumference (inches) | 49.1 ± 6.1 | 44 ± 4.2 |
| Blood pressure (mmHg) | 120/75 ± 13.4/8.4 | 119/74 ± 14.0/9.8 |
| Hemoglobin A1c | 6.2 ± 1.2 | N/A |
*n=25 for baseline data except for hemoglobin A1c (n=23)
Table 1: Patient demographics and metabolic profiles both before and after LAGB. Data is shown as mean values ± one standard deviation.
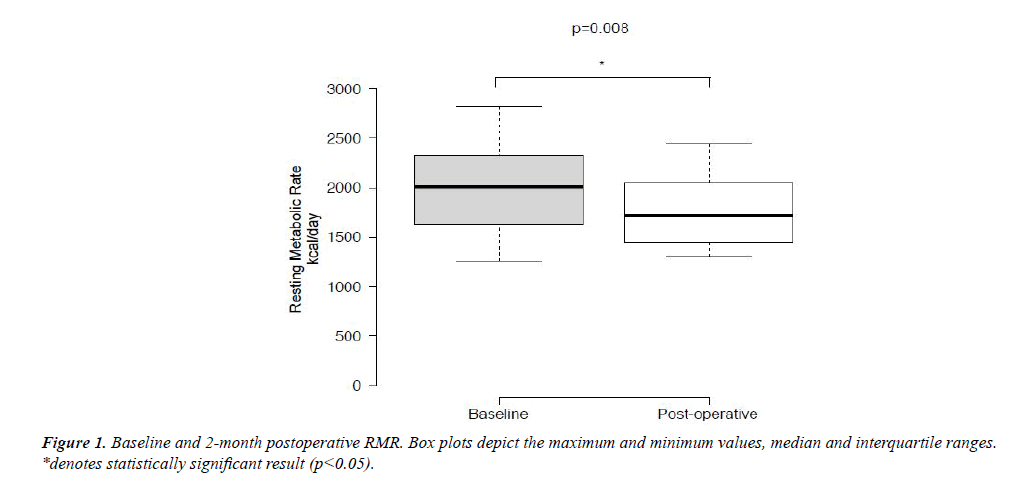
Median RMR before and after LAGB decreased significantly from 2010 kcal/day to 1715 kcal/day (p=0.008) (Figure 1). Decrease in RMR correlated positively with the amount of weight loss (ρ=0.432). There was no correlation between age and either preoperative RMR or change in RMR (p=0.62 and 0.69 respectively).
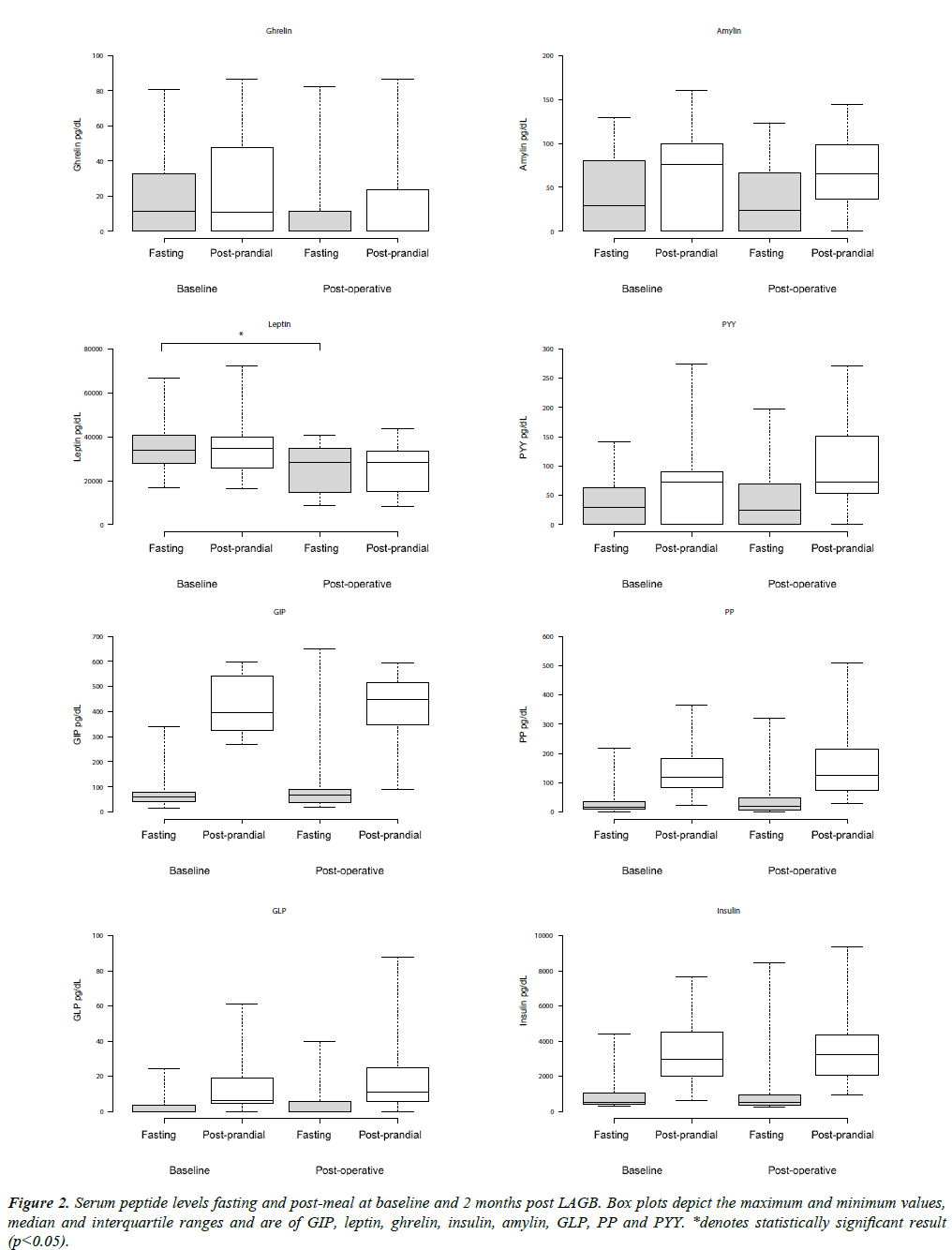
Fasting hormone levels and changes in hormone levels in response to the standardized meal are shown in Tables 2 and 3, respectively. There was a significant decrease in median intrapatient serum fasting leptin levels before and after LAGB which fell from a median of 33885 pg/ml pre-operatively to 28253 pg/ ml post operatively (p<0.001). There was no significant change in median intra-patient post-operative