Research Article - Annals of Cardiovascular and Thoracic Surgery (2021) Volume 4, Issue 1
Catheter-Directed Thrombolysis for Patients with Intermediate-high-risk Pulmonary Embolism: Is It Safe and Effective?
Abdelmaksoud A Elganady1*, Abeer M Shawky1 and Ahmed Dawood2
1Cardiology Department, Al-Azhar University, Egypt
2Cardiology Department, National Heart Institute, Egypt
- *Corresponding Author:
- Abd-Elmaksoud Elganady
Department of Cardiology
Al-Azhar University
Egypt
E-mail: elganady_1965@hotmail.com
Accepted: September 29, 2020
Abstract
Background: Intermediate-high-risk Pulmonary Embolism (PE) is common and carries a risk of progression to hemodynamic collapse and death. Catheter-Directed Thrombolysis (CDT) has become a recommended treatment option in intermediate-high-risk PE. For such cases, CDT ensures recovery of echocardiographic and haemodynamic parameters and may be characterized by a better safety profile. We aimed to clarify the effectiveness of CDT in improving the short term disease-outcomes without increasing the risk of bleeding in intermediate-high-risk PE. Methods and Results: This is a single-centre retrospective observational study of consecutive fifty patients with a mean age of 55+04 with a primary diagnosis of sub massive PE with high-risk features, admitted to an intensive care unit between November 2018 and April 2020. We identified patients with intermediate-high-risk PE to be treated with CDT of Tissue Plasminogen Activator (tPA), by our Pulmonary Embolism Response Team (PERT). We compared the outcome of patients before and after treatment with CDT. There was a significant improvement in symptoms and a decrease in laboratory markers of myocardial injury (proBNP and Troponin-T). Also, comparing baseline echocardiographic parameters including RV:LV ratio, RV-TAPSE and sPAP with the same parameters after completion of CDT revealed a significant reduction in these parameters with improvement in right ventricular function. There were no in-hospital deaths secondary to haemorrhage or procedure-related complications in the studied patients. During the follow-up range of three months after CDT, only 3 minor bleeding episodes were encountered but no hemodynamic decompensation, recurrent venous thromboembolism, major bleeding complications or death. Conclusion: CDT can be used in patients with intermediated-high-risk PE safely and effectively. Future studies will further define the role of CDT in comparison to other revascularization strategies in the management of PE patients at increased risk.
Keywords
Pulmonary embolism, Catheter-directed thrombolysis, Pulmonary embolism response team, Tissue plasminogen activator.
Abbreviations
PE: Pulmonary Embolism; CDT: Catheter-Directed Thrombolysis; tPA: Tissue Plasminogen Activator; PERT: Pulmonary Embolism Response Team; CTEPH: Chronic Thromboembolic Pulmonary Hypertension; BNP: Brain Natriuretic Peptide; CTPA: Computed Tomographic Pulmonary Angiography; RV-TAPSE: Right Ventricular Systolic Function-Tricuspid Annular Plane Systolic Excursion; TR: Tricuspid Regurgitation; sPAP: Systolic Pulmonary Artery Pressure.
Introduction
Pulmonary Embolism (PE) is one of the most common causes of morbidity and mortality worldwide, making an early diagnosis and treatment is crucial. Pulmonary embolisms are classified into three main risk groups: low risk, sub-massive (intermediate), and massive (high risk). Intermediate (sub-massive) PEs are further sub-divided into intermediate-high and intermediatelow risk PEs [1,2]. For each category, the diagnosis of PE is made based on clinical presentation, abnormal biomarkers, echocardiography/imaging evidence and hemodynamic instability. An intermediate high risk PE is diagnosed when there is a characteristic of an embolus/emboli causing right ventricular strain without significant hypotension (systolic blood pressure <90 mmHg) and elevated biomarkers [3].
Risk stratification guides subsequent treatment and the expected outcome of a patient presenting with PE. For patients with intermediate-high-risk PE, signs of right ventricular overload or dysfunction are associated with unfavourable short-term prognosis [4]. Thromboemboli in patients with intermediatehigh- risk pulmonary embolism increase pulmonary vascular resistance by obstruction and vasoconstriction. Also, residual thrombi and persistent right ventricular dysfunction may contribute to post-PE functional impairment, and influence the risk of developing Chronic Thromboembolic Pulmonary Hypertension (CTEPH). This deteriorates symptoms and hemodynamic status in those groups of the patient that leads to increase risk of mortality. According to one of meta-analysis focusing on intermediate-risk patients, systemic thrombolysis can improve survival but with a higher risk of fatal (or intracranial) haemorrhage, when compared with anticoagulation alone [5]. Hence, reperfusion therapy can reverse the haemodynamic burden that acute PE imposes on the pulmonary circulation and the right heart but on the expense of increased bleeding risk [6].
