Research Article - Journal of Infectious Diseases and Medical Microbiology (2018) Volume 2, Issue 1
Carrier rate of urinary tract infections in apparently healthy individuals in Ikare-Akoko, Ondo State, Nigeria.
Fadipe DO1*, Olajubu FA2, Akinseye JF3, Fadipe AO41Health Centre, Adekunle Ajasin University, Akungba-Akoko, Ondo-State, Nigeria
2Department of Microbiology, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria
3Department of Medical Laboratory Science, Afe Babalola University, Ado-Ekiti, Ekiti-State, Nigeria
4Department of Medical Laboratory Science, Ondo State School of Health Technology, Akure Ondo State, Nigeria
- *Corresponding Author:
- Fadipe DO
Adekunle Ajasin University
Akungba-Akokol
Ondo-State
Nigeria
Tel: +2348072905144
E-mail: dipedare@gmail.com
Accepted Date: January 10, 2018
Citation: Fadipe DO, Olajubu FA, Akinseye JF, et al. Carrier rate of urinary tract infections in apparently healthy individuals in Ikare-Akoko, Ondo State, Nigeria. J Infectious Disease Med Microbiol. 