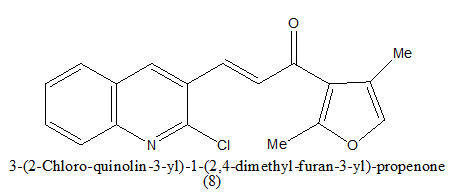
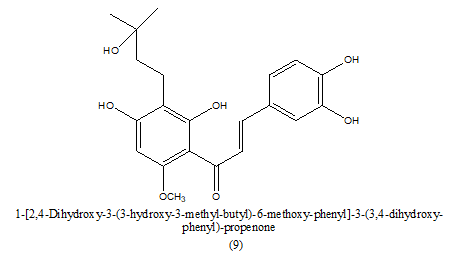
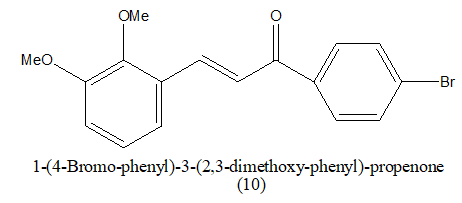
Review Article - Journal of Pharmaceutical Chemistry & Chemical Science (2022) Volume 6, Issue 5
Chalcone: a biologically active scaffold.
Poonam Jundre*, Sachin Bhosale
Department of Pharmaceutical Chemistry, Savitribai Phule Pune University, Nashik, India
- Corresponding Author:
- Poonam Jundre
Department of Pharmaceutical Chemistry
Savitribai Phule Pune University
Nashik
India
E-mail: poonamjundre.14.p@gmail.com
Received: 13-Sep-2022, Manuscript No. AAPCCS-22-62938; Editor assigned: 15-Sep-2022, AAPCCS-22-62938(PQ); Reviewed: 30-Sep-2022, QC No. AAPCCS-22-62938; Revised:04-Oct-2022, Manuscript No. AAPCCS-22-62938(R); Published: 11-Oct-2022, DOI: 10.35841/AAPCCS.6.5.121
Citation: Jundre P, Bhosale S. Chalcone: a biologically active scaffold. J Pharm Chem Chem Sci. 2022;6(5):121.
Abstract
Chalcones and their analogues has become a popular target in the recent years. Researchers have discovered new chalcones which produce a variety of medicinal and biological effects. This chalcones synthesized and evaluated so far have shown antimicrobial, antifungal, anti-mycobacterial, antimalarial, antiviral, anti-inflammatory, and antioxidant, antileishmanial, anti-tumor and anticancer properties. This review highlights the synthesis and pharmacological properties of chalcones.
Keywords
Chalcones, Synthesis, Pharmacological properties
Introduction
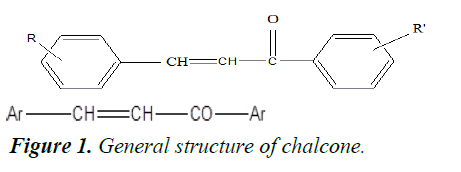
The 1, 3-diphenylprop-2-en-1-one frame is best known as‘‘chalcone’’.It is also known as benzalacetophenone and benzylidene acetophenone. It belongs to a flavonoid family. It is a type of open chain flavonoid having two aromatic rings that are linked by an aliphatic three carbon chain. The presence of a double bond in conjugation with a carbonyl group is believed to be responsible for its biological functions, and the removal of the double bond makes it biologically inactive. Chalcone is a unique template associated with several biological functions such as antioxidant, anticancer, antiinflammatory, antibacterial, antifungal, antiviral, antitubercular, antimalarial, antileishmanial, antihyperglycemic, tyrosine inhibitory and vasorelaxant activity.
Literature Review
Chemistry of chalcones
In chalcones, two aromatic rings and an electrophilic α, β- unsaturated carbonyl systems are in a continuous bond. It may be the reason for their low redox potential, stability, electron transfer reaction and more importantly for its promising biological functions (Figure 1). Therefore, chalcones are a class of compounds that have great therapeutic potential against various diseases. Chalcones have the ability to exist in both the forms that is cis and trans. Chalcone is a synthon for various heterocycles with excellent pharmaceutical activities. Interestingly, chalcones are associated with a good yield with Claisen-Schmidt acid or base-catalysed condensation.