Catheter-Directed Thrombolysis (CDT) is one of the reperfusion treatment options for sub massive pulmonary embolisms with high-risk features, given its ability to rapidly reduce right heart strain with an acceptably low rate of major haemorrhagic complication [7]. Therefore, CDT is emerging as a promising, and possibly safer option to improve short-term effectiveness in intermediate-high-risk PE patients [8]. CDT involves the infusion of a low dose of thrombolytic agent intravascular adjacent to the clot burden through a percutaneous trans catheter. Thrombolytic agents dissolve blood clots by activating plasminogen. Clot dissolution and relieving obstruction may restore pulmonary arterial flow, which improves right ventricular hemodynamics and cardiac output [9]. This effect may have a positive impact on patient outcomes.
In our study, we aimed to clarify the clinical significance and effectiveness of catheter-directed thrombolysis in improving short-term disease-specific quality of life without increasing the risk of bleeding in patients with intermediate-high-risk PE with a follow-up period for 3 months.
Patients and Methods
Study Cohort
We performed a single-centre retrospective study of all patients admitted to an Intensive Care Unit (ICU) in Dr Erfan and Bagedo General Hospital in Jeddah, between November 2018 and April 2020, with a primary diagnosis of intermediate-high-risk PE. Patients were initially identified by our Pulmonary Embolism Response Team (PERT). Baseline admission demographics, vital signs, elevated Pro-Brain Natriuretic Peptide (BNP), evidence of myocardial injury as detected by elevated cardiac biomarkers (troponin T), evidence of embolus located in at least one main or lower lobe pulmonary artery as assessed by Computed Tomography Pulmonary Angiography (CTPA) or conventional pulmonary angiography and evidence of Right Ventricular (RV) dysfunction detected by echocardiography including RV-to-left ventricle (RV:LV) diameter ratio more than or equal to one [1] but without need for vasopressors and other RV data were extracted from the electronic medical record. All these data were evaluated before CDT and after completion of CDT. All other patients not meeting these criteria were excluded from our study including patients with PE symptom duration >14 days; insufficient echocardiographic image quality that interferes with the measurement of the RV:LV ratio; known significant bleeding risk; active bleeding; known coagulation disorder; platelet count <100000/mm3; previous use of vitamin K antagonists with international normalized ratio >2.5 on admission; a history of any intracranial or intra spinal surgery or trauma or bleeding; intracranial neoplasm, arterio venous malformation, or aneurysm; gastrointestinal bleeding <3 months; internal eye surgery or hemorrhagic retinopathy <3 months; or major surgery. This study was approved by the hospital ethical committee.
Transthoracic Echocardiography
Transthoracic Echocardiography (TTE) was performed before and after completion of CDT. Recorded echocardiographic loops were analysed by an experienced non-invasive cardiologist for signs of right ventricular dysfunction [10]. Right and left ventricular end-diastolic dimensions were obtained from the end-diastolic apical four chamber image by measuring the ventricular endocardial borders at the subannular plane located 1cm above the annular plane and perpendicular to the interventricular septal axis [11]. The difference in RV:LV ratio from baseline and after completion of CDT was obtained; particular attention was paid to obtain at least 3 adequate cine loops from the apical 4-chamber view for the measurement of RV:LV ratio. An RV:LV ratio >1 was an inclusion criterion. Besides, from the apical 4-chamber views, Right Ventricular Systolic Function- Tricuspid Annular Plane Systolic Excursion (RV-TAPSE) and the Systolic Pulmonary Artery Pressure (sPAP) from baseline and after completion of CDT were obtained. TAPSE was assessed with M-mode, measuring the distance of tricuspid annular movement between end-diastole to end-systole. TAPSE was measured by placing the 2D cursor at the tricuspid lateral annulus and measuring the distance of systolic annular RV excursion along a longitudinal line defining the end of systole as the end of the T wave in the electrocardiogram. A TAPSE was as low as 18 mm has been shown to correlate with RV systolic dysfunction. A Tricuspid Regurgitation (TR) jet was identified in apical 4-chamber view with the help of colour Doppler to assess Right Ventricular Systolic Pressure (RVSP). CW Doppler is used with a sweep speed of 100 mm/s to achieve a satisfactory envelope. Traditionally, Right Atrial Pressure (RAP) is assumed by the size and distensibility of Inferior Vena Cava (IVC) during inspiration at rest and during forced inhalation, and this value is added to the peak TR velocity [12] to estimate the sPAP.
