Research Article - Biomedical Research (2017) Volume 28, Issue 8
Celecoxib and quercetin induce apoptosis in human hepatocarcinoma
Chun-Peng Yu1, Ruo-Guang Qiu2, Lei Shi3 and Jun Liang4,5*
1Department of Interventional Medicine, the Affiliated Hospital of Qingdao University, Shangdong, PR China
2Department of Computed Tomography, Jimo Hospital of Traditional Chinese Medicine, Shangdong, PR China
3Department of Emergency, the Affiliated Hospital of Qingdao University, Shangdong, PR China
4Department of Oncology, the Affiliated Hospital of Qingdao University, Shangdong, PR China
5Department of Oncology, Peking University International Hospital, Beijing, PR China
- *Corresponding Author:
- Jun Liang
Department of Oncology
The Affiliated Hospital of Qingdao University, PR China
Accepted on December 26, 2016
Abstract
Introduction: Celecoxib and quercetin are reported as an anticancer agent in different malignancies. In present investigation, we have screened celecoxib and quercetin in different human hepatocarcinoma cells such as MDBK, HepG2 and Huh-7 for anticancer activity.
Methods: In present study, human hepatocellular carcinoma cells grown on medium treated with quercetin and celecoxib. In this effect on apoptosis and cell growth has been evaluated with help of by MTT assay and apoptosis in MDBK, HepG2 and Huh-7 cells and DAPI staining in MDBK cells. The apoptotic pathway induction by treatment group assessed with Bcl-2 and Bax protein expressional changes.
Results: Quercetin and celecoxib significantly increased apoptosis in human hepatocarcinoma MDBK, HepG2 and Huh-7 cells. Further, Bcl-2 and Bax-1 protein expression has been studied in MDBK cells exposed to treatments. The immunofluorescence of DAPI showed inhibition in proliferation human liver cancer cells by celecoxib and quercetin.
Conclusion: In conclusion, quercetin and celecoxib showed increase in apoptosis and decrease in hepatocellular proliferation. Further, immunofluorescence showed increase in fluorescence levels of treatment group confirmed apoptosis. The apoptosis might be through caspase pathway through changes in Bcl-2 and Bax proteins.
Keywords
Quercetin, Celecoxib, Apoptosis, Bcl-2, MTT.
Introduction
In United States of America, hepatocellular carcinoma increased around 71% in last few decades. Millions of people died annually because of Hepatocellular Carcinoma (HCC) globally which established it as leading cause of cancer related death. It may occur due to environmental factors such as hepatitis, carcinogen as well lifestyle and population with high consumption of alcohol. Liver cancer has less chances of diagnosis at earlier stages made it fatal disease. Oxidative stress and pro-inflammatory mediators are associated with various disease conditions such as diabetes and cancer. It has been reported oxidative stress and inflammatory mediators has its role in liver cancer [1-3]. HCC lacks for standard therapy by which researcher exploring different molecular targets for better treatment options. Molecular targets with differential action related to metastatic mechanism and apoptosis need to be explored. Oxidative stress and inflammation induces mechanism of metastasis hence antioxidants and antiinflammatory agents will be better combination.
Celecoxib, selectively inhibiting COX-2 reported to act as proliferation inhibitor through induction of apoptosis in cancerous cell lines and animal models of bladder, cancer and colon. Celecoxib also prompted apoptosis through death receptors D5 in human NSCLC (Non-Small-Cell Lung Carcinoma) cells [4-8]. Quercetin, a polyphenolic flavonoid constituent of vegetables and fruits having anti-oxidant, cardioprotective, anti-carcinogenic and bacteriostatic pharmacological actions [9,10]. There are several reports which shows Quercetin anti-cancer action in wide variety of cancers including colon, breast, lung, liver and oral cancers. Earlier studies also indicate that Quercetin induces apoptosis in MCF-7 breast cancer cells. Quercetin was found to arrest the cell cycle at the sub-G1. Quercetin is also reported to regulate a number of signalling proteins including MAPKs, Akt/protein kinase B, and PI3K. Celecoxib and quercetin has been established apoptotic inducer through Bcl-2 and Bax pathway in cell lines [11-14].
The present study Celecoxib and quercetin were found to be very effective anticancer activity in hepatocellular carcinoma cell lines.