Biological activities
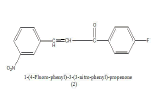
Antimicrobials: Synthesized antimicrobial agents, a series of chalcones. All compounds are tested for their antibacterial and antifungal activities by cup plate method. 3-hydroxybenzaldehyde and 1-(4-fluoro–phenyl)-ethanone used as a starting materials they are converted to chalcones in the presence of alkali and yield a product 1-(4-fluoro-phenyl)-3-(3- hydroxy-phenyl)-propanone (1). 1-(4-fluoro-phenyl)-3-(3- Nitro-phenyl)-propanone (2). Compounds were tested for their in vitro antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa and antifungal activity against Aspergillus niger, Aspergillus flavus. By measuring the blocking area in mm. Showed antimicrobial activity is performed by paper disc plate at a concentration of 100 μg/ml [1].
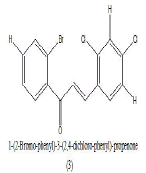
Synthesized series of chalcones in which they were tested for their antibacterial activity against both gram-positive bacteria namely, Bacillus pumilis, Bacillus subtilis and twogram negative bacterium Escherichia coli, Proteus vulgaris using the cup plate method. Compounds containing electron releasing groups such as methoxy and hydroxyl have shown better anti-bacterial activity than others without those groups. Compounds with pharmacophores such as chloro, dichloro and fluoro groups showed additional antifungal activity. The presence of active keto group, unsaturated in chalcones, is found to be responsible for its anti-bacterial activity (Table 1). The synthesized compound 1-(2 bromo phenyl)-3-(2, 4 dichloro–phenyl)-propanone (3) gives important activity against B .pumilis, B. subtilis and E. coli [2].
| Compound | MIC | Antifungal against | Antibacterial against (Gram positive) | (Gram negative) | Reference |
|---|---|---|---|---|---|
 |
100 ug/mL | Aspergillus niger | - | Escherichia coli | [1] |
 |
100 ug/mL | Aspergillus flavus | - | Pseudomonas aeruginosa | [1] |
 |
- | - | Bacillus pumilis | Escherichia coli | [2] |
| Proteus vulgaris | Bacillus subtilis | ||||
 |
1.9 ug/ml 7.8 ug/ml |
- | Staphylococcus aureus 1. methicillin susceptible 2. methicillin Resistant |
- | [3] |
 |
5.2ug/ml | - | Staphylococcus aureus | Pseudomonas aeruginosa | [4] |
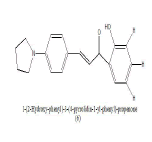 |
10 mm 15 mm |
- | Staphylococcus aureus | Pseudomonas aeruginosa | [4] |
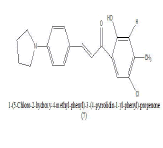 |
15 mm | Candilaalbicans | - | - | [4] |
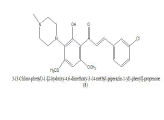 |
6.25 ug/ml | Staphylococcus aureus | - | [5] | |
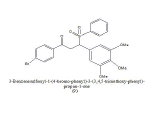 |
1.95 ug/ml | - | S. typhimurium | - | [5] |
Table 1. Antibacterial activity and antifungal activity against both gram-positive and gram negative bacteria.
Anticancer: In 2013, a new report showed that chalcones were also able to inhibit polymerization of tubulin, providing cytotoxicity and destruction of tumor vasculature. Ahmed kamal synthesized a novel chalcones linked imidazolones and evaluated for their anti-cancer activity against a panel of 53 human tumour cell lines derived from nine different cancer types that is leukemia, lung, colon, CNS, melanoma, ovarian, renal, prostate and breast [3-5]. The compounds were screened for their anticancer activity by measuring their effects on percentage growth of more than two different lines for variety of cancer. from overall observation it was observed that among the compound (1) where R=4-OH-3-OMe; R1=phenyl, were this compound shows antitumor activity (GI50) in the range of 1.35-6.81, from the cell cycle analysis this compound exhibited significant cell cycle arrest and it is most potent compound then the other. Where R=4-OH-3-OMe; R1= 4- methoxy phenyl (2) were this compound shows antitumor activity (GI50) in the range of 1.35-13.9 (Table 2). Which is less potent then the other compound [6].