Computed Tomographic Pulmonary Angiography
Computed Tomographic Pulmonary Angiography (CTPA) scans were performed with contrast to all studied patients using a Siemens Somatom AST and Toshiba Aquillion One. The baseline CTPA scans confirmed the presence of thrombus/ thrombi in all enrolled patients. The acquisition was performed during a single breath-hold or shallow breathing depending on the patient's level of dyspnea, from the diaphragm to lung apices in a supine position.
Catheter Directed Thrombolysis Technique
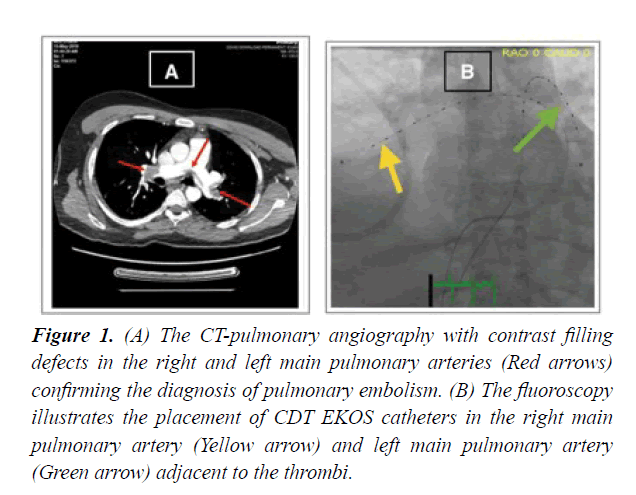
CDT technique has gained popularity in the last decade. The insertion of the catheter system was performed at the cardiac catheterization laboratory with continuous hemodynamic and electrocardiographic monitoring. Vascular access for placement of the CDT catheter (EKOS) is obtained. Typically a femoral approach was chosen. Begin with the insertion of the introducer needle into the common femoral vein with the use of one or two 6F introducer sheath for patients who were scheduled for unilateral or bilateral CDT, followed by the threading of a 6F multipurpose catheter over a 0.035 guide-wire through the right heart into the pulmonary vasculature adjacent to the thrombus. The guide-wire was then guided up inside, using fluoroscopic guidance; the infusion catheter is passed over the guide-wire and across the treatment site. Once positioned correctly, the guide-wire is removed. Lytic therapy then administered utilizing low-dose infusions of Tissue Plasminogen Activator (tPA). tPA slowly infused through the catheter simultaneously with the emission of the ultrasonic wave from the EKOS catheter, which is left in place for the duration of the treatment Figure 1.
Figure 1: (A) The CT-pulmonary angiography with contrast filling defects in the right and left main pulmonary arteries (Red arrows) confirming the diagnosis of pulmonary embolism. (B) The fluoroscopy illustrates the placement of CDT EKOS catheters in the right main pulmonary artery (Yellow arrow) and left main pulmonary artery (Green arrow) adjacent to the thrombi.
Systemic arterial oxygen saturation was recorded through transcutaneous oxygen saturation measurement. A 6F pigtail catheter used if EKOS catheter was not available for local delivery of tPA without ultrasonic source. A continuous infusion of tPA at 1 mg per lung per hour and of saline coolant at 35 mL/h per catheter was initiated.