Materials and Methods
Chemicals
Experimental Chemicals such as Dulbecco's modified Eagle's medium (DMEM), Dimethyl Sulfoxide (DMSO), penicillin, propidium iodide, streptomycin and tryspin-EDTA for were purchased from Sigma Inc. (St. Louis, MO, USA). Fetal Bovine Serum (FBS) was obtained from TBD Biotechnology Development (Tianjin, China). All other chemicals purchased from Sigma Ltd USA with highest grade of purity.
Cell culture
HepG2, MDBK and Huh-7 human hepatocarcinoma cell lines were purchased from American Type Cell Culture Collection (ATCC). Cell lines were grown on Dulbecco’s modified eagle medium supplemented with fetal bovine serum (10%), antibiotics such as penicillin and streptomycin, at 37°C carbogen supply. Cells were grown upto 90% confluence observed with inverted microscope.
Antiproliferative assay
Human HCC cells grown on culture medium and trypsinised and counted by cell count method using tryphan blue on haemocytometer. Cells were trypsinised and solubilized for counting with haemocytometer. After seeding cells were settled to grow and allowed to attach on surface and medium were regularly changed with 0.1 ml. Culture cells were treated with 0.1 μl quercetin and celecoxib in dose dependent manner were DMSO (0.1%) was used as control and cells were supplemented with 5% of carbon dioxide at 37°C for 72 hours. The assay was performed in triplicates. The effect of drug treatment on proliferation cells was determined with help of methylene blue method measuring absorbance by ELISA plate reader at 660 nm.
MTT cell viability assay
The proliferative or cytotoxic effects of drugs were evaluated using the MTT tetrazolium assay, which involves the reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) to produce formazan crystals. HCC were seeded at a density of 3 × 103 cells/well into 96 well tissue culture plates. The cells were then treated combination of drugs in triplicate and incubated for at 37°C. Thereafter, 20 μl of MTT (5 mg ml-1 in PBS) was added to each well and incubated for 4 h in dark at 37°C. After 4 h, the formazan crystals were solubilized in 50 μl of DMSO and incubated at room temperature for 15 min. Absorbance was measured at 570 nm in multimode plate reader (ModelVictor X3, Perkin Elmer) (Table 1). Absorbance given by untreated cells was considered as 100% cell growth.
| Drug | Cell Viability in MDBK cells | Cell Viability in HepG2 cells | Cell Viability in Huh-7 cells | |||
|---|---|---|---|---|---|---|
| 6 h | 12 h | 6 h | 12 h | 6 h | 12 h | |
| Vehicle Control 10 umol/l | 2.48 ± 0.60 | 3.44 ± 0.98 | 2.55 ± 0.45 | 3.58 ± 0.84 | 2.7 ± 0.55 | 3.6 ± 0.9 |
| Vehicle Control 20 umol/l | 2.5 ± 0.55 | 3.55 ± 0.90 | 2.64 ± 0.58 | 3.65 ± 0.70 | 2.8 ± 0.6 | 3.75 ± 0.84 |
| Celecoxib 10 umol/l | 7.40 ± 1.1* | 9.3 ± 1.27* | 7.2 ± 1.06* | 9.1 ± 1.21* | 7.10 ± 1.16* | 9.25 ± 1.2* |
| Celecoxib 20 umol/l | 8.25 ± 1.2* | 9.8 ± 1.3* | 8.4 ± 1.05* | 9.6 ± 1.26* | 8.38 ± 1.24* | 10.8 ± 1.4* |
| Quercetin 10 umol/l | 5.35 ± 1.34* | 6.3 ± 1.42* | 5.45 ± 1.14* | 6.5 ± 1.34* | 5. 5 ± 1.4* | 6.6 ± 1.2* |
| Quercetin 20 umol/l | 5.85 ± 1. 4* | 6.8 ± 1.56* | 5.9 ± 1. 34* | 7.4 ± 1.6* | 5.7 ± 1. 3* | 8.2 ± 1.64* |
| Celecoxib ± Quercetin 10 umol/l | 8.2 ± 1.1* | 9.9 ± 1.26* | 8.4 ± 1.26* | 10.4 ± 1.28* | 8.9 ± 1.36* | 11.3 ± 1.21* |
| Celecoxib ± Quercetin 20 umol/l | 10.2 ± 1.3* | 11.6 ± 1.4* | 10.6 ± 1.15* | 11.9 ± 1.6* | 10.9 ± 1.45* | 11.8 ± 1.55* |
Experimental values are expressed as mean SEM, n=6-8, P value less than 0.05 was statistically significant.
