- Biomedical Research (2010) Volume 21, Issue 1
Cell numbers in the dorsal and median raphe nuclei of AS and AS/AGU rats
A. Aldahmash*Stem Cell Unit, Anatomy Department, College of Medicine and King Khalid University Hospital, King Saud University. Riyadh, Saudi Arabia
- *Corresponding Author:
- A. Aldahmash
Stem Cell Unit, Anatomy Department
College of Medicine & King Khalid University Hospital
King Saud University
P.O. 2925, Riyadh 11461
Saudi Arabia
E-mail: a.aldahmash@gmail.com
Accepted date: July 28 2009
Abstract
The AS/AGU rat is a spontaneous recessive mutation derived from a closed colony of Albino Swiss (AS) rats. This mutation generates a stop codon within the coding region of the PKC-gamma gene. The rats exhibit locomotor dysfunc-tion, which is progressive with age. The nigrostriatal system has already been shown to be affected by this mutation that a) there is marked (80-90%) reduction in extracellular dopamine in the dorsal caudate- putamen as revealed by in vivo microdialysis and HPLC-ECD and b) there is a loss of tyrosine hydroxylase immunoreactive cells in the substantia nigra pars compacta Because PKC-gamma may be involved in the packaging or release of vesicles, and because other aminergic neurons may also be involved in basal ganglia disorders, we investigated the raphe-striatal serotonergic sys-tem in these mutants and the parent AS strain. Animals aged 12-15 months (6 pairs) were killed by administration of sodium pentobarbitone and were perfused with Ringer’s solution + Lignocaine followed by 4% paraformaldehyde. 30 m sections were cut, rostral to caudal, in a one in five series. The collected sections were processed for immu-nostaining using the Avidin-Biotin-Peroxidase Complex (ABC) technique. All sections of dorsal raphe and median raphe nuclei were counted using the light microscope and also by unbiased Sterological methods using “Stereologer”. In each case, AS/AGU rats possessed an average of 20-25% fewer cells in the dorsal raphe than AS control rats (p<0.05); cell count in the median raphe were unaffected.
Keywords
AS/AGU rat raphe-striatal serotonergic system.
Introduction
The cell bodies of most serotonergic neurons are located within the brainstem [1,2,3]. Those in the hindbrain pro-ject down to the spinal cord whilst another group in the midbrain projects rostrally [2]. It is the group in the mid-brain which was looked at in this experiment.
The area of the midbrain called the raphe nucleus can be divided into dorsal raphe and median raphe nuclei (DRN and MRN, respectively) and it is within these that the se-rotonergic neurons are concentrated [4,1,2,5], being most abundant in the dorsal raphe nucleus.
The cells of the dorsal and median raphe nuclei provide the vast majority of axonal processes containing serotonin (5-hydroxy tryptamine, 5-HT) that innervate the forebrain of the rat [4,6,2]. However, the pattern of termination of 5-HT fibres to the forebrain differs between the DRN and MRN. Thus, whereas 5-HT cells within the DRN mainly innervate brain structures related to motor activity such as the basal ganglia, those in the MRN project preferentially, though not exclusively, to limbic regions such as the me-dial septum and hippocampus [7,4,8,6,2,9].
In addition, the axons of 5-HT cells of the MRN are thicker and have larger varicosities compared to seroton-ergic cells in the DRN [10,11]. The two nuclei also show differences in their sensitivity to neurotoxins [12,13], ago-nists at somatodendritic 5-HT1A autoreceptors [14,15,16,17] and in their activation as a response to aver-sive stimuli [18,19]. However, conflicting studies exist which have found no difference in the response to neuro-toxins [20] or the sensitivity to 5-HT1A agonists or selec-tive 5-HT reuptake inhibitors [21].
As described in the Introduction, Parkinson’s disease was initially defined as a loss of both dopaminergic and sero-tonergic neurons, with particular loss of large 5-HT cells in the dorsal raphe found post-mortem [22]. Other related syndromes (e.g. MSA, PSNP) show cell loss in multiple aminergic systems [23].
The purpose of this experiment, therefore, was to see if AS/AGU mutants had reduced 5-HT neurons as well as reduced DA neurons as previously reported [24].