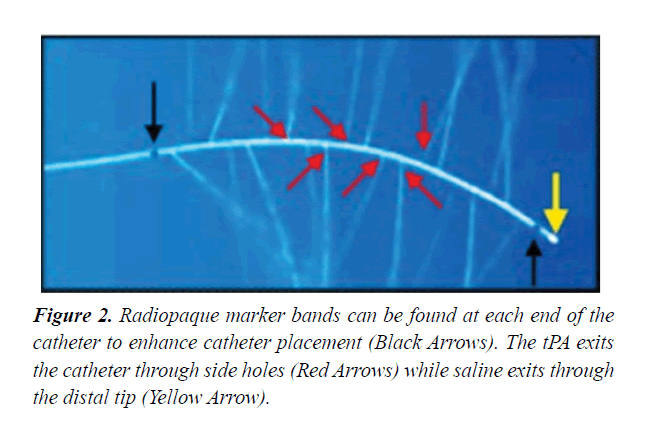
Note that radiopaque marker bands can be found at each end of the catheter to enhance catheter placement and the thrombolysis exits the catheter through side holes while saline exits through the distal tip Figure 2. During CDT, we used low-profile catheters; less than <10 French (EKOS catheter or pigtail catheter) to decrease clot burden via local infusion of thrombolytic agents. After catheter placement, patients were transferred to the intensive care unit for continuous monitoring. The maximum suggested tPA dose was 24 mg along with intravenous unfractionated heparin or low molecular weight heparin. Typically, thrombolysis was administered for a standard 18 hours. However, the duration of thrombolysis may vary depending on the degree of hemodynamic instability and clot burden. After completion of thrombolytic therapy, a repeat CTPA was performed to evaluate the improvement in the clot burden. Depending on the results, the decision was made to pursue further thrombolytic therapy versus the withdrawal of the CDT catheter. When the therapy was deemed complete, the ultrasonic core was removed, and the guide-wire was replaced inside the catheter. Then we removed the catheter leaving the guide-wire in place. Finally, the guide-wire was also replaced, and a compression device to the access site was applied until local haemostasis was achieved.
Study Endpoints
Our primary endpoint was overall mortality. Secondary endpoints included improvement in right ventricular end diastolic diameter/left ventricular end-diastolic diameter (RV:LV) ratio, right ventricular systolic function (RV-TAPSE), Systolic Pulmonary Artery Pressure (sPAP), recurrent venous thromboembolism, major bleeding defined as overt bleeding plus haemoglobin drop ≥ 5 g/dL (provided haemoglobin drop is related to the bleed); or bleeding requiring surgical intervention for control or bleeding requiring vasoactive drugs or bleeding causing intracranial haemorrhage with a follow-up period for 3 months.
Statistical methods
Data were entered in Microsoft Excel and then converted to an SPSS version for statistical analysis. For descriptive purposes, data are presented as maximum/minimum and average with means ± standard deviations as median values with ranges or absolute numbers and percentages for continuous and categorical variables, respectively. p-values for differences between before and after completion of CDT were calculated from unpaired t-tests.
Results
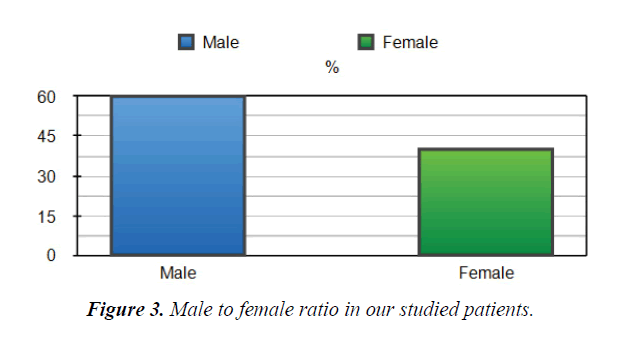
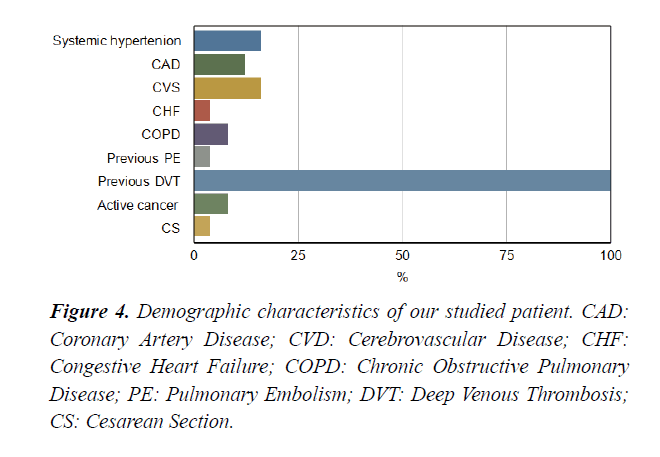
Between November 2018 and April 2020, fifty patients admitted to an ICU at Dr Erfan and Bagedo General Hospital in Jeddah, with the primary admission diagnosis of intermediate-highrisk PE that was identified by our PERT team. The mean age of the enrolled patients was 55+04 years. Sixty% of the studied patients were males Figure 3. Demographic characteristics of our studied patients are demonstrated in Figure 4. Baseline admission proBNP and Troponin T; as an evidence of myocardial strain and injury, were elevated and markedly reduced after completion of CDT Table 1.