Table 1. Effect of Celecoxib and Quercetin on cell viability HCC cells.
DAPI staining
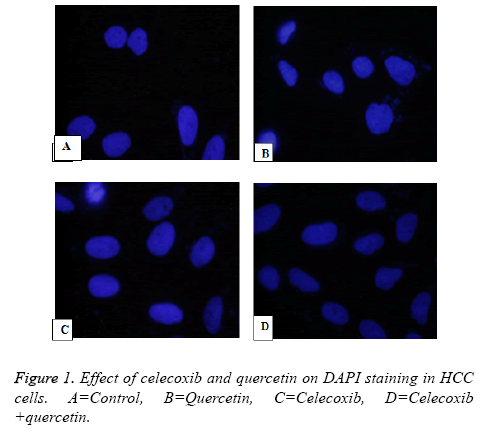
Human HCC MDBK Cells were fixed on glass slide with cold methanol after washing with PBS buffer. The slides were kept for fixing for 15 minutes at room temperature. After fixation slide was washed with cold PBS twice and blocked with goat serum and further treated with Triton-X and 100 ml cold PBS for 60 min at room temperature. The slide was treated with primary antibody caspase. After 12 h slides were washed with PBS and 0.1% Tween-20 and incubated with secondary antibody horse radish peroxidase Alexa Fluor 594 for 1 h. Further slides were mounted with DAPI (4, 6-diamidino-2- phenylindole).
Expression level of Bax, Bcl-2 and p53
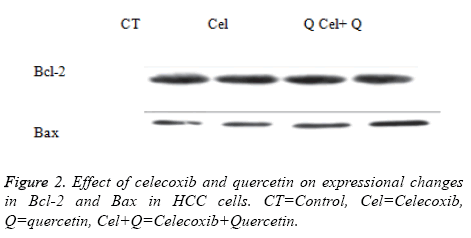
Briefly Human HCC cells were incubated with the combination of drugs for indicated times. The cells were washed once with DPBS. The cells were harvested and lysed in protein lysis buffer containing protease inhibitors (10 μg ml-1 leupeptin, 2 mM Phenylmethylsulfonylfluoride (PMSF) and 2 mM Na3VO4). The protein concentration was estimated by Bradford reagents. Equal protein was run on 10% SDS gel. The proteins were transferred to a nitrocellulose membrane. The blot was subjected to immunodetection with antibody against Bcl-2, Bax, p53. The densitometric analysis was performed with molecular analyst and β-actin was used as positive control.
Statistical analysis
All result data was expresses as mean ± SEM in the different treatment groups were compared. Statistical analyses were performed by Analysis of Variance (ANOVA). Significance level measured as P value less than 0.05 was considered significant.
Results
Effects of quercetin co incubation with celecoxib on HCC cell proliferation
MTT assay was performed to analyse the effect of drugs combination on the proliferation of human hepatocarcinoma. The IC50 of the drug is concentration at which 50% inhibition of cell proliferation has been observed. The IC50 values of querctin and celecoxib in human HepG2 cells was 3.40 ± 0.026 μg/ml and 3.10 ± 0.042 μg/ml. Quercetin and celecoxib also exhibited antiproliferative effect MDBK cells with an IC50 values of 5.96 ± 0.045 μg/ml and 7.1 ± 0.08 μg/ml respectively. The IC50 values of quercetin and celecoxib in Huh-7 cell was 7. 6 ± 0.062 μg/ml and 7.7 ± 0.074 μg/ml.
Beneficial effects of combination of quercetin and celecoxib on apoptosis
In investigation of cell death was either because of apoptosis or necrosis effect of quercetin and celecoxib on human HepG2, MDBK and MG-62 hepatocellular carcinoma cells were observed. In this case DMSO was vehicle control while Cisplatin is used as standard treatment. In the present investigation it has been observed that anticancer action is trough induction of apoptosis as fragmented DNA get labelled with 12dUTP in the nucleus as yellow fluorescence level significantly increased from 48 h to 72 h.