Both midbrain raphe nuclei, the dorsal raphe nucleus and median raphe nucleus were examined. The reasons for choosing these two nuclei is to see:
A. whether the serotonergic system is affected at all in the AS/AGU rat and
B. whether the damage is general or specific to neu-rons projecting to the basal ganglia.
So numbers of serotonergic cells have been counted in both nuclei in AS and AS/AGU rats.
The numbers of cells in particular brain regions or nuclei is a fundamental piece of information and cell counts have been carried out in many brain areas in many differ-ent species and using many different methods. Serotoner-gic neurons in the brain and the raphe nuclei in particular have been counted in rat [25,26,27,28,29,30], cat [31], monkey [32] and in human [33,23].
Quantitive methods for analyzing the morphology of the brain have undergone a revolution in the past fifteen years. The unique elements of this revolution include sys-tematic random sampling, measuring three-dimensional quantities such as total neuronal number and volume, and using unbiased methods to estimate these three-dimensio-nal quantities [34,35,36,37,38,39,40,41]. One of these unbiased methods is the Cavalieri principle, which en-ables the determination of the total volume of the brain structure of interest. Another is the optical disector method, which enables the determination of the number of neurons in a sub-volume of the brain structure. The product of the total volume and the number of neurons in a sub-volume (i.e., the neuronal density, or the Nv) by these methods yields an unbiased estimate of the total number of neurons.
Such studies of total numbers permit reliable compari-sons to be made between species, between normal and diseased human conditions, and between control and ex-perimentally injured animals [42,43,41,44]. Total neu-ronal numbers are also important for theoretical ap-proaches such as the computer modeling of brain func-tion.
Data on total neuronal numbers within the rat raphe nuclei [25,26,27,28,29,30,31,32,33] as well as counts involving human control and PD subjects [23] have not been made using unbiased stereological methods. Therefore, analysis of the DRN and MRN of AS and AS/AGU rats were done by:
A. using conventional cell counts in an interrupted se-ries of sections in order to compare findings with pre-vious authors.
B. using unbiased Cavalieri and optical disector meth-ods.
Materials and MethodsMaterials and Methods
Animals
Six male AS and six male AS/AGU rats were used, aged 12 months.
Immunocytochemistry (ICC)
In order to ensure that 5-HT cells were counted, these cells were identified by ABC immunocytochemistry, a technique first employed [45].
The rats were deeply anaesthetized with an overdose of Sodium pentobarbitone BP (Vet) (Rhone-Merieux, Spire Greencentre, Harlow, Essex, 60mg/1ml). They were then perfused through the left ventricle with 100ml Ringer’s solution containing the vasodilator Lignocaine, followed by 500ml 4% Paraformaldehyde (2944744 BDH) in 0.1M phosphate buffer. The brains were removed and post-fixed in the perfusion fixative for 3 hours. They were then washed three times for 10 minutes in PBS and immersed in 7% Sucrose (10274, BDH) and Sodium azide (S2002, SIGMA) in PBS before storage overnight at 4 oC.
The brains were embedded in tissue-tek (4583, Miles) frozen in liquid-nitrogen and then cut at 30 µm thickness on a cryostat at –20 oC (Leica, Jung Frigocut 2800E). Coronal sections were taken in a rostral to caudal direc-tion and collected serially in bottles containing 0.1M phosphate buffer (PB). The sections were processed for 5-HT immunostaining.
Immunocytochemistry on cryostat sections
The sections were rinsed in PBS and incubated initially in Goat anti-serum in 0.3 Triton X-100 (30632, BDH) for one hour. This is used to bind charged proteins within the tissue and allow enhanced penetration of primary anti-body. This was followed by incubation with the Primary antibody, at an optimal dilution in PBS containing 0.3% Triton X-100.
The primary antibody was Rabbit anti-Serotonin (Z02597, AFFINITI) at a dilution of 1:10,000, which reliably labels serotonergic neurons in the midbrain. Initially, different dilutions of antibodies were tested under the same condi-tions in order to determine the optimum antibody concen-trations.
PBS was used to dilute the antibodies and for all washes. Triton X-100 increased the penetration of antibodies [46]. Incubation was performed in a humidified chamber at room temperature overnight.
