Research Article - Biomedical Research (2017) Volume 28, Issue 18
CHA2DS2-VASc score predicts intracoronary thrombus burden in patients with ST-elevation myocardial infarction
Sabri Seyis1*, Özge Kurmuş2, Salih Kiliç3, Sezen Bağlan Uzunget2 and Ebru Akgül Ercan2
1Department of Cardiology, Istinye University Liv Hospital, Istanbul, Turkey
2Department of Cardiology, Ufuk University, Ankara, Turkey
3Department of Cardiology, Nizip State Hospital, Gaziantep, Turkey
- *Corresponding Author:
- Sabri Seyis
Department of Cardiology
Istinye University Liv Hospital, Istanbul, Turkey
Accepted on August 28, 2017
Abstract
Objective: To evaluate the role of CHA2DS2-VASc score in predicting the intracoronary thrombus burden in patients with ST-Elevation Myocardial Infarction (STEMI).
Background: High intracoronary thrombus burden is associated with reduced procedural success, larger infarct size, and mortality after Primary Percutaneous Coronary Intervention (PPCI). Prediction of thrombus burden before PPCI might be useful by enabling the detection of patients at risk for these complications.
Methods: We retrospectively evaluated 164 consecutive patients who presented with STEMI within 12 h after the onset of chest pain between January 2015 and June 2016. PPCI was performed within 1 h after admission. Thrombus burden was graded according to Thrombolysis in Myocardial Infarction (TIMI) thrombus score. The patients were stratified into low (grades 1, 2 and 3) and high thrombus burden groups (grades 4 and 5). CHA2DS2-VASc score was calculated for each patient.
Results: Thrombus burden was low in 94 (57%) patients and high in 70 (43%) patients. CHA2DS2-VASc score was higher in patients with high thrombus burden compared to patients with low thrombus burden (4.41 ± 1.7 vs. 1.47 ± 1.1, p<0.001). Logistic regression analysis revealed that one-point increment in CHA2DS2VASc score was associated with three times higher risk of having high thrombus burden (odds ratio 3.28, 95% CI: 2.57-5.70). The area under the ROC curve for a cut-off value of CHA2DS2- VASc score>2 to predict high thrombus burden was 0.925, with a sensitivity of 91% and a specificity of 82%.
Conclusion: CHA2DS2-VASc score is a simple tool that predicts thrombus burden in STEMI patients undergoing PPCI.
Keywords
CHA2DS2-VASc score, ST-elevation myocardial infarction (STEMI), Thrombus burden.
Introduction
ST-Elevation Myocardial Infarction (STEMI) comprises approximately 25-40% of myocardial infarction presentations [1]. Essential mechanism of coronary occlusion is atherosclerotic plaque rupture or erosion and subsequent thrombus formation. Large intracoronary thrombus burden is known to be associated with reduced procedural success during the Primary Percutaneous Coronary Intervention (PPCI), larger infarct size, increased ischemic complications and mortality [2-5]. Prediction of thrombus burden may be useful before starting intervention because it enables detection of patients at risk for these complications.
CHA2DS2-VASc score is easily applied in daily practice to predict thromboembolic risk in atrial fibrillation patients. Furthermore, it predicts major adverse cardiac events after PPCI, and it is associated with increased 1 y mortality rate in patients with Acute Coronary Syndrome (ACS) [6,7].
We aimed to evaluate the role of CHA2DS2-VASc score in predicting the amount of intracoronary thrombus burden in STEMI patients undergoing PPCI.