| Compound | Antitumor Activity (GI50 range ) | Reference | |
|---|---|---|---|
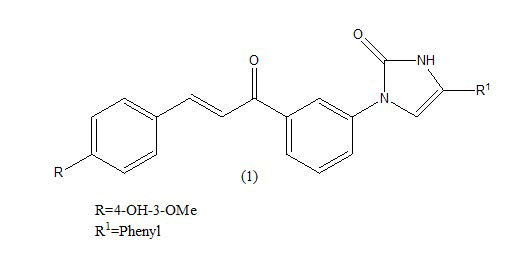 |
1.35-6.81 μM | [6] | |
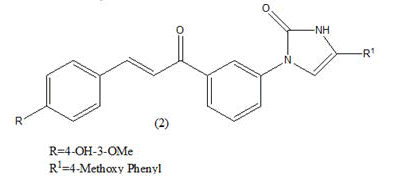 |
1.35-13.9 μM | [6] | |
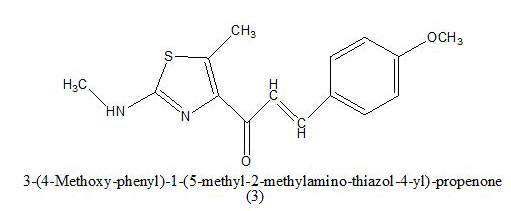 |
0.78–1.97 μM | [7] | |
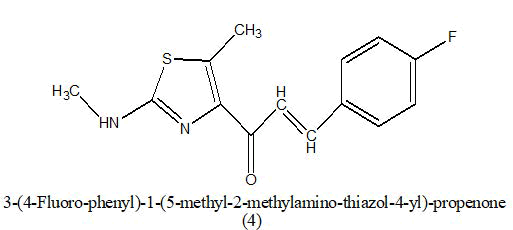 |
0.78–1.97 μM | [7] | |
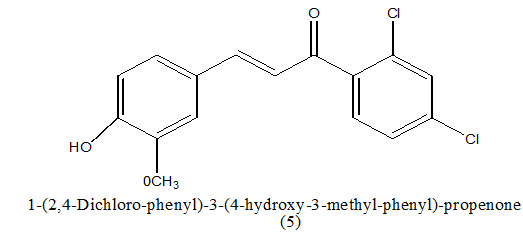 |
5.4 ± 0.7 μM | [8] | |
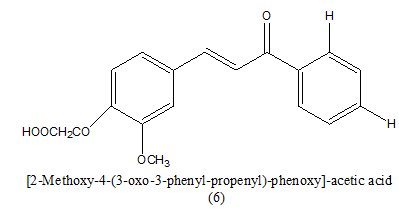 |
>100 μM | [8] | |
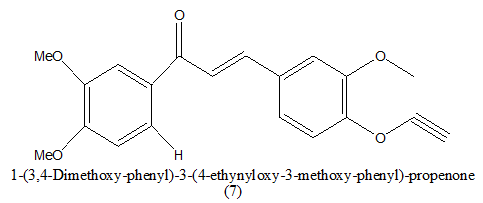 |
20 μM | [9] | |
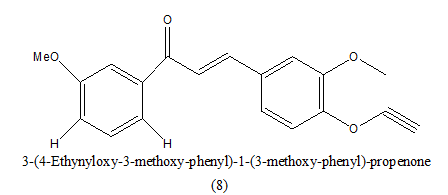 |
20 uΜ | [9] | |
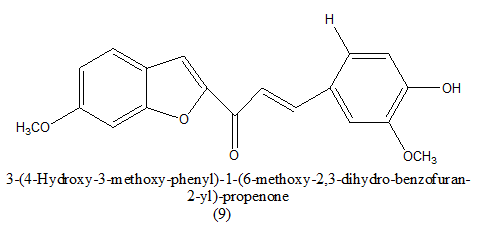 |
46 uM | [10] | |
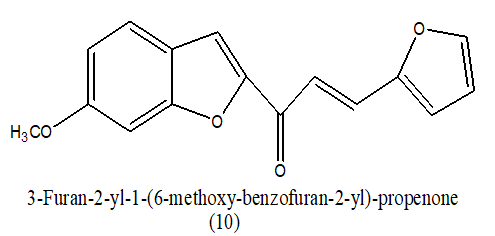 |
26.9 uM | [10] | |
Table 2. Antitumor activity of (GI50)
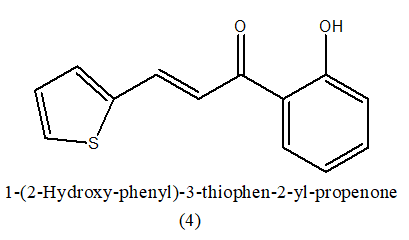
Anti-inflammatory: A chalcones Mannich base and evaluated for their anti-inflammatory activity. Anti-inflammatory action of chalcone Mannich base derivatives has been tested and appears to be associated with the suppression of inflammatory mediators, such as nitric oxide and Tumor Necrosis Factor (TNF), produced by macrophages activated by Lipopolysaccharide (LPS). This protective mechanism may result from the simultaneous inhibition of the production of various inflammatory mediators and direct action to inhibit the activation of transcription factors (NF-BAP-1) that regulate inflammatory reactions [7]. In this showed a variation of the variety acyclic conjugated styryl ketones e.g. chalcones, in Mannich's compatible bases were generally accompanied by an increase in bioactivity both in vitro and in vivo. Synthesized (E)-1 (2-hydroxyphenyl)-3 (thiophen-2-yl) prop-2-en-1-one,(4) a chalcone derivatives tested in vitro for its inhibitory activity in chemical extracts from mast cells, neutrophils, macrophages and microglial cells have satisfactory effects [8-10]. In this Lipoxygenase is a class of non-heme iron containing enzymes that contribute to the eicosanoid pathway. This enzyme is a precursor to inflammatory mediators, leukotrienes and lipoxins so we found it interesting to test our compounds for their Lipooxygenase blocking activity. Perusual of IC50 values shows that compound 4 and 5 is more active and more potent [11].
Assemble three nitro-substituted chalcones and test their antiinflammatory activity in the model of carrageenan induced edema in mice. In these three compounds were tested for biological activity at a dose of 200 mg/kg and showed a protective effect against oral and intraperitoneal inflammation. This effect was dependent on time. To perform this test, an arrangement of 7 groups, each with 6 mice, was performed. Three of these groups received a corresponding chalcones by oral administration. While the other three received a consistent chalcones by intraperitoneal injection. Meloxicam was identified as a reference drug a group of 6 mice acquired oral administration. One dose (10 mg kg-1) for reference. After this treatment, one