The sites of polyclonal antibody-antigen reaction were visualized by the application of biotinylated donkey anti-rabbit immunoglobulin (IG) (RPN 1004, AMERSHAM) at a dilution of 1:250 for one hour at room temperature, followed by Avidin-Biotin-Peroxidase complex (20 l of solution A and 20? l of solution B in 1ml of PBS Vector ABC elite kit) which binds to the secondary antibody as the Avidin portion of the complex contains 4 binding sites specific to biotin. Finally, 5-HT-immunoreactivity was visualised using 3,3'-diaminobenzidine (DAB). This re-acts with the peroxidase portion of the ABC complex to form a brown reaction product after 3-5 minutes. As DAB is a carcinogen, care was taken to ensure that this part of the experimental protocol was carried out in a fume cup-board, using disposable laboratory equipment.
Cell Counting techniques
Conventional counts
The rostral-caudal length of the DRN/MRN is approxi-mately 3mm thus yielding c. 100 x 30 m thick coronal section. Every fifth section was collected, stained and counted using a microscope with a 10x eyepiece fitted with a graticule. All stained cells were counted through-out the dorsal and median raphe nuclei, yielding a value of the average number of 5-HT-ir cells per 30 µm section.
Stereology
The optical disector technique [47] was used to estimate 5-HT cell numbers in DRN & MRN. Total raphe nuclei volumes were estimated using the Cavalieri Principle [48]. Slides used to estimate the number of cells were also used to estimate raphe nuclei volume to avoid any re-quirement for correction factors due to tissue shrinkage. A computer using AutoCADlt97 software (Autodesk lnc, San Rafael, CA) and a digitising Tablet was used to esti-mate the surface area of selected sections (every fifteenth section). The total surface area of these selected sections is designated SA. The thickness of each section is known (h), as is the distance between the sections (d), and total raphe nuclei volume (tv) can be calculated by tv = SA x h x d. The numerical density was estimated using an Olym-pus BX50 microscope fitted with a motorized stage (Prior Scientific Instruments, Cambridge) and Stereologer soft-ware (Systems Planning Analysis, Alexandria, VA).
The absolute number of cells in each nucleus was then calculated by multiplying its volume by the cells numeri-cal density.
Results
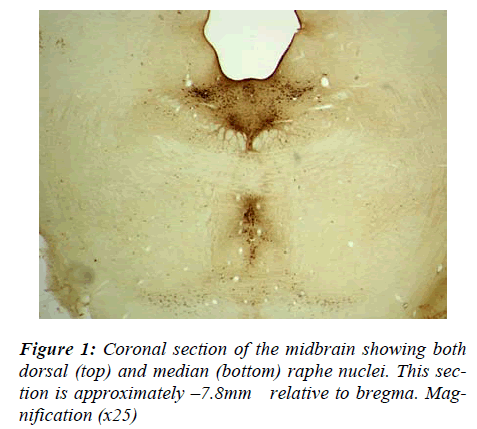
To ensure optimal cell staining, it was first necessary to determine the appropriate concentration of primary anti-body required. The concentration of primary antibody that resulted in clearly visible serotonergic cells was 1:10,000 and this was used throughout the successive ICC experi-ments. At this concentration, serotonergic cells within the dorsal and median raphe nuclei stained positively and could easily be identified (Fig. 1). A clear distinction could readily be seen between the cells in the median ra-phe nucleus and those contained in the larger, dorsal ra-phe nucleus. Cells clusters were carefully examined at high magnification with repeated re-foucusing until the number of cells forming the cluster could be determined.
There was no difference in raphe nucleus size between two group of animals (AS and AS/AGU), nor any obvious difference in cell size or shape.
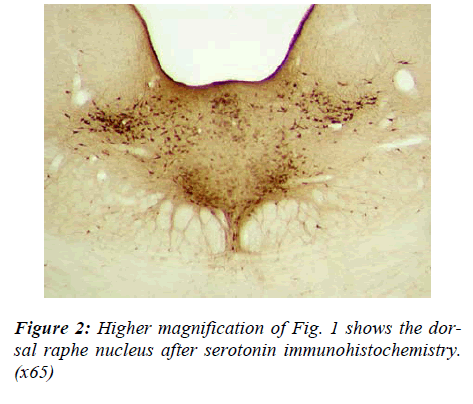
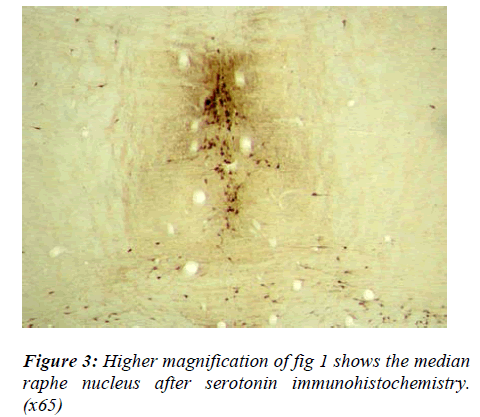
There appeared to be slight regional differences in cell morphology in the two raphe nuclei. Cells of the DRN were more densely stained, tended to have a triangular shape and their dendritic processes were highly visible (Fig 2). In contrast, cell bodies contained in the MRN were rounder, smaller and paler and the neuronal projec-tions were less visible.