Methods
We retrospectively evaluated 164 consecutive patients admitted to our hospital with STEMI between January 2015 and June 2016. STEMI was diagnosed based on a history of a typical chest pain lasting at least 30 min and ST-segment elevation of 1 mm or more in at least two contiguous leads or 2 mm or more in leads V1 through V3 on the electrocardiography. Patients admitted to the hospital within 12 h after the onset of chest pain were enrolled. All patients underwent PPCI within 1 h after hospital admission. All PPCI procedures were performed using the standard femoral approach. Aspirin 300 mg, clopidogrel 600 mg, and heparin 100 u/kg were administered before the procedure. None of the patients were pre-treated with a thrombolytic agent or a glycoprotein IIb/IIIa inhibitor. Exclusion criteria were as follows: chest pain longer than 12 h, stent thrombosis, cardiogenic shock, previous revascularization, pacemaker implantation, oral anticoagulation use, atrial fibrillation during or before admission, severe renal or hepatic insufficiency and bleeding diathesis. This study protocol was approved by the local ethics committee. Data from subjects were analyzed retrospectively.
Angiographic analysis
Coronary angiograms that were stored in hospital database were reviewed. All angiograms prior to the intervention were assessed for Infarct-Related Artery (IRA), which was determined by angiographic and electrocardiographic features, and for thrombus grade, which was based on visual estimation. The angiographic sequence of IRA that best demonstrated the thrombus was selected for analysis. Analysis of the angiograms was performed by 2 experienced cardiologists who were blinded to CHA2DS2-VASc scores of patients. In case of disagreement, another cardiologist’s opinion was obtained. Thrombus burden was graded according to TIMI thrombus score as follows: Grade 0, no thrombus; Grade 1, possible thrombus; Grade 2, the thrombus' greatest dimension is <1/2 vessel diameter; Grade 3, greatest dimension>1/2 to <2 vessel diameters; Grade 4, greatest dimension>2 vessel diameters; Grade 5, total vessel occlusion due to thrombus [8]. The patients were stratified into low thrombus burden (grades 1, 2 and 3) and high thrombus burden groups (grades 4 and 5) according to thrombus score.
CHA2DS2-VASc score
CHA2DS2-VASc score was calculated for each patient using the data available in the patient files recorded during hospitalization. According to CHA2DS2-VASc scoring system, patients were given 1 point for congestive heart failure (signs/ symptoms of heart failure and ejection fraction<40%), hypertension (taking anti-hypertensive medicine or systolic and diastolic blood pressure ≥ 140/90 mmHg), diabetes mellitus (defined as a fasting blood glucose level>126 mg/dl or blood glucose level ≥ 200 mg/dl or using anti-diabetic drugs), history of vascular disease (peripheral artery disease defined as stenosis of at least 50% in non-coronary artery circulation), age 65-74 y, female sex and 2 points for age 75 y or older and previous stroke or transient ischemic attack [9].
Statistical analysis
The data are presented as mean ± standard deviation or n (%). The Kolmogorov-Smirnov test was used to evaluate the distribution of continuous variables. Continuous variables were analyzed with either Student’s t-test or Mann-Whitney U-test. Categorical variables were analyzed with either the Chi-square test or Fisher’s exact test. In logistic regression analysis, high thrombus burden was accepted as dependent variable. Optimal cut-off value was calculated by using Youden index. The Receiver Operating Characteristics (ROC) curve was used to demonstrate the sensitivity and specificity of CHA2DS2-VASc score and its cut-off value for predicting high thrombus burden.
Results
A total of 164 patients (117 male (71.3%), mean age: 61.2 ± 11.6 y) were included in this study. Of the patients, 94 (57%) had low thrombus burden and 70 (43%) had high thrombus burden. The baseline clinical, laboratory and angiographical characteristics of patients in low and high thrombus burden groups were presented in Table 1. Patients with high thrombus burden were significantly older (65.8 ± 11.6 y vs. 57.8 ± 10.3 y, p<0.001) and had higher prevalence of diabetes mellitus and hypertension (p<0.001 and p<0.001, respectively). Patients with high thrombus burden had more commonly multi-vessel disease than patients with low thrombus burden (75.7% vs. 54.3%, p=0.005). There wasn’t a significant difference between two groups in terms of presence of anterior versus non-anterior myocardial infarction. The duration between the onset of chest pain and angiography was similar in two groups (262 ± 190 min vs. 284 ± 205 min, p=0.51).