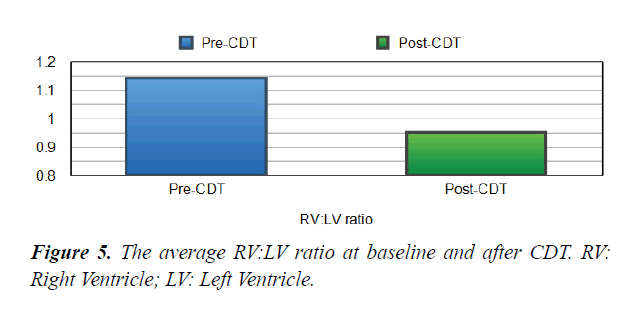
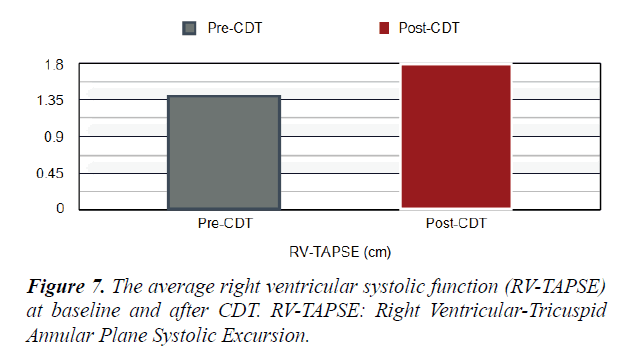
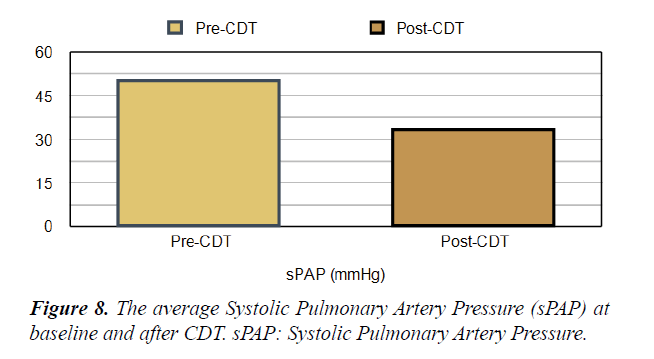
Table 1. Echo and laboratory parameters of the study patients at the baseline and after CDT. RV: LV, Right Ventricular to Left Ventricular Ratio, RV-TAPSE: Right Ventricular-Tricuspid Annular Plane Systolic Excursion, sPAP: Systolic Pulmonary Artery Pressure.
| Pre- CDT | Post-CDT Average | P value | |
| Average | |||
| RV:LV ratio | 1.1 | 0.9 | 0.001> |
| RV-TAPSE (cm) | 1.3 | 1.8 | 0.05> |
| sPAP (mmHg) | 50.22 | 33.3 | 0.05> |
| Troponin | 73.5 | 51.7 | |
| Pro-BNP | 5520 | 615 |
Safety and Efficacy of CDT
Short Term Mortality: There were no in-hospital deaths secondary to haemorrhage or procedure-related complications in the studied patients. Also, no mortality was reported in all included patients during the follow-up period for 3 months after CDT.
Short Term Outcomes: There was a notable improvement in symptoms and right ventricular function after CDT in our studied population. A significant difference was found between the baseline echocardiographic parameters and these parameters after completion of CDT; preferring the CDT Table 1. There was a significant reduction in RV:LV ratio after completion of CDT comparing with the baseline ratio (p<0.001) Figures 5 and 6. The average right ventricular systolic function was significantly improved (RV-TAPSE; and the p-value was <0.05) Figure 7. In comparison to baseline, there was a significant reduction in Systolic Pulmonary Artery Pressure (sPAP). sPAP before treatment was 50.22 mmHg in average and after completion of CDT was 33.3 mmHg (p-value <0.05) Figure 8. During the follow-up range of three months after CDT, only 3 minor bleeding episodes were encountered but no hemodynamic decompensation, recurrent venous thromboembolism, or major bleeding complications.