Conventional Cell counts
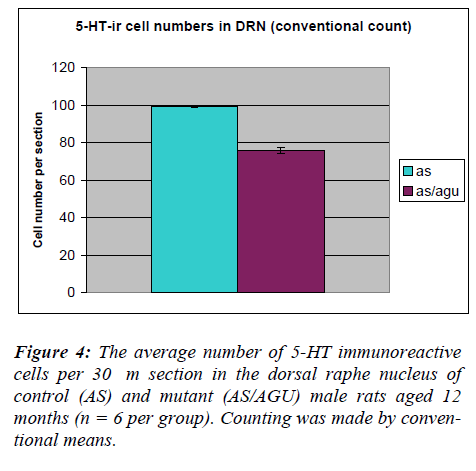
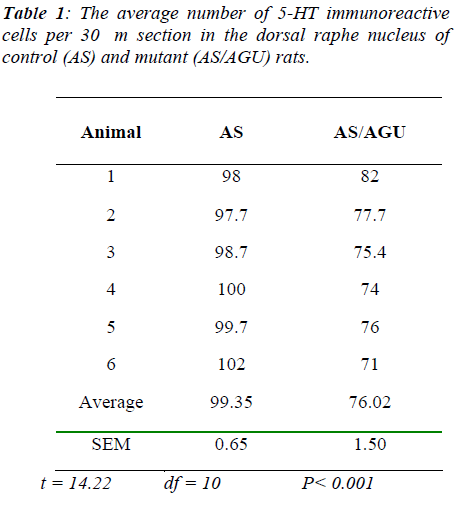
The number of serotonergic cell bodies within the DRN and MRN of AS and AS/AGU rats can be seen in fig 4 and fig 5 respectively. A student t-test for independent samples was performed, using the Minitab statistics pack-age. The results are summarized in Table 1 and shown pictorially in Fig. 4. The data show a significant differ-ence between the AS (control) and AS/AGU (mutant) rats, with mutants possessing c. 23% fewer 5-HT-ir cells in the dorsal raphe than controls (p<0.001).
Nevertheless, all serotonergic cells could be easily counted in both regions.
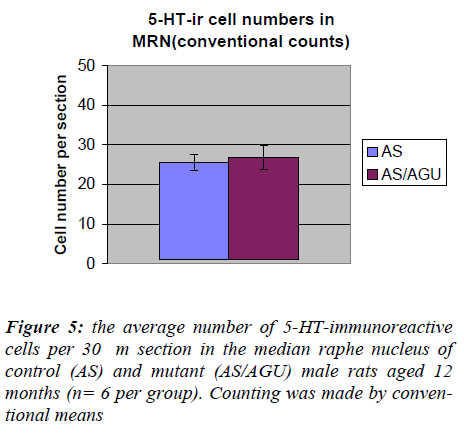
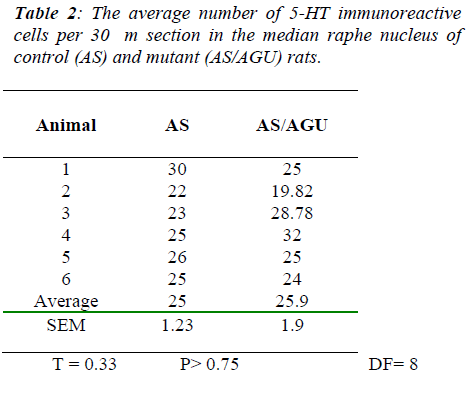
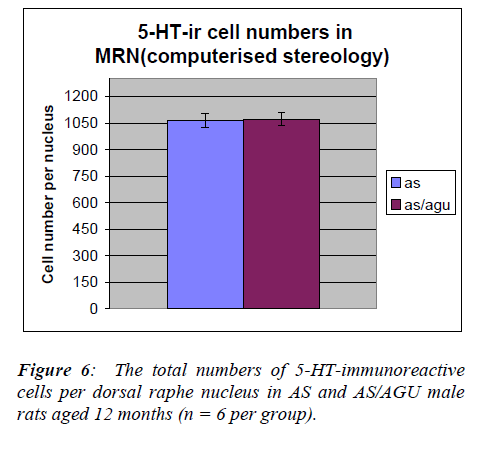
By contrast, Table 2 and Fig. 5 show that there was no significant difference between AS and AS/AGU rats in the numbers of serotonergic cells in the median raphe.