| Variables | All patients n=164 | Low thrombus n=94 | High thrombus n=70 | p |
|---|---|---|---|---|
| Age (y) | 61.2 ± 11.6 | 57.8 ± 10.3 | 65.8 ± 11.6 | <0.001 |
| Men (%) | 71.3 | 73.6 | 68.6 | 0.498 |
| Diabetes mellitus (%) | 47 | 27.7 | 72.9 | <0.001 |
| Hypertension (%) | 52.8 | 42.6 | 80 | <0.001 |
| Smokers (%) | 65.2 | 66 | 64.3 | 0.824 |
| Hyperlipidaemia (%) | 42.1 | 38.3 | 47.1 | 0.256 |
| Mean ejection fraction (%) | 43.5 ± 8.7 | 47.5 ± 7.3 | 38.0 ± 7.5 | <0.001 |
| CHA2DS2-VASc score (median ± IQR) | 2 ± 3 | 1 ± 2 | 4 ± 3 | <0.001 |
| Anterior MI (%) | 41.5 | 37.1 | 47.1 | 0.351 |
| Multi-vessel disease (%) | 63.4 | 54.3 | 75.7 | 0.005 |
| Chest pain to angiography time (min) | 271 ± 196 | 262 ± 190 | 284 ± 205 | 0.51 |
| Mean haemoglobin(g/dl) | 13.0 ± 1.3 | 13.1 ± 1.6 | 12.9 ± 1.6 | 0.39 |
| Mean white blood cell count (/ml3) | 10768 ± 3040 | 10782 ± 3108 | 10750 ± 2967 | 0.945 |
| Mean platelet count (/ml3) | 253124 ± 71433 | 257000 ± 70000 | 247000 ± 73000 | 0.305 |
| Mean total cholesterol level (mg/dl) | 192.3 ± 41.3 | 193.0 ± 41 | 190.0 ± 40 | 0.585 |
| Mean low density lipoprotein level (mg/dl) | 117.9 ± 36.5 | 116.1 ± 37 | 119.5 ± 35 | 0.693 |
| Mean high density lipoprotein level (mg/dl) | 38.8 ± 9.8 | 38.5 ± 9 | 39.0 ± 10 | 0.231 |
| Mean triglyceride level (mg/dl) | 172.0 ± 108.9 | 189.6 ± 142 | 148.1 ± 110 | 0.48 |
Table 1. Clinical, laboratory and angiographical characteristics among high thrombus burden and low thrombus burden groups.
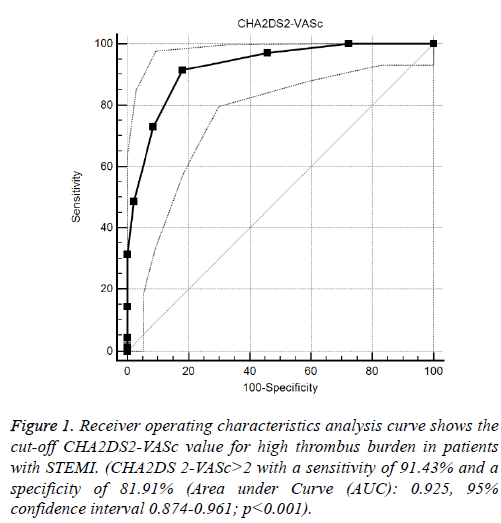
Median CHA2DS2-VASc score of the study population was 2 ± 3 (Median ± IQR). CHA2DS2-VASc score was higher in patients with high thrombus burden compared to patients with low thrombus burden (4 ± 3 vs. 1 ± 2, p<0.001). Logistic regression analysis revealed that one-point increment in CHA2DS2-VASc score was associated with three times higher risk of having high thrombus burden in IRA (odds ratio 3.28, 95% CI: 2.57-5.70). ROC analysis revealed the cut-off value of CHA2DS2-VASc score>2 as a predictor of high thrombus burden with a sensitivity of 91% and a specificity of 82% (AUC:0.925, 95% CI 0.874-0.961; p<0.001) (Figure 1).