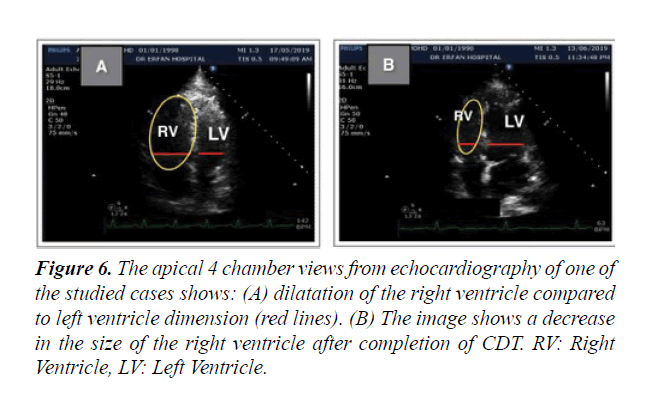
Figure 6: The apical 4 chamber views from echocardiography of one of the studied cases shows: (A) dilatation of the right ventricle compared to left ventricle dimension (red lines). (B) The image shows a decrease in the size of the right ventricle after completion of CDT. RV: Right Ventricle, LV: Left Ventricle.
Discussion
Pulmonary Embolism (PE) is a life-threatening entity and a common condition that causes substantial morbidity and mortality. Early diagnosis followed by early treatment is the key to improve survival rates. Once the diagnosis of PE is made, the treatment strategies should be based on the categorization of pulmonary embolism [3]. The goals of treatment are primarily focused on the reduction of mortality and secondarily on the prevention of RV dysfunction, PE recurrence and late-onset CTEPH. In our study, we focused on patients with intermediatehigh- risk PE to clarify the clinical significance and effectiveness of CDT in improving outcomes and quality of life without increasing the risk of bleeding.
Pulmonary Embolism Response Team (PERT)
Our patients were initially identified by our Pulmonary Embolism Response Team (PERT) and diagnosed as having an intermediate-high-risk PE based on elevated cardiac biomarkers (e.g., Pro-BNP and troponin) and right ventricular strain without hemodynamic compromise [13]. The guidelines recommend a PERT approach at all healthcare facilities treating PEs. This is an inter-professional team that is alerted in the event of intermediate or high-risk pulmonary embolism diagnosis [14]. In our hospital, the team members include specialists from various clinical fields including emergency medicine, critical care, cardiology, internal medicine, vascular surgery and radiology specialities. Our radiology specialist was consulted to know his recommendations as regards the size and burden of the pulmonary embolism in all of our patients. The team also consists of speciality-trained nurses and clinical pharmacists especially those trained in critical care, cardiac care, and anticoagulation. The emergency care and critical care nurses assisted in monitoring our patients during the procedure as well as during subsequent infusion therapy aiming to monitor for hemodynamic or neurologic complications. The clinical pharmacists assisted the team by providing appropriate medication and dosing for the procedure and adjusting other concurrent medications to minimize side-effects. By working as a collaborative team, they helped in doing CDT efficiently and effectively to all of our studied patients aiming to improve patient care. Once CDT was chosen as a management course, our team counselled and discussed with our patient and their family the risk and benefits of the procedure and informed consent was obtained from them. Our studies concordant with multiple studies have shown that the institution of a PERT team can reduce adverse events in regards to PE care [15].
Safety and Efficacy of CDT
Currently, CDT is a promising strategy that is more efficacious than anticoagulation alone and safer than systemic thrombolysis in patients with intermediate-high-risk PE [11]. The use of this therapy is on the rise in the world as more healthcare professionals are becoming trained in the art of CDT [16]. To perform CDT in our study, a trained professional interventional cardiologist familiar with the EKOS catheter system was present. In this study, we utilized an endovascular approach to do CDT to locally deliver a thrombolytic agent within the thrombus itself to facilitate fibrinolysis. This technique has shown to accelerate the reversal of right heart strain caused by PE while also reducing the bleeding risk associated with systemic thrombolysis [13]. By delivery of a lytic agent directly into and around the clot can; this reduces the amount of drug being used and thereby reducing the risk of subsequent systemic bleeding. Large studies have investigated the use of CDT for PE. The Pulmonary Embolism Thrombolysis (PEITHO) trial in 2014 showed an increase in major bleeding including hemorrhagic stroke during their investigation of tenecteplase versus Unfractionated Heparin (UFH) in the intermediate-risk population [17]. In our cases, we used tPA as a lytic agent and not tenecteplase like PEITHO trial. However, the results of our study concordant with 2015 PERFECT trial in that, CDT appears to be safer with a lower risk of bleeding as compared to conventional systemic therapy and also procedural complications are uncommon [18].