Cell count (using computerised stereology)
Estimation of the total volume of each raphe nucleus (Tv):
This was calculated according to the Cavalieri principle by multiplying three factors:
1. Surface area of selected sections (Sa)
2. The thickness of each section (H)
3. The distance between sections (D).
The surface area was measured by a computer with Auto CAD1t97 software and digitising Tablet. So the total volume (Tv) of each raphe nucleus is calcu-lated as:
Tv= Sa x H x D
Estimation of the numerical density (Nv) of 5-HT-ir cells
Pilot studies were performed to determine appropriate measurement factors, which will reduce a coefficient of error (CE) to less than 0.10 (Gundersen and Jensen, 1987). Then the full study of calculating the Nv was achieved automatically using an Olympus BX50 micro-scope fitted with a motorized stage and stereologer soft-ware.
Total neuronal number in dorsal and median raphe nuclei (Tn)
The absolute number of cells in each nucleus was calcu-lated by multiplying the cell density by the volume Tn = Nv x Tv
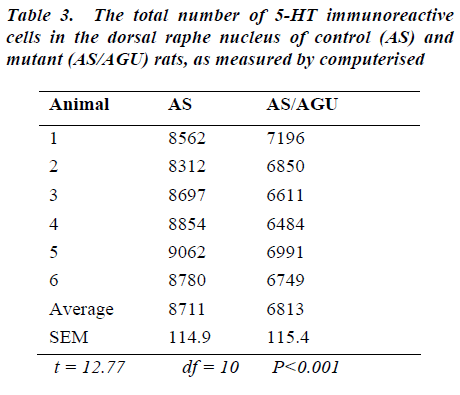
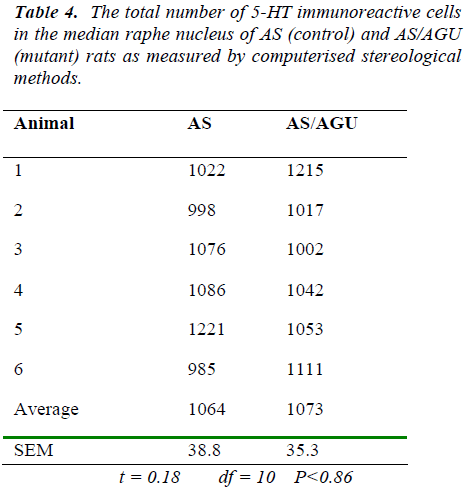
The total number of serotonergic cells in the dorsal raphe nucleus of the two groups is shown in Table 3. stereological methods.
Counts were obtained by computerised stereological means.
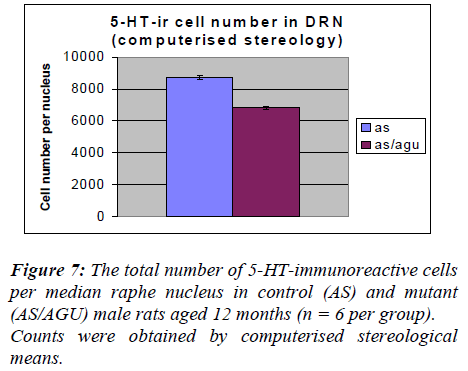
From Table 3 and Fig. 6, it is apparent that there is a very significant difference in the number of 5-HT-ir cells in the dorsal raphe nucleus of AS (control) and AS/AGU (mu-tant) rats. Mutants possess 22% fewer serotonergic cells than controls.