fasting GLP-1, GIP, PYY, PP, ghrelin, insulin or amylin before and after LAGB, although there was an observed decrease in post-operative fasting ghrelin levels that did not reach statistical significance (11.24 pg/ml to 0 pg/ml, p value 0.066) (Figure 2). Hormonal response to the standardized meal was not shown to be significantly changed post-operatively.
| Hormones | Pre-operative fasting serum hormone concentration (pg/mL) (n=17) |
Post-operative fasting serum hormone concentration (pg/mL) (n=17) |
P-value |
|---|---|---|---|
| Ghrelin | 11.24 (0-34.05) | 0 (0-16.86) | 0.066 |
| Gastric Inhibitory Polypeptide | 58.15 (38.32-85.73) | 66.91 (35.17-92.24) | 0.463 |
| Leptin | 33884.55 (26096.04-40935.17) | 28253.49 (14333.0-34992.25) | <0.001* |
| Pancreatic Polypeptide | 16.05 (8.0-39.36) | 20.63 (7.01-66.72) | 0.717 |
| Peptide YY | 29.04 (0-74.93) | 25.11 (0-81.09) | 1.000 |
| Amylin | 28.77 (0-83.6) | 24.06 (0-67.56) | 0.239 |
| Glucagon-like Peptide 1 | 0 (0-4.02) | 0 (0-7.20) | 0.866 |
| Insulin | 503.65 (399.96-1094.27) | 520.43 (349.41-983.27) | 0.619 |
*denotes statistically significant result (p<0.05).