Among the determinants of VASc scoring system; Heart Failure (HR: 5.6, p=0.003), Hypertension (HR: 8.9, p=0.002), Diabetes Mellitus (HR: 7.9, p=0.001) and Multi-vessel disease (HR: 7.7, p=0.002) are found to be related to High thrombus burden in multivariate binary logistic regression analysis, with a classification accuracy of 90%.
When additional variables are added to the model (Smoking, Hyperlipidemia, MI type, IRA, Number of Vessels, HG, WBC, Platelet, MPV, LDL, HDL, TG, Anti-agregan use) they are not found to be significant determinants of high thrombus burden.
In another multivariate binary logistic model where VASc score group were added to these additional variables it is found that Higher VACS score (HR:48, p<0.001) is related to High thrombus burden, with a classification accuracy of 86%.
Discussion
In this study, we found that CHA2DS2-VASc was increased in STEMI patients with high thrombus burden and one-point increment in CHA2DS2-VASc score was associated with three times higher risk of having high thrombus burden in IRA.
The main mechanism of acute coronary occlusion in the setting of STEMI is intracoronary thrombus formation at the site of plaque rupture or erosion. Previous studies demonstrated that large thrombus burden is present in 38-53% of myocardial infarction patients [10-12]. In our study, 43% of the patients had high thrombus burden. Importantly, high intracoronary thrombus burden is known to be associated with increased procedural complications such as unsuccessful angiographic reperfusion. Reduced epicardial and myocardial perfusions, slow flow and no-reflow were higher in patients with large thrombus burden than patients with small thrombus burden [13,14]. Patients with large thrombus were treated with longer and larger diameter stents [15]. Distal embolization during PPCI occurs more often in patients with high thrombus burden [16]. Angiographically evident thrombus was associated with reduced frequency of ≥ 70% ST-segment resolution in STEMI patients [8]. Also, a correlation was found between infarct size and thrombus burden. A study with cardiac magnetic resonance imaging has shown that the presence of large thrombus burden in the setting of PPCI is related to larger myocardial necrosis regardless of angiographically detectable distal embolization [4].
Baseline thrombus and residual thrombus after aspiration thrombectomy predicts adverse events and myocardial damage. NSTEMI-ACS patients with baseline thrombus had significantly higher rates of death, myocardial infarction, and stent thrombosis at 30 d and at 1 y than patients without baseline thrombus [5]. Large thrombus burden was an independent predictor of mortality and IRA stent thrombosis in STEMI patients treated with drug-eluting stents [17]. STEMI patients with greater residual thrombus after aspiration thrombectomy had worse reperfusion and greater myocardial damage [3].
CHA2DS2-VASc score is a well-known score, and it has been widely used to assess the risk of thrombus formation and embolism in patients with non-valvular atrial fibrillation. It is simple to use, time saving, and easily applied at bedside. It includes some of the traditional risk factors for coronary artery disease. Besides the application for the assessment of thromboembolic risk in patients with atrial fibrillation, it was also studied in patient groups with coronary artery disease. It was demonstrated that higher CHA2DS2-VASc score was independently associated with increased 1-y all-cause mortality in a study consisting of 13422 ACS patients [7]. Several other studies have evaluated whether CHA2DS and CHA2DS2- VASc score can be used to predict the risk of adverse events among ACS patients. Similar findings were reported [18-20]. CHA2DS and CHA2DS2-VASc score had prognostic value in ACS patients both with and without atrial fibrillation [20-22]. A retrospective analysis of 15681 patients with acute myocardial infarction revealed that CHA2DS2-VASc score was associated with long-term cardiac events such as myocardial infarction and all-cause death and it was found to be a more important predictor in STEMI patients than NSTEMI patients [23]. In a study of 12785 consecutive patients who underwent PCI, CHA2DS2-VASc score predicted all-cause mortality and death and nonfatal myocardial infarction in a significant and linear fashion [6]. CHA2DS2- VASc score correlated significantly with the number of diseased vessels and the severity of coronary artery disease [24]. Also, it is an independent predictor of no-reflow in STEMI patients [25]. But, data about the value of CHA2DS2- VASc score for prediction of baseline thrombus burden in STEMI patients is lacking. We found that CHA2DS2-VASc score was increased in patients with high thrombus burden compared to patients with low thrombus burden and CHA2DS2-VASc>2 had a good sensitivity and specificity to predict thrombus burden. There are some factors that are known to affect the thrombus formation, but not included in the calculation of this score. However, in multivariate analysis it is found that either the score itself, or the variables that are forming this score is the only significant predictor. This shows that in clinical practice instead of using multiple variables to come up to a conclusion, it may be better to rely on this score.