dose of carrageenan 0.3% vas was used as intraperitoneal. In the right hind of each mouse and every hour the volume of the right hind paw of the specimen is measured up to 7 hrs. and calculated the maximum anti-inflammatory protective effect for every hour. In which compound the maximum effect (58.2 ± 3.2%) was achieved 1 hour after administration. Maximum anti-inflammatory protective effect for (1) was maintained between 50% and 60% within 7 hours of testing. The compound (2) recorded the highest MAPE of the three chalcones (68.0 ± 3.8%) and were achieved within the first hour of testing, although 5 hours after administration (Table 3). A compound (3) reached its highest maximum antiinflammatory Protective Effect within 2 hours after administration (43.2 ± 3.3%); this effect was maintained steady during testing. The highest MAPE of reference drugs (59.3 ± 3.8%) was reached 2 hours after administration and was maintained unchanged within 7 hours of testing [12].
Table 3. This compound shows Anti-inflammatory activities.
Antimalarials: Malaria is a public health problem in countries where the disease is endemic. These countries constitute over one-fifth of the world's population. About 1 million people die every year from Plasmodium falciparum malaria infection. Synthesized a serious of chalcones and evaluated for their antimalarial activity against Plasmodium falciparum. Asexual blood stages were determined. Antiplasmodial IC50 (halfmaximal inhibitory concentration) activity of a compound against malaria parasites first in vitro provides a screen to detect antimalarial potential in the compound. (4- Benzimidazol-1-yl-phenyl)-3-(2,4-dimethoxy-phenyl)-propen-1 -one has shown most potent antiplasmodial activity in vitro with IC50 of 1.1 Ug⁄mL, compared to licochalcone 1.43 ug ⁄mL and based on methoxy group the compound which is having a methoxy substitution on 2 and 4th position also shows optimum for antimalarial activity as compare to methoxy substitution on 3 and 4 position [13-15]
Synthesized a series of sulfonamide chalcones derivatives and investigated for their ability to inhibit the formation of b-hematin in vitro and its activity against cultured Plasmodium falciparum parasites. In this a novel opportunity for the development of sulfonamide release as antimalarials directing the formation of b-hematin and inhibiting the development of cultured P. falciparum parasites, which should help delay the rapid onset of drug action in single site. The inhibition of bhematin formation was minimal in an aromatic ring of chalcones moiety as it was found in some compounds and larger in compounds (IC50 0.48 μM) and 14 (IC50 0.50 μM) with a substitution of 3, 4, 5-trimethoxyl and 3-pyridinyl, respectively (Table 4). Among the entire most active compound lead 1 [4-N (2, 5-dichloro phenyl) sulfonyl-amide phenyl]-3-(4-methylphenyl)-2-propen-1-one, acting as antimalarial agent by inhibition of parasite P. falciparum at (1 μM) [16].