Table 2 : Baseline and post-operative levels of measured gastrointestinal hormones pre and post LAGB. Data is shown as a median value with interquartile range.
| Hormones | Pre-operative prandial change in serum hormone concentration (pg/mL) (n=17) |
Post-operative prandial change in serum hormone concentration (pg/mL) (n=17) |
P value |
|---|---|---|---|
| Ghrelin | 0 (0-5.05) | 0 (0-2.54) | 0.721 |
| Gastric Inhibitory Polypeptide | 330.67 (254.0-438.30) | 356.43 (229.22-439.33) | 0.758 |
| Leptin | -720.56 (-1451.25-1040.91) | -661.0 (-1645.74-246.72) | 0.943 |
| Pancreatic Polypeptide | 86.09 (43.21-141.84) | 72.8 (31.72-209.83) | 0.758 |
| Peptide YY | 23.54 (0-44.78) | 42.5 (1.94-86.99) | 0.211 |
| Amylin | 14.71 (0-52.46) | 21.2 (10.12-58.75) | 0.501 |
| Glucagon-like Peptide 1 | 5.47 (0-11.72) | 7.11 (0.97-24.39) | 0.334 |
| Insulin | 2522.08 (1098.89-3552.82) | 2718.08 (1567.77-4047.97) | 0.136 |
p value of <0.05 was considered statistically significant.
Table 3 : Baseline and post-operative prandial response of measured gastrointestinal hormones to a standardized meal. Data is shown as a median value with interquartile range.
Discussion
In this study, patients were used as their own controls. As expected, LAGB led to rapid weight loss over the first two months. We had hypothesized that an altered hormonal response to meals might contribute to the weight loss seen after LAGB, as seen after Roux-en-Y gastric bypass (RYGB) [14]. No significant difference was demonstrated, suggesting that an altered gastrointestinal hormonal response profile does not contribute to weight loss at 2 months after LAGB. Previous studies have shown an altered response to ghrelin at 52 weeks and to PPY at 26 weeks after LABG [5]. It is possible that an altered prandial hormonal response after RYGB could contribute to the more significant weight loss in RYGB compared to LAGB. Whether or not an altered postprandial response plays an important role in LAGB is yet to be clarified and further research is required in this area to fully characterize the role of altered postprandial hormonal response after LAGB and other bariatric surgeries and how this evolves with time.
Fat free mass (FFM) has been shown to be the most reliable indicator of RMR in normal adults, although total fat mass (FM) also plays a significant role [15]. The majority of weight loss after LAGB is due to loss of FM, with a relatively preserved FFM [8]. Decreases in RMR following bariatric procedures have been well documented in the literature. This effect is likely explained, at least in part, by alterations in body composition, with a greater reduction in total body fat than loss of lean mass and subsequent reduction in energy expenditure [8,16]. Our study was limited in that only RMR was measured, so limited conclusions can be drawn regarding the relationship between RMR and lean body mass or body composition.
Leptin is predominantly produced in white adipose tissue and primarily acts on the hypothalamus to decrease food intake and body weight [17]. Leptin also increases sympathetic nervous activity and RMR through increased thermogenesis in brown adipose tissue [18]. A reduction in fasting leptin levels that parallels weight loss has been demonstrated in multiple studies in both surgical and non-surgical weight loss, likely due to decreased total body adipose tissue and the reduction in calorie ingestion seen after LAGB [6,8,19]. As levels fall, the anorexigenic and hypermetabolic effects of leptin are reduced, creating the orexigenic, hypometabolic state that likely represents an energy conserving response to weight loss in starvation [18,20].
Our study demonstrated a nonsignificant trend towards a reduction in circulating fasting ghrelin levels 2 months after LAGB, despite significant weight loss, which could suggest a contributing mechanism towards the increased satiety seen after LAGB
Serum levels of ghrelin rise substantially in diet-induced weight loss and has been implicated in the subsequent weight gain which is so often seen in non-surgical weight loss, likely due to increased food intake and reduction in RMR due to inhibition of thermogenesis in brown adipose tissue [21,22]. This rise in ghrelin has been inconsistently reported to be attenuated in patients who have undergone RYGB, an effect which could contribute to the sustained effectiveness of this procedure [22,23]. As with RYGB, there is inconsistency in the published literature regarding ghrelin levels after LAGB [11]. One proposed mechanism is that the placement of a gastric band impairs the function of the ghrelin producing cells in the fundus and that alterations in surgical technique could explain the observed variance in the literature [11].