By contrast, we can see from Table 4 and Fig. 7 that there are no differences between AS (control) and AS/AGU (mutant) rats with respect to the numbers of 5-HT-ir cells in the median raphe nucleus.
Discussion
Two points require initial discussion. Firstly, are total cell numbers comparable to those found in previous studies and, secondly, are there differences in cell numbers be-tween control and mutant animals?
Cell numbers in the midbrain have been counted using conventional methods in different species. Descarries, Lemay, Doucet, Berger reported that the total population of serotonergic cells in the DRN of adult rat was 11,428 [29]. In cat DRN the number was estimated to be 24,257 [31]. More recent work has indicated that there are 11,500 serotonergic neurons in the DRN of adult rat while there are 1100 serotonergic neurons in the MRN (49). Cell numbers in primates may be more equal in the two nuclei. Thus, 5-HT-ir cell counts in the DRN and MRN of squir-rel monkeys showed that the numbers were 8,269 and 7,034 respectively (32).
In the present study the total numbers of serotonergic neu-rons were analysed using unbiased computerised sterolo-gical software and Cavalieri principle [47,48 respectively]. The data obtained by unbiased methods in the present study showed that serotonergic neuron numbers were 8,711 for the DRN and 1064 for the MRN of AS animals. These numbers are slightly lower than those re-ported with conventional methods [29] but this may be accounted for by age and strain difference as well as the different methodology employed.
To answer the second question, this study shows that there are fewer 5-HT-ir cells in the DRN of the mutant rat compared to the AS control rats. The reduction is ap-proximately 23% when the total numbers were obtained using unbiased methods and 22% when counted by more conventional means. By contrast with the DRN, counts of the MRN serotonergic cells showed no difference be-tween strains when measured either by conventional counts or by unbiased methods.
The extent of 5-HT cell loss in the mutant rat is less than found previously for DA-ergic cells (C. 40%) in 1 year old animals (49), but is consistent with a general deple-tion of aminergic cells.
The reduction of 5-HT cells in DRN along with 40% loss in DA-ergic cells in the mutant rat strongly suggests that the AS/AGU rat is a valuable model for studying neu-rodegenerative processes. Cell reductions of this magni-tude have been reported in many human conditions re-lated to movement disorders.
The change in cell numbers is likely to affect the target regions to which these cells project. The DRN is the prin-cipal source of striatal and cortical serotonin and axons from the DRN project mainly to the caudate-putamen, amygdala and substantia nigra pars compacta [4,6]. There are also projections to the thalamus [4] and to the locus coeruleus [6]. In contrast, the MRN was found to send projections mainly to the hippocampus and the anterior hypothalamus [4], and also the mammillary body [50]. Additionally, there was no evidence of axonal projection from the MRN to the striatum or amygdala [50] or the substantia nigra (6). Moreover, lesions of the DRN were found to cause much greater decreases of serotonin in the striatum than lesions of the MRN [51].
The reduction in cell numbers in the mutant DRN leads to hypothesis that this will affect levels of 5-HT in the DRN and the striatum.
References
- Chojnacka-Wojcik E. 5-Hydroxytryptamine in the cen-tral nervous system. Pol J Pharmacol 1995; 47 (3):219-235.
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 1978; 179 (3): 641-667.
- Morgan DG, May PC, Finch CE. Dopamine and sero-tonin systems in human and rodent brain: effects of age and neurodegenerative disease. J Am Geriatr Soc 1987; 35(4): 334-345.
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 1978; 179 (3):641-667.
- van der Kooy D, Hattori T. Dorsal raphe cells with collateral projections to the caudate-putamen and sub-stantia nigra: a fluorescent retrograde double labeling study in the rat. Brain Res 1980; 186 (1):1-7.
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol 1986; 243 (3): 363-380.
- Bobillier P, Pettijean F, Salvert D, Ligier M, Seguin S. Differential projections of the nucleus raphe dorsalis and nucleus raphe centralis as revealed by autoradio-graphy. Brain Res 1975; 85 (2): 205-210.
- Kohler C, Chan-Palay V, Steinbusch H. The distribu-tion and origin of serotonin-containing fibers in the septal area: a combined immunohistochemical and flu-orescent retrograde tracing study in the rat. J Comp Neurol 1982; 209 (1): 91-111.
- McQuade R, Sharp T. Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J Neurochem 1997; 69 (2): 791-796.