| Compound | Antiplasmodial IC50 range | Reference |
|---|---|---|
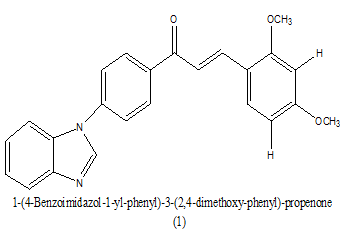 |
1.1 Ug ⁄ mL | [15] |
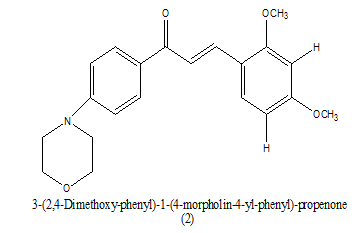 |
1.43 ug ⁄ mL | [15] |
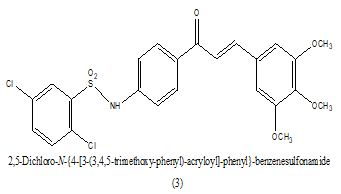 |
0.48 uM | [16] |
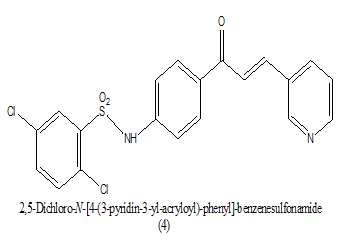 |
0.50 uM | [16] |
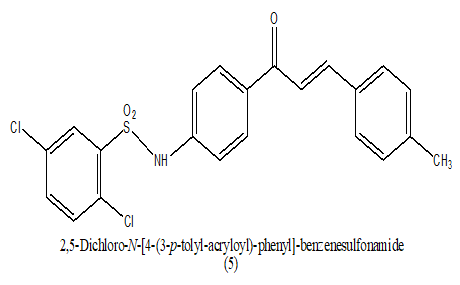 |
1 uM | [16] |
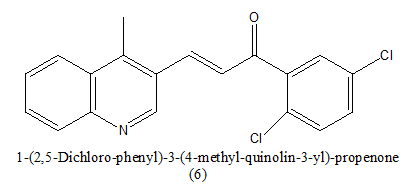 |
200 nM | [17] |
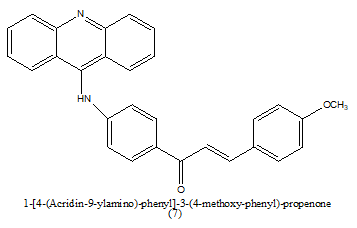 |
2 ug/mL | [18] |
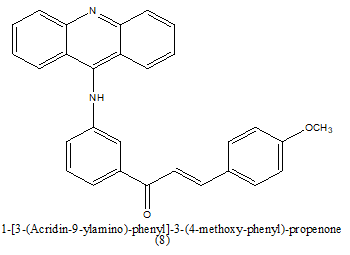 |
2 ug/mL | [18] |
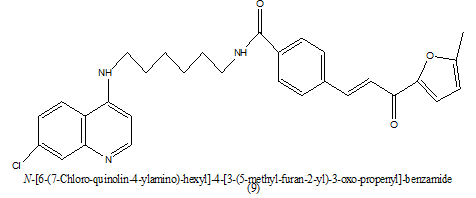 |
0.04 uM | [19] |
Table 4. Antiplasmodial range of IC50.
Antivirals: Prepared or discovering new antiviral agents, from a novel chalcones containing purine moiety was designed and integrated by combining bioactive subunits. An antiviral activity of derivatives was tested against Tobacco Mosaic Virus (TMV) and Cucumber Mosaic Virus (CMV). Among all synthesized purine moity chalcone derivatives the compound (1) and (2) shows an excellent antiviral activity. Compounds (1) and (2) have shown excellent treatment, a protective and non-protective activity against TMV as well has shown remarkable therapeutic and protective functions against CMV in vivo [17-19]. The introduction of suitable groups to remove electrons from the fragrant ring strengthened the anti-viral activity against TMV [20].