Quercetin and celecoxib co-treatment and caspase activation
In the slide caspase treated MDBK cells treated with quercetin, celecoxib and combination showed increase in caspase levels indicates combination of Quercetin and celecoxib resulted in significant increase in apoptosis through activation of caspase.
Caspases activation is an important event during apoptosis, There is doubt which caspase was analysed, is it caspase-3. Since the caspase as present as pre-caspase form and upon cleavage they are activated. It is important to see the active fragment of caspase, Immunoblotting is ideal to show the activation of caspase, or used specific antibody for activated caspase-3 which does not bind to precursor caspase.
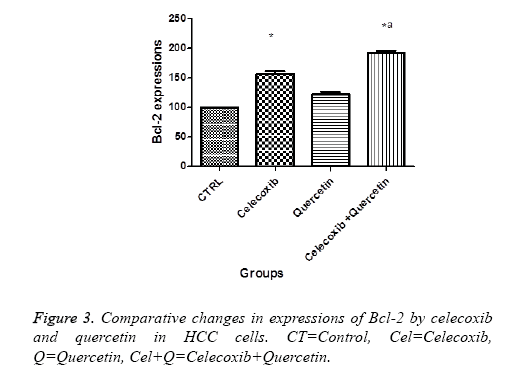
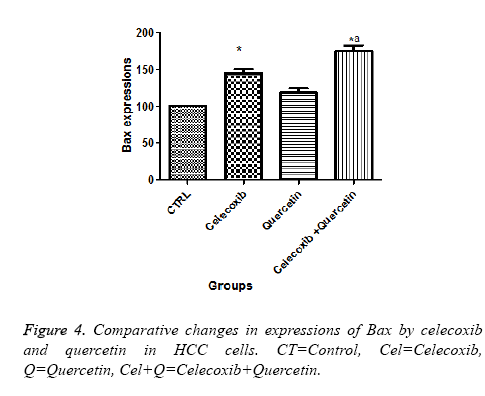
Effect of quercetin and celecoxib on Bax and Bcl-2 protein expressions
The present investigation is focused to analyse apoptotic cell death induced by quercetin and celecoxib in different hepatocarcinoma cells (Table 2). The mechanism of apoptosis mostly associated with activation of caspase and altered expression of p53, Bax and Bcl-2 protein. In present study, human MDBK cells were treated with quercetin and celecoxib for 0, 3, 6, 12 and 48 h. As seen from the Figures 1-4 quercetin and celecoxib combination induced increased expression of Bax which proapoptotic while Bcl-2 (anti-apoptotic protein expression) get decreased. In the present study quercetin and celecoxib treatment group also induced p53 tumor inhibitory protein. It is not clear if there is increase or decrease in p53 level since p53 is tumor suppressor protein it is expected that the p53 level is decreased as a result the DNA damage repair is inhibited and induce apoptosis.
| Drug | Apoptotic cell death in MDBK cells | Apoptotic cell deathHepG2 cells | Apoptotic cell death Huh-7 cells |
|---|---|---|---|
| Vehicle control 10 umol/l | 2.50 ± 1.12 | 2.60 ± 1.4 | 2.7 ± 1.25 |
| Celecoxib 10 umol/l | 8.1 ± 1.7* | 8.4 ± 1.2* | 9.2 ± 1.25* |
| Quercetin 10 umol/l | 6.3 ± 1.44* | 6.5 ± 1.28* | 6.7 ± 1.26* |
| Celecoxib+quercetin 10 umol/l | 9.6 ± 1.62* | 9.3 ± 1.29* | 9.4 ± 1.35* |
Table 2. Effect of celecoxib and quercetin on apoptotic cell death HCC cells after 12 h.