Short Term Mortality: Despite the benefits of using thrombolytics in intermediate-high-risk PE, the most feared complication is fatal bleeding (e.g., intracranial or gastrointestinal bleeding). Systemic thrombolysis has been investigated in patients with submassive PE, showing that while there was a decrease in hemodynamic collapse, a corresponding increase in the risk of significant haemorrhage was noted, with a resulting net neutral effect on mortality [19]. Therefore, a less harmful but effective alternative should be sought; these include catheter-based, local administration of the lytic agent, surgical embolectomy, and fragmentation or aspiration of the thrombus [20]. In our study concordant with ULTIMA trial in 2014, there were no deaths secondary to haemorrhage or to procedurerelated complications in the studied patients. Also, no mortality for three months after CDT [21].
Short Term outcomes: Once thromboembolic lodged in the pulmonary vasculature, the blockage may increase pulmonary vascular resistance by obstruction and vasoconstriction, resulting in hemodynamic heart strain along with a decrease in blood supply to the downstream pulmonary parenchyma. This deteriorates symptoms and hemodynamic status. Some common hemodynamic changes include an increase in right ventricular systolic pressure (which expressed by systolic pulmonary artery pressure; sPAP) up to 40 mmHg (nearly doubles from the baseline). Some patients with higher right ventricular systolic pressure than 40 mmHg may be attributable to coexisting pulmonary hypertension [22]. In our study, the sPAP was 50.22 mmHg on average at the time of diagnosis. The net effect of this increase in sPAP is dilatation and dyskinesia of the Right Ventricle (RV strain). This leads to right ventricular ischemia/micro-infarction (the cause of troponemia) and pressure overload (elevated pro-BNP) [23,24]. The RV strain was assessed in our cases by focused echocardiography. Like other studies, the characteristic findings included RV dyskinesia and dilatation (sometimes exceeding the dimensions of the left ventricle), flattening of the septum (the right ventricle appears like alphabet letter “D”), paradoxical movement of septum towards left ventricle, and increase in sPAP [19]. In our cases, troponin and pro-BNP were markedly elevated at the time of diagnosis. Studies have shown that elevated biomarkers (troponin and pro-BNP) and RV dysfunction have been associated with increased risk of mortality [25,26]. Therefore, rapid and effective treatment was necessary to “unload” right ventricle. CDT could be effective against the acute burden on the right ventricle and will improve hemodynamic status and relief of symptoms. The lytic agent is delivered directly into the pulmonary artery and once binds to the clot; it does not migrate into the circulatory system [25]. In our study, we noted that Troponin and proBNP were markedly reduced after completion of CDT. Therefore, CDT can prevent the progression of clot burden; improve the hemodynamic status, and relief from the symptoms and long-term complications such as CTEPH in the intermediate-risk PE population. In those group of patients, ULTIMA trial in 2014 compared ultrasound-assisted CDT to Intravenous (IV) heparin. They concluded that CDT was superior to anticoagulation in reversing RV dilatation in the intermediate-risk PE population [21]. Another trial in 2015; SEATLE II study revealed that CDT decreased RV dilation, lessened pulmonary hypertension, reduced clot burden, and minimized intracranial bleeding in acute submassive PE [27]. In our study, there was an improvement in symptoms and right ventricular function after CDT. Also, there was a significant reduction in RV:LV ratio and sPAP after CDT.
Although the main determinant of acute morbidity in PE is related to the degree of RV dysfunction, there are several possible complications from CDT for pulmonary embolus. One of the most common and most feared complications is a hemorrhagic stroke. Also, the safety of CDT may be associated with specific external factors, including technical expertise. A post hoc analysis of the SEATTLE II trial suggested multiple venous access attempts were associated with a higher risk of major bleeding during or after the catheter-directed procedure [28]. Other common complications include vascular access complications such as hematoma, pulmonary haemorrhage, retroperitoneal haemorrhage followed by cardiogenic shock, perforation of the pulmonary artery, arrhythmias, pericardial tamponade, and contrast-induced nephropathy [29]. In our studied cases, only 3 minor bleeding episodes were encountered during follow up period but no hemodynamic decompensation or major bleeding complications. No major bleeding at 90 days after CDT. Therefore, our centre and many others implement CDT therapy for patients with intermediate-high-risk submassive PE.