- Kosofsky BE, Molliver ME. The serotoninergic inner-vation of cerebral cortex: different classes of axon ter-minals arise from dorsal and median raphe nuclei. Syn-apse 1987; 1 (2): 153-168.
- Mamounas LA, Mullen CA, O'Hearn E, Molliver ME. Dual serotoninergic projections to forebrain in the rat: morphologically distinct 5-HT axon terminals exhibit differential vulnerability to neurotoxic amphetamine derivatives. J Comp Neurol 1991; 314 (3): 558-586.
- Mamounas LA, Molliver ME. Evidence for dual sero-tonergic projections to neocortex: axons from the dor-sal and median raphe nuclei are differentially vulner-able to the neurotoxin p-chloroamphetamine (PCA). Exp Neurol 1988; 102 (1): 23-36.
- Blier P, Serrano A, Scatton B. Differential responsive-ness of the rat dorsal and median raphe 5-HT systems to 5-HT1 receptor agonists and p-chloroamphetamine. Synapse 1990; 5 (2): 120-133.
- Sinton CM, Fallon SL. Electrophysiological evidence for a functional differentiation between subtypes of the 5-HT1 receptor. Eur J Pharmacol 1988; 157 (2-3): 173-181.
- Hillegaart V. Effects of local application of 5-HT and 8-OH-DPAT into the dorsal and median raphe nuclei on motor activity in the rat. Physiol Behav 1990; 48 (1): 143-8.
- Invernizzi R, Carli M, Di Clemente A, Samanin R. Administration of 8-hydroxy-2-(Di-n-propylamino) tet-ralin in raphe nuclei dorsalis and medianus reduces se-rotonin synthesis in the rat brain: differences in potency and regional sensitivity. J Neurochem 1991; 56 (1): 243-247.
- Casanovas JM, Lesourd M, Artigas F. The effect of the selective 5-HT1A agonists alnespirone (S-20499) and 8-OH-DPAT on extracellular 5-hydroxytryptamine in different regions of rat brain. Br J Pharmacol 1997; 122 (4): 733-741.
- Dilts RP, Boadle-Biber MC. Differential activation of the 5-hydroxytryptamine-containing neurons of the midbrain raphe of the rat in response to randomly pre-sented inescapable sound. Neurosci Lett 1995; 199 (1): 78-80.
- Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology 1997; 36 (4-5): 735-741.
- Hensler JG, Ferry RC, Labow DM, Kovachich GB, Frazer A. Quantitative autoradiography of the serotonin transporter to assess the distribution of serotonergic projections from the dorsal raphe nucleus. Synapse 1994; 17 (1): 1-15.
- Hajos M, Gartside SE, Sharp T. Inhibition of median and dorsal raphe neurones following administration of the selective serotonin reuptake inhibitor paroxetine. Naunyn Schmiedebergs Arch Pharmacol 1995; 351 (6): 624-629.
- Jellinger KA. Pathology of Parkinson's disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 1991; 14 (3):153-97.
- Hedera P, Whitehouse PJ. Neurotransmitters in neurod-egeneration. In: Calne DB, editor. Neurodegenerative disease 1994.
- Payne AP, Campbell JM, Russell D, Favor G, Sutcliffe RG, Bennett NK, et al. The AS/AGU rat: a spontaneous model of disruption and degeneration in the nigrostria-tal dopaminergic system. J Anat 2000; 196 (Pt 4): 629--633.
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia 1964; 20(7):398-399.
- Beaudet A, Descarries L. Quantitative data on sero-tonin nerve terminals in adult rat neocortex. Brain Res 1976; 111 (2):301-309.
- Nygren LG, Olson L. Intracisternal neurotoxins and monoamine neurons innervating the spinal cord: acute and chronic effects on cell and axon counts and nerve terminal densities. Histochemistry 1977; 52 (4): 281-306.
- Lorez HP, Saner A, Richards JG. Evidence against a neurotoxic action of halogenated amphetamines on se-rotoninergic B9 cells. A morphometric fluorescence histochemical study. Brain Res 1978; 146 (1):188-194.
- Descarries L, Lemay B, Doucet G, Berger B. Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience 1987; 21 (3): 807-824.
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev 1992; 72 (1): 165-229.