Synthesized a series of novel 4-thioquinazoline derivatives containing the chalcone moiety and tested for their antiviral activity against TMV. In this bioassay results showed that most of these compounds showed good moderate activity against Tobacco Mosaic Virus. Among the all combinations compounds (3) and (4) have shown appreciable protective functions in TMV in vivo at 500 ug/ml with a 50% active concentration level (EC50) of 138.1 and 154.8 μg/mL, (Table 5) respectively, which was better than the commercial anti-viral agent Ribavirin? It also provided an effective modification and optimization tool for 4-thioquinazoline-containing chalcone moiety to enhance antiviral activity [21].
Antioxidant: Synthesis various derivatives of chalcones. These newly prepared compounds were tested for in-vitro antioxidant activity by the Diphenyl Picryl Hydrazine (DPPH) model. The method used was to determine the ability to block free radicals of various antioxidants using stable free radical such as 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) in methanol. Most of the compound shows a 2, 4-Dihydroxy acetophenon IC50=31.48 μg/mL. Among all compound (1) shows a most potent compound for the antioxidant activity. the hydroxyl group substitution pattern of rings A and B may be very important in improving their antioxidant activity, in this 2,4- Dihydroxy acetophenon and 4 hydroxy benzaldehyde is used as a starting material to obtained a compound 1-(2,4- dihydroxy-phenyl)-3-(4-hydroxy phenyl)-propanone. This is the most potent compound [22-24].
Synthesized chalcone derivatives and tested for antioxidant activity. This antioxidant activity are generally tested by four methods that is DPPH free radical scavenging assay, iron chelating assay, reducing power assay and hydrogen peroxide scavenging assay. Compound (2) (2,5-dihydroxy-4- dimethylamino chalcone) with p-dimethylamino group on Bring and 2,5’-dihydroxy group on A-ring of chalcone has shown the most promising antioxidant activity in DPPH free radical scavenging assay (Table 6), iron chelating assay and reducing power assay and other compounds shows moderate activity. The antioxidant activity of this compound (2) increases with the dimethylamino group on position 4 [25].
Antileishmanial: Earlier it was found that Licochalcone A (1) is an oxygenated chalcone and it has antileishmanial and antimalarial activity and it also alters the ultrastructure and function of the mitochondria of Leishmania species. Lin Zhai and co-workers investigated the antileishmanial activity and the mechanism of action of a group of new oxygenated chalcones; it was found that they inhibited the growth of Leishmania major promastigotes and Leishmania donovani amastigotes. Treatment of hamsters infected with L. Donovani with intraperitoneal administration of two oxygenated chalcones resulted in a significant reduction of parasite load in the liver and spleen compared with untreated control animals [26-28].
Tadigoppula Narender and Co-workers has synthesized a large number of a novel chromenochalcones. These chromenochalcones were screened against in vitro extracellular promastigotes and intracellular amastigotes of Leishmaniadonovani. The most potent compound in this series was compound (2) with a pyridine ring-A, which showed 99% inhibition of promastigotes at 10 ug/ml, 82% at 0.25 ug/ml and 96% at 10 ug/ml concentration against amastigotes (Table 7). Our results disclose that chromenochalcones have potent antileishmanial activity and further work is in progress to improve the potency of these compounds [29].
Anthelmintic: A novel quinazolinonyl chalcones and carried out for anthelmintic evaluation in vitro against helminth (Peritima posthuma). The compound was synthesized by claisen-schmidt condensation reaction. These anthelmintic activities are tested based on the paralytic and lethal time taken for individual worms observation and 2 hrs time period as the maximum time for parasite response to compound. Lethality was terminated or confirmed when the worms lost their mobility with a dull body colour. Compounds (1), (2) have shown an important anthelmintic function and most potent compared to standard Albendazole at 10 mg/ml [30-32].
Discussion
A hybrid benzimidazolyl-chalcone derivatives and evaluated for their anthelmintic activity as compared to the anthelmintic reference drugs that is fenbendazole and ivermectin and also then check their nematicidal functions in vitro against Haemonchus contortus. Structural activity relationship studies and initial work have helped to highlight the structural features of the nematicidal functions. This suggests that the transformation of the phenyl group into position 1 to 1, 3- diphenyl-2-propen-1-one with a benzimidazole ring induces the possible anthelmintic activity. Among all the compound (3) showed good nematicidal activity (LC100) at 0.002 and 0.0092 μg/ml (Table 8). The activities of these four benzimidazolyl-chalcones is about the same as that of fenbendazole and also show anti-haemonchus function which is equal or more efficient then ivermectin and compound (4) shows a potent anthelmintic activity at 0.68 and 0.16 μg/ml [33-42].