Conclusion
CDT can be used in patients with intermediated-high-risk PE safely and effectively. By these encouraging results, CDT may become the preferred reperfusion method for the treatment of intermediate-high-risk PE. Futu re researches are needed to justify the role of CDT in comparison to other revascularization strategies in the management of PE patients at intermediatehigh- risk.
Funding
The authors received no funding regarding this article.
Methodology
Performed at one institution.
Authorship Contribution
1. Abd-Elmaksoud A Elganady: Contributed to study design, study selection, collection of data, statistical analyses, data interpretation, and provided vital reviews of the manuscript
2. Abeer M Shawky: Contributed to study design, interpreted the data and writing the manuscript
3. Ahmed Dawod: Contributed to study selection and data collection
References
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788-1830.
- Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: The task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29:2276-2315.
- Liang J, Goldberg A, Afessa B. A 51-year-old woman with dyspnea. Cleveland Clinic J Med. 2013;80:487-499.
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
- Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: A systematic review and meta-analysis. Eur Heart J. 2015;36:605-614.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311:2414-2421.
- Dilektasli AG, Demirdogen Cetinoglu E, et al. Catheter-Directed Therapy in Acute Pulmonary Embolism with Right Ventricular Dysfunction: A Promising Modality to Provide Early Hemodynamic Recovery. Medical science monitor. Med Sci Monit. 2016;22:1265-1273.
- Konstantinides SV, Barco S. Prevention of early complications and late consequences after acute pulmonary embolism: focus on reperfusion techniques. Thromb Res. 2018;164:163-169.
- Valerio L, Klok FA, Barco S. Immediate and late impact of reperfusion therapies in acute pulmonary embolism. Eur Heart J Suppl. 2019; 21:I1-I13.
- Kuo WT, Gould MK, Louie JD, et al. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;20:1431-1440.
- Kucher N, Rossi E, DeRosa M, et al. Massive pulmonary embolism. Circulation. 2006;113:577-582.
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685-713.
- Giri JS, Piazza G. A midterm report card for pulmonary embolism response teams. Vascular medicine. 2018;23:72-74
- Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: Initial 30 month experience with a novel approach to the delivery of care to patients with submassive and massive pulmonary embolism. Chest. 2016;150:384-393.
- Porres-Aguilar M, Anaya-Ayala JE, Heresi GA, et al. Pulmonary embolism response teams: A novel approach for the care of complex patients with pulmonary embolism. Clin Appl Thromb Hemost. 2018;24:48S-55S.
- Barco S, Konstantinides SV. Pulmonary embolism: Contemporary medical management and future perspectives. Ann Vasc Dis. 2018;11:265-276.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. The New Eng J Med. 2014;370:1402-1411.
- Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial results from a prospective multi-center registry. Chest. 2015;148:667-673.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-1411.
- Piazza G, Goldhaber SZ. Management of submassive pulmonary embolism. Circulation. 2010;122:1124-1129.
- Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479-486.
- Goldhaber SZ, Elliott CG. Acute pulmonary embolism: part I: Epidemiology, Pathophysiology, and Diagnosis. Circulation. 2003;108:2726-2729.
- Konstantinides S, Geibel A, Olschewski M, et al. Importance of cardiac troponins I and T in risk stratification of patients with acute pulmonary embolism. Circulation. 2002;106:1263-1268.
- Kucher N, Printzen G, Doernhoefer T, et al. Low pro-brain natriuretic peptide levels predict benign clinical outcome in acute pulmonary embolism. Circulation. 2003;107:1576-1578.
- Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: A systematic review and meta-analysis. Am J Respir Crit Care Med. 2008;178:425-430.
- Bajaj A, Rathor P, Sehgal V, et al. Prognostic value of biomarkers in acute non-massive pulmonary embolism: A systematic review and meta-analysis. Lung. 2015;193:639-651.
- Piazza G, Hohlfelder B, Jaff MR, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv actions. 2015;8:1382-1392.
- Sadiq I, Goldhaber SZ, Liu PY, et al. Submassive, massive pulmonary embolism treatment with ultrasound accelerated thrombolysis therapy I. Risk factors for major bleeding in the SEATTLE II trial. Vasc Med. 2017;22:44-50.
- Mostafa A, Briasoulis A, Telila T, et al. Treatment of massive or submassive acute pulmonary embolism with catheter-directed thrombolysis. Am J Cardiol. 2016;117:1014-1020