The optimal approach for the management of lesions with large thrombus burden is still evolving. Thrombus aspiration during PPCI remains controversial [26-28]. The advantage of predicting the amount of thrombus lies in several aspects: 1) Pre-procedural: identification of patients at high risk for angiographic complications; 2) Procedural: guiding management of high thrombus lesions with specific therapies; 3) Post procedural: prognosis and determining stent thrombosis risk.
Study Limitations
Our study has several limitations. The main limitation is the retrospective design. Another major limitation of our study is small sample size. Although angiographically visible thrombus is a predictor of intraprocedural complications in ACS, angiographic assessment of thrombus amount is less sensitive and less specific than intravascular ultrasound or optical coherence tomography. Also, some of the patients may have had undiagnosed peripheral artery disease at the time of the enrolment and this may affect CHA2DS2-VASc score. For these reasons, further prospective studies with larger sample sizes are necessary. But, our study is a hypothesis-generating study and provides rapid and easy clues to predict coronary thrombus burden before the beginning of the invasive procedure.
Conclusions
Our findings suggest that CHA2DS2-VASc score can predict thrombus burden in STEMI patients undergoing PPCI. CHA2DS2-VASc score has an easy to remember formula and can be applied quickly in emergent patients. Further studies are needed to evaluate the potential of CHA2DS2-VASc score as an additive and simple tool for identification of cases at risk for complications during PPCI due to high thrombus burden.
Disclosure Statement
The authors have no conflict of interest to declare.
References
- O’Gara PT, Kushner FG, Ascheim DD. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circ 2013; 127: 529-555.
- Kirma C, Izgi A, Dundar C. Clinical and procedural predictors of no-reflow phenomenon after primary percutaneous coronary interventions: experience at a single center. Circ J 2008; 72: 716-721.
- Higuma T, Soeda T, Yamada M. Does residual thrombus after aspiration thrombectomy affect the outcome of primary PCI in patients with ST-segment elevation myocardial infarction? An optical coherence tomography study. JACC Cardiovasc Interv 2016; 9: 2002-2011.
- Napodano M, Dariol G, Al Mamary AH. Thrombus burden and myocardial damage during primary percutaneous coronary intervention. Am J Cardiol 2014; 113: 1449-1456.
- Goto K, Lansky AJ, Nikolsky E. Prognostic significance of coronary thrombus in patients undergoing percutaneous coronary intervention for acute coronary syndromes: a sub-analysis of the ACUITY (Acute Catheterization and Urgent Intervention Triage strategy) trial. JACC Cardiovasc Interv 2011; 4: 769-777.
- Orvin K, Bental T, Assali A. Usefulness of the CHA2DS2-VASC score to predict adverse outcomes in patients having percutaneous coronary intervention. Am J Cardiol 2016; 117: 1433-1438.
- Rozenbaum Z, Elis A, Shuvy M. CHA2DS2-VASc score and clinical outcomes of patients with acute coronary syndrome. Eur J Intern Med 2016; 36: 57-61.
- Gibson CM, de Lemos JA, Murphy SA. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 sub-study. Circ 2001; 103: 2550-2554.
- Camm AJ, Kirchhof P, Lip GY. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery. Eur Heart J 2010; 31: 2369-2429.