Conclusion
Chalcones are a dynamic medium for the development of various heterocyclic systems such as pyrazoline, oxazoline, thiazine, oxazine, pyrimidine derivatives. In 2013, a new report showed that chalcones were also able to inhibit polymerization of tubulin, providing cytotoxicity and destruction of tumor vasculature.
References
- Tiwari B, Pratapwar AS, Tapas AR, et al. Synthesis and Antimicrobial Activity of Some Chalcone derivatives. 2010;2(1):499-503.
- Rajendra PY, Praveen KP, Ravi Kumar P, et al. Synthesis and antimicrobial activity of some new chalcones of 2-acetyl pyridine.E-Journal Chem. 2008;5(1):144–148.
- Garcia MAR, Theodoro RS, Sardi JCO, et al. Design, synthesis and antibacterial activity of chalcones against MSSA and MRSA planktonic cells and biofilms. Bioorg Chem. 2021;116(8):105279.
- Pardeshi SD, Sonar JP, Dokhe SA, et al. Synthesis and Anti-microbial Activity of Novel Pyrrolidine Containing Chalcones and Pyrazolines. Int J Chem Phys Sci. 2016;5:34-40.
- Xu M, Wu P, Shen F, et al. Chalcone derivatives and their antibacterial activities: Current development. Bioorg Chem. 2019;91(7):103133.
- Kamal A, Ramakrishna G, Raju P, et al. Synthesis and anti-cancer activity of chalcone linked imidazolones. Bioorganic Med Chem Lett. 2010;20(16):4865-4869.
- Farghaly TA, Masaret GS, Muhammad ZA, et al. Discovery of thiazole-based-chalcones and 4-hetarylthiazoles as potent anticancer agents: Synthesis, docking study and anticancer activity. Bioorg Chem. 2020;98(3):103761.
- Raghavan S, Manogaran P, Kalpattu Kuppuswami B, et al. Synthesis and anticancer activity of chalcones derived from vanillin and isovanillin. Med Chem Res. 2015;24(12):4157-4165.
- Ngameni B, Cedric K, Mbaveng AT, et al. Design, synthesis, characterization, and anticancer activity of a novel series of O-substituted chalcone derivatives. Bioorganic Med Chem Lett. 2021;35.
- Coskun D, Dalkilic S, Dalkilic LK, et al. The Synthesis, Characterization, and Anticancer Activity of New 2-acetylbenzofuran-Chalcone Hybrids. Iran J Sci Technol Trans A Sci. 2021;45(5):1561-1569.
- Maria K, Dimitra H-L, Maria G, et al. Synthesis and Anti-Inflammatory Activity of Chalcones and Related Mannich Bases. Med Chem (Los Angeles). 2008;4(6):586-596.
- Gómez-Rivera A, Aguilar-Mariscal H, Romero-Ceronio N, et al. Synthesis and anti-inflammatory activity of three nitro chalcones. Bioorganic Med Chem Lett. 2013;23(20):5519-5522.
- Herencia F, Ferrándiz ML, Ubeda A, et al. Synthesis and anti-inflammatory activity of chalcone derivatives. Bioorganic Med Chem Lett. 1998;8(10):1169-1174.
- Vogel S, Barbic M, Jürgenliemk G, et al. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur J Med Chem. 2010;45(6):2206-2213.
- Yadav N, Dixit SK, Bhattacharya A, et al. Antimalarial Activity of Newly Synthesized Chalcone Derivatives In Vitro. Chem Biol Drug Des. 2012;80(2):340-347.
- Domínguez JN, León C, Rodrigues J, et al. Synthesis and antimalarial activity of sulfonamide chalcone derivatives. Farmaco. 2005;60(4):307-311.
- Li R, Kenyon GL, Cohen FE, et al. In vitro antimalarial activity of chalcones and their derivatives. J Med Chem. 1995;38:5031-5037.
- Tomar V, Bhattacharjee G, Rajakumar S, et al. Synthesis of new chalcone derivatives containing acridinyl moiety with potential antimalarial activity. Eur J Med Chem. 2010;45(2):745–751.