Discussion
The present study was focus to elucidate anticancer mechanism of quercetin and celecoxib in various human hepatocarcinoma cell lines. The various leads from natural resources have been explored for chemo preventive action of hepatic carcinoma [15-17]. Quercetin and celecoxib was also found to exert anticancer activity and cytoprotective activity by its antiinflammatory, inhibition of oxidative stress and apoptosis. Quercetin is classified as a bioflavonoids present in various fruits and vegetables [18,19]. It is found that apple an onion has the highest amount of quercetin. In addition to anticancerous properties anti-oxidant and anti-artherogenic properties are also associated with quercetin. One of the study shows quercetin induced apoptosis in HeLa cell through an ERalpha-dependent mechanism involving caspase- and p38 kinase activation [20]. In another report it was shown that quercetin treatment on human breast carcinoma SK-Br3, MDA-MB-453, and MDA-MB-231 generates mild DNA damage and activates Chk2 kinase, which plays a crucial role in p21 induction. In addition, transcriptional repression of cyclin B1 is revealed as another mechanism of the antiproliferation effect of a low dose of quercetin [21]. Recently quercetin was shown to induced cytotoxicity in leukemic and breast cancer cells in a dose-dependent manner. It was observed that quercetin arrest the cell cycle progression in S phase tested cancer cells [22]. Moreover, it is also observed that quercetin induces apoptosis in a caspase-3-dependent pathway by inhibiting Cox-2 expression and regulates the expression of downstream apoptotic components, including Bcl-2 and Bax. Therefore Quercetin is proposed as a potent and promising medicine which might be safely used in leukaemia therapy [23]. At the same time there are numbers of reports where celecoxib is being used to target apoptosis in cancer cells. Celecoxib is basically an anti-inflammatory drug with potent anti-tumor activity against various human epithelial tumor types including colorectal, breast, non-small cell lung, and prostate cancers, this drug is found to very effective in patients with familial adenomatous polyposis. Celecoxib was reported as anticancer in colon, breast, lung testes and ovary cancer models while quercetin was also protective in various cancer models such as head, lung, neck and colon cancer. Celcoxib was selective cox-inhibitor and quercetin has an antioxidant action with potential to induce apoptosis. These drugs have been shown to effective drug resistant cancer cells even with high expression of Bcl-2. However there is big controversy with the effect these drugs in in-vitro and in animal studies. This could be because of low oral bioavailability of these drugs. Therefore there is the need to investigate the mechanism involved with these drugs in combination, since drug resistance is one of the biggest challenges. In present study, HCC were taken for the investigation. It was observed in hepatocellular carcinoma, OSU-03012 resulted in autophaghic cell death by partly accumulation of ROS instead of apoptosis. In HCC, COX-2 inhibitors induce different apoptosis signalling pathway through death receptor and caspase activation. Celecoxib through decreased production PGE2 and increased PPAR inhibit in HCC cells. Cox-2 inhibitor celecoxib and MG132 synergistically inhibited proliferation and proapoptotic in hepatocellular carcinoma cells. In the present study the effect of quercetin and celecoxib induced apoptosis in human hepatocarcinoma cells and its mechanism was studied. It was observed that celecoxib and quercetin showed inhibition of hepatocellular proliferation as alone as well in combination. The synergistic effect of cell proliferation was induced through apoptosis. Study on protein expression indicated quercetin and celecoxib induced apoptosis through changes in expression of Bcl-2 and bax expressions.
In conclusion, present study combination of celecoxib and quercetin confirmed for anticancer action through apoptosis which might be resultant caspase mediated Bcl-2 and bax changes. Combination of celecoxib and quercetin resulted in synergistic action in human hepatocellular carcinoma cells. In addition these findings indicate new potential chemopreventive actions of flavonoids in combination with antiinflammatory drug on cancer growth.
Conflict of Interest
There are no conflicts.
References
- Dajas F. Life or death: neuroprotective and anticancer effects of quercetin. J Ethnopharmacol 2012; 143: 383-396.
- Kern MA, Haugg AM, Koch AF, Schilling T, Breuhahn K, Walczak H, Fleischer B, Trautwein C, Michalski C, Schulze-Bergkamen H, Friess H. Cyclooxygenase-2 inhibition induces apoptosis signalling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res 2006; 66: 7059-7066.
- Alía M, Mateos R, Ramos S, Lecumberri E, Bravo L, Goya L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur J Nutr 2006; 45: 19-28.
- Grubbs CJ, Lubet RA, Koki AT, Leahy KM, Masferrer JL, Steele VE, Kelloff GJ, Hill DL, Seibert K. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res 2000; 60: 5599-5602.