- Miranda-Guardiola F, Rossi A, Serra A. Angiographic quantification of thrombus in ST-elevation acute myocardial infarction presenting with an occluded infarct-related artery and its relationship with results of percutaneous intervention. J Interv Cardiol 2009; 22: 207-215.
- Martí D, Salido L, Mestre JL. Impact of thrombus burden on procedural and mid-term outcomes after primary percutaneous coronary intervention. Coron Artery Dis 2016; 27: 169-175.
- Vavuranakis M, Vrachatis DA, Papaioannou TG. Residual platelet reactivity after clopidogrel loading in patients with ST-elevation myocardial infarction undergoing an unexpectedly delayed primary percutaneous coronary intervention. Impact on intracoronary thrombus burden and myocardial perfusion. Circ J 2011; 75: 2105-2112.
- Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol 2010; 22: 6-14.
- Yip HK, Chen MC, Chang HW. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and no-reflow phenomenon. Chest 2002; 122: 1322-1332.
- Costa RA, Abizaid A, Lotan C. Impact of thrombus burden on outcomes after standard versus mesh-covered stents in acute myocardial infarction (from the MGuard for acute ST elevation reperfusion trial). Am J Cardiol 2015; 115: 161-166.
- Napodano M, Ramondo A, Tarantini G. Predictors and time-related impact of distal embolization during primary angioplasty. Eur Heart J 2009; 30: 305-313.
- Sianos G, Papafaklis MI, Daemen J. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J Am Coll Cardiol 2007; 50: 573-583.
- Huang SS, Chen YH, Chan WL. Usefulness of the CHADS2 score for prognostic stratification of patients with acute myocardial infarction. Am J Cardiol 2014; 114: 1309-1314.
- Kiliszek M, Szpakowicz A, Filipiak KJ. CHA2DS2-VASc and R2CHA2DS2-VASc scores have predictive value in patients with acute coronary syndromes. Pol Arch Med Wewn 2015; 125: 545-552.
- Poci D, Hartford M, Karlsson T. Role of the CHADS2 score in acute coronary syndromes: risk of subsequent death or stroke in patients with and without atrial fibrillation. Chest 2012; 141: 1431-1440.
- Hudzik B, Szkodzi?ski J, Hawranek M. CHA2DS2-VASc score is useful in predicting poor 12-month outcomes following myocardial infarction in diabetic patients without atrial fibrillation. Acta Diabetol 2016; 53: 807-815.
- Podolecki T, Lenarczyk R, Kowalczyk J. Stroke and death prediction with CHA2DS2-vasc score after myocardial infarction in patients without atrial fibrillation. J Cardiovasc Med (Hagerstown) 2015; 16: 497-502.
- Kim KH, Kim W, Hwang SH. The CHA2DS2VASc score can be used to stratify the prognosis of acute myocardial infarction patients irrespective of presence of atrial fibrillation. J Cardiol 2015; 65: 121-127.
- Cetin M, Cakici M, Zencir C. Prediction of coronary artery disease severity using CHADS2 and CHA2DS2-VASc scores and a newly defined CHA2DS2-VASc-HS score. Am J Cardiol 2014; 113: 950-956.
- Ipek G, Onuk T, Karatas MB. CHA2DS2-VASc Score is a predictor of no-reflow in patients with ST-segment elevation myocardial infarction who underwent primary percutaneous intervention. Angiol 2016; 67: 840-845.
- Vlaar PJ, Svilaas T, van der Horst IC. Cardiac death and re-infarction after 1 year in the thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction study (TAPAS): a 1-year follow-up study. Lancet 2008; 371: 1915-1920.
- Fröbert O, Lagerqvist B, Gudnason T. Thrombus Aspiration in ST-Elevation myocardial infarction in Scandinavia (TASTE trial). A multi-center, prospective, randomized, controlled clinical registry trial based on the Swedish angiography and angioplasty registry (SCAAR) platform. Study design and rationale. Am Heart J 2010; 160: 1042-1048.
- Jolly SS, Cairns JA, Yusuf S. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med 2015; 372: 1389-1398.