Given this was a pilot study, it has several limitations. First, our sample size was small. We had available for our analysis a convenience sample of only 17 subjects. With our sample size of 17, it would not be possible to detect any but a small effect on results. Graphical representation of the data (Figures 1 and 2) can be used to assess the effect on statistical power of the relatively large variability produced by our small sample size. These figures show the substantial overlap between interquartile ranges. A larger sample size, resulting in smaller IQRs, might reveal significant differences.
Our sample was limited to LAGB patients and we did not have a non-surgical control group. This thereby limits our ability to make observations and conclusions only to this type of metabolic surgery. Not having a non-surgical control group also makes it difficult to attribute RMR and hormonal changes solely to the surgical procedure as these changes have also been seen in the setting of weight loss from calorie restriction alone. Additionally, long term changes in RMR, hormonal levels, and hormone responses after LAGB cannot be determined as the patients were not followed in the study beyond 2 months after surgery.
In our study, RMR was calculated using the Med Gem indirect calorimeter and while the device itself has been validated, measurement of RMR can be difficult [24]. Numerous factors can influence the measured RMR, including prandial status, intake of alcohol, nicotine and caffeine as well as subject activity prior to measurement [25]. To minimize inter-observer variability, the same team of experienced clinicians were responsible for measurement of RMR. However, while subjects were instructed to follow a protocol prior to measurement, they were not observed for the hours prior to indirect calorimetry.
Despite the limitations, our study provides insight into the potential metabolic and hormonal changes that could influence weight loss and weight regain after LAGB. Overall, the results of our study suggest that a suppressed or altered ghrelin response to weight loss rather than an altered hormonal response to meals could be contributing to the weight loss and increased satiety seen immediately after LAGB, while the observed decrease in fasting leptin and RMR could have a deleterious effect on weight loss and explain the plateau in weight loss observed in many longer-term studies of LAGB. More studies are required to investigate the contribution of mealtime response of gastrointestinal hormones to weight loss following LAGB and how this changes in the long term after surgery [26-37].
Conflicts of Interest and Disclosure Statement
Dr. Schwack, Dr. Ren-Fielding and Dr. Fielding report grants, speaking fees and consulting fees from Apollo Endo surgery, Inc, outside the submitted work. Dr. Boxer, Dr. Weinshel, Dr. Mitchell, Dr. Chua and Dr. Lofton have nothing to disclose.
References
- Ogden CL, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth in United States. 2011-2014 NCHS Data Brief. 2015:1-8.
- Whitlock G, Lewington S. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. The Lancet. 2009;373:1083-096.
- O'Brien PE, McPhail T, Chaston TB, et al. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16:1032-040.
- Johnson EE, Simpson AN, Harvey JB, et al. Trends in bariatric surgery, 2002-2012: Do changes parallel the obesity trend? Surgery for obesity and related diseases. Official Journal of the American Society for Bariatric Surgery. 2015.
- Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes. 2009;33(7):786-95.
- Busetto L, Valente P, Pisent C, et al. Eating pattern in the first year following adjustable silicone gastric banding (ASGB) for morbid obesity. Int J Obes Relat Metab Disord. 1996;20:539-46.
- Leonetti F, Silecchia G, Iacobellis G, et al. Different plasma ghrelin levels after laparoscopic gastric bypass and adjustable gastric banding in morbid obese subjects. J Clin Endocrinol Metab. 2003;88:4227-231.
- Coupaye M, Bouillot JL, Coussieu C, et al. One-year changes in energy expenditure and serum leptin following adjustable gastric banding in obese women. Obes Surg. 2005;15:827-33.