- Orengo IF, Gerguis J, Phillips R, Guevara A, Lewis AT, Black HS. Celecoxib, a cyclooxygenase 2 inhibitor as a potential chemopreventive to UV-induced skin cancer: a study in the hairless mouse model. Arch Dermatol 2002; 138: 751-755.
- Shen F, Weber G. Synergistic action of quercetin and genistein in human ovarian carcinoma cells. Oncol Res 1997; 9: 597-602.
- Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through AKT activation: Evidence for AKT inhibition in celecoxib-induced apoptosis. Hepatology 2003; 38: 756-768.
- Zhu J, Huang JW, Tseng PH, Yang YT, Fowble J, Shiau CW, Shaw YJ, Kulp SK, Chen CS. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res 2004; 64: 4309-4318.
- Welder A, Acosta D. Enzyme leakage as an indicator of cytotoxicity in culture cells. In vitro toxicity indicators: methods in toxicology. New York Academic Press 1994: 46-49.
- Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Rad Biol Med 1999; 27: 612-616.
- Sunjic SB, Ana C, Filip R, Renate W, Neven Z. The influence of 4-hydroxy-2-nonenal on proliferation, differentiation and apoptosis of human osteosarcoma cells. Biofactors 1999; 24: 141-148.
- Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Nat Cancer Inst 2004; 96: 1769-1780.
- Li W, Xiao J, Zhou X, Xu M, Hu C, Xu X, Lu Y, Liu C, Xue S, Nie L, Zhang H. STK4 regulates TLR pathways and protects against chronic inflammation-related hepatocellular carcinoma. J Clin Investig 2015; 125: 4239-4254.
- Granado AB, Martín MA, Bravo L, Goya L, Ramos S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). J Nutr 2006; 136: 2715-2721.
- Cusimano A, Azzolina A, Iovanna JL, Bachvarov D, McCubrey JA, DAlessandro N, Montalto G, Cervello M. Novel combination of celecoxib and proteasome inhibitor MG132 provides synergistic antiproliferative and proapoptotic effects in human liver tumor cells. Cell Cycle 2010; 9: 1399-1410.
- Gao M, Yeh PY, Lu YS, Hsu CH, Chen KF, Lee WC, Feng WC, Chen CS, Kuo ML, Cheng AL. OSU-03012, a novel celecoxib derivative, induces reactive oxygen species–related autophagy in hepatocellular carcinoma. Cancer Res 2008; 68: 9348-9357.
- Cervello M, Bachvarov D, Cusimano A, Sardina F, Azzolina A, Lampiasi N, Giannitrapani L, McCubrey JA, Montalto G. COX-2-dependent and COX-2-independent mode of action of celecoxib in human liver cancer cells. Omics J Integr Biol 2011; 15: 383-392.
- Cui W, Yu CH, Hu KQ. In vitro and in vivo effects and mechanisms of celecoxib-induced growth inhibition of human hepatocellular carcinoma cells. Clin Cancer Res 2005; 11: 8213-8221.
- Roy KR, Reddy GV, Maitreyi L, Agarwal S, Achari C, Vali S, Reddanna P. Celecoxib inhibits MDR1 expression through COX-2-dependent mechanism in human hepatocellular carcinoma (HepG2) cell line. Cancer Chemother Pharmacol 2010; 65: 90.
- Galluzzo P, Martini C, Bulzomi P, Leone S, Bolli A, Pallottini V, Marino M. Quercetin-induced apoptotic cascade in cancer cells: Antioxidant versus oestrogen receptor a-dependent mechanisms. Mol Nutr Food Res 2009; 53: 699-708.
- Jeong JH, An JY, Kwon YT, Rhee JG, Lee YJ. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J Cell Biochem 2009; 106: 73-82.
- Srivastava S, Somasagara RR, Hegde M, Nishana M, Tadi SK, Srivastava M, Raghavan SC. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep 2016; 6.
- Niu G, Yin S, Xie S, Li Y, Nie D, Ma L, Wu Y. Quercetin induces apoptosis by activating caspase-3 and regulating Bcl-2 and cyclooxygenase-2 pathways in human HL-60 cells. Acta Biochim Biophysica Sinica 2011; 43: 30-37.