- Smit FJ, N’Da DD. Synthesis, in vitro antimalarial activity and cytotoxicity of novel 4-aminoquinolinyl-chalcone amides. Bioorganic Med Chem.2014;22(3):1128-1138.
- Gan X, Wang Y, Hu D, et al. Design, Synthesis, and Antiviral Activity of Novel Chalcone Derivatives Containing a Purine Moiety. Chinese J Chem. 2017;35(5):665-672.
- Wan Z, Hu D, Li P, et al. Synthesis, antiviral bioactivity of novel 4-thioquinazoline derivatives containing chalcone moiety. Molecules. 2015;20(7):11861-11874.
- Chen Z, Li P, Hu D, et al. Synthesis, antiviral activity, and 3D-QSAR study of novel chalcone derivatives containing malonate and pyridine moieties. Arab J Chem. 2019;12(8):2685-2696.
- Elkhalifa D, Al-Hashimi I, Al Moustafa AE, et al. A comprehensive review on the antiviral activities of chalcones. J Drug Target. 2021;29(4):403-419.
- Murti Y, Goswam A, Mishra P. Synthesis and antioxidant activity of some chalcones and flavanoids. IntJPharmTech Res. 2013; 5:811-8.
- Narsinghani T, Sharma MC, Bhargav S, et al. Synthesis, docking studies and antioxidant activity of some chalcone and aurone derivatives. Med Chem Res. 2013;22(9):4059-4068.
- Sökmen M, Akram Khan M. The antioxidant activity of some curcuminoids and chalcones. Inflammopharmacology. 2016;24(2-3):81-86.
- Gacche RN, Dhole NA, Kamble SG, et al. In-vitro evaluation of selected chalcones for antioxidant activity. J Enzyme Inhib Med Chem. 2008;23(1):28-31.
- Zhai L, Chen M, Blom J, et al. The antileishmanial activity of novel oxygenated chalcones and their mechanism of action. J Antimicrob Chemother. 1999;43(6):793-803.
- Narender T, Khaliq T, Goyal N, et al. Synthesis of chromenochalcones and evaluation of their in vitro antileishmanial activity. Bioorganic Med Chem. 2005;13(23):6543-6550.
- Zheoat AM, Alenezi S, Elmahallawy EK, et al. Antitrypanosomal and antileishmanial activity of chalcones and flavanones from polygonum salicifolium. Pathogens. 2021;10(2):1-9.
- de Mello MVP, Abrahim-Vieira BA, Domingos TFS, et al. A comprehensive review of chalcone derivatives as antileishmanial agents. Eur J Med Chem. 2018;150:920-929.
- Lakshmi K, Rama Rao N, Basaveswararao MV, et al. Synthesis, antimicrobial and anthelmintic evaluation of novel quinazolinonyl chalcones. Rasayan J Chem. 2014;7(1):44-54.
- Ouattara M, Sissouma D, Koné MW, et al. Synthesis and anthelmintic activity of some hybrid benzimidazolyl-chalcone derivatives. Trop J Pharm Res. 2011;10(6):767-775.
- Gaonkar SL, Vignesh UN. Synthesis and pharmacological properties of chalcones: a review. Res Chem Intermed. 2017;43(11):6043–6077.
- Rozmer Z, Perjési P. Naturally occurring chalcones and their biological activities. Phytochem Rev. 2016;15(1):87–120.
- Singh P, Anand A, Kumar V, et al. Recent developments in biological activities of chalcones: A mini review. Eur J Med Chem. 2014;85:758–777.
- Mathew B, Suresh J, Anbazghagan S, et al. Heteroaryl chalcones: Mini review about their therapeutic voyage. Biomed Prev Nutr. 2014;4(3):451–458.
- Nowakowska Z. A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem. 2007;42(2):125–137.
- Rammohan A, Reddy JS, Sravya G, et al. Chalcone synthesis, properties and medicinal applications:a review. Environ Chem Lett. 2020;18(2):433–458.
- Farooq S, Ngaini Z. Recent Synthetic Methodologies for Chalcone Synthesis (2013-2018). Curr Organocatalysis. 2019;6(3):184–192.
- Sahu NK, Balbhadra SS, Choudhary J, et al. Exploring Pharmacological Significance of Chalcone Scaffold: A Review. Curr Med Chem. 2012;19(2):209–225.
- Rajput SS, Patole S. Synthesis and Uses of Chalcone in Hetrocyclic synthesis. World J Pharm Res. 2015;4(7):1566–1591.