- Wiklund L, Leger L, Persson M. Monoamine cell dis-tribution in the cat brain stem. A fluorescence histo-chemical study with quantification of indolaminergic and locus coeruleus cell groups. J Comp Neurol 1981; 203 (4): 613-647.
- Hatzidimitriou G, McCann UD, Ricaurte GA. Altered serotonin innervation patterns in the forebrain of mon-keys treated with (+/-) 3,4-methylenedioxymethamphe-tamine seven years previously: factors influencing ab-normal recovery. J Neurosci 1999; 19 (12):5096-107.
- Agid Y, Javoy-Agid, F. and Ruberg, M, editor. Bio-chemistry of neurotransmitters in parkinson's disease. London: Butterworth; 1987.
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 1984; 134 (Pt 2): 127-136.
- Gundersen HJ. Stereology of arbitrary particles. A re-view of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc 1986; 143 (Pt 1): 3-45.
- Swaab DF, Uylings HB. Comments on review by Cole-man and Flood, 'Neuron Numbers and Dendritic Extent in Normal Aging and Alzheimer's Disease.' Density measures: parameters to avoid. Neurobiol Aging 1987; 8 (6): 574-576.
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, et al. The new stereological tools: disector, fractionator, nucleator and point sam-pled intercepts and their use in pathological research and diagnosis. Apmis 1988; 96 (10): 857-881.
- Bolender RP, Charleston J, Mottet K, McCabe JT. Quantitative morphology of the nervous system: ex-panding horizons. Anat Rec 1991; 231 (4): 407-415.
- Oorschot DE, Peterson DA, Jones DG. Neurite growth from, and neuronal survival within, cultured explants of the nervous system: a critical review of morphometric and stereological methods, and suggestions for the fu-ture. Prog Neurobiol 1991; 37 (6): 525-546.
- Mayhew TM. A review of recent advances in stereol-ogy for quantifying neural structure. J Neurocytol 1992; 21 (5): 313-328.
- Oorschot DE. Are you using neuronal densities, synap-tic densities or neurochemical densities as your defini-tive data? There is a better way to go. Prog Neurobiol 1994; 44 (3): 233-247.
- Pakkenberg B, Moller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in pa-tients with Parkinson's disease estimated with an unbi-ased stereological method. J Neurol Neurosurg Psy-chiatry 1991; 54 (1): 30-33.
- Bjugn R. The use of the optical disector to estimate the number of neurons, glial and endothelial cells in the spinal cord of the mouse--with a comparative note on the rat spinal cord. Brain Res 1993; 627 (1): 25-33.
- Raadsheer FC, Oorschot DE, Verwer RW, Tilders FJ, Swaab DF. Age-related increase in the total number of corticotropin-releasing hormone neurons in the human paraventricular nucleus in controls and Alzheimer's disease: comparison of the disector with an unfolding method. J Comp Neurol 1994; 339 (3): 447-457.
- Hsu SM, Raine L, Fanger H. The use of antiavidin an-tibody and avidin-biotin-peroxidase complex in immu-noperoxidase technics. Am J Clin Pathol 1981; 75 (6): 816-821.
- Grube D. Immunoperoxidase methods: increased effi-ciency using fluorescence microscopy for 3,3-diamino-benzidine (DAB) stained semithin sections. Histoche-mistry 1980; 70 (1): 19-22.
- Wreford NG. Theory and practice of stereological techniques applied to the estimation of cell number and nuclear volume in the testis. Microsc Res Tech 1995; 32 (5):423-436.
- Mayhew TM. A review of recent advances in stereol-ogy for quantifying neural structure. J Neurocytol 1992; 21 (5): 313-328.
- Payne AP, Sutcliffe RG, Campbell JM, Favor G, Rus-sell D, Bennett NK, et al. Disordered locomotion in the AS/AGU mutant rat and the effects of L-dopa or fetal midbrain grafts. Mov Disord 1998; 13 (5): 832-834.
- Bobillier P, Seguin S, Degueurce A, Lewis BD, Pujol JF. The efferent connections of the nucleus raphe cen-tralis superior in the rat as revealed by radioautography. Brain Res 1979; 166 (1): 1-8.
- Jacobs BL, Trimbach C, Eubanks EE, Trulson M. Hip-pocampal mediation of raphe lesion- and PCPA-induced hyperactivity in the rat. Brain Res 1975; 94 (2): 253-261.