- Dixon AF, Dixon JB, O'Brien PE. Laparoscopic adjustable gastric banding induces prolonged satiety: A randomized blind crossover study. J Clin Endocrinol Metab. 2005;90:813-19.
- Uzun H, Zengin K, Taskin M, et al. Changes in leptin, plasminogen activator factor and oxidative stress in morbidly obese patients following open and laparoscopic Swedish adjustable gastric banding. Obes Surg. 2004;14:659-65.
- Shak JR, Roper J, Perez-Perez GI, et al. The effect of laparoscopic gastric banding surgery on plasma levels of appetite-control, insulinotropic, and digestive hormones. Obes Surg. 2008;18:1089-096.
- Stoeckli R, Chanda R, Langer I, et al. Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res. 2004;12:346-50.
- Langer FB, Reza Hoda MA, Bohdjalian A, et al. Sleeve gastrectomy and gastric banding: Effects on plasma ghrelin levels. Obes Surg. 2005;15:1024-029.
- Human Metabolic Hormone Magnetic Bead Panel-Metabolism Multiplex Assay, Available at: http://www.emdmillipore.com/US/en/product/MILLIPLEX-MAP-Human-Metabolic-Hormone-Magnetic-Bead-Panel--Metabolism-Multiplex-Assay,MM_NF-HMHMAG-34K#anchor_TI. Accessed 5th February 2016.
- Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY and insulin. J Clin Endocrinol Metab. 2005;90:359-65.
- Cunningham JJ. A reanalysis of the factors influencing basal metabolic rate in normal adults. Am J Clin Nutr. 1980;33:2372-374.
- Johnstone AM, Murison SD, Duncan JS, et al. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr. 2005;82:941-48.
- Coupaye M, Bouillot JL, Poitou C, et al. Is lean body mass decreased after obesity treatment by adjustable gastric banding? Obes Surg. 2007;17:427-33.
- Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-32.
- Seeley RJ, Van Dijk G, Campfield LA, et al. Intraventricular leptin reduces food intake and body weight of lean rats but not obese Zucker rats. Horm Metab Res. 1996;28:664-68.
- Elias CF, Kelly JF, Lee CE, et al. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423:261-81.
- Enriori PJ, Sinnayah P, Simonds SE, et al. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189-2197.
- Considine RV, Sinha MK, Heiman ML, et al. Serum immune reactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292-95.
- Rosenbaum M, Nicolson M, Hirsch J, et al. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. 1997;82:3647-654.
- Weigle DS, Duell PB, Connor WE, et al. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. Clin Endocrinol Metab. 1997;82:561-65.
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-604.
- Buzga M, Evzen M, Pavel K, et al. Effects of the intra gastric balloon Med Sil on weight loss, fat tissue, lipid metabolism, and hormones involved in energy balance. Obese Surg. 2014;24:909-15.
- Chan JL, Heist K, DePaoli AM, et al. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409-421.
- Kaiyala KJ, Morton GJ, Leroux BG, et al. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes. 2010;59:1657-666.
- Labayen I, Ortega FB, Ruiz JR, et al. Role of baseline leptin and ghrelin levels on body weight and fat mass changes after an energy-restricted diet intervention in obese women: Effects on energy metabolism. J Clin Endocrinol Metab. 2011;96:E996-1000.
- Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992.
- Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623-630.
- Druce MR, Wren AM, Park AJ, et al. Ghrelin increases food intake in obese as well as lean subjects Int J Obes (Lond). 2005;29:1130-136.
- Lin L, Saha PK, Ma X, et al. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell. 2011;10:996-1010.
- Fruhbeck G, Rotellar F, Hernandez Lizoain JL, et al. Fasting plasma ghrelin concentrations 6 months after gastric bypass are not determined by weight loss or changes in insulinemia. Obes Surg. 2004;14:1208-215.
- McDoniel SO. Systematic review on use of a handheld indirect calorimeter to assess energy needs in adults and children. Int J Sport Nutr Exerc Metab. 2007;17:491-500.
- Compher C, Frankenfield D, Keim N, et al. Best practice methods to apply to measurement of resting metabolic rate in adults: A systematic review. J Am Diet Assoc. 2006;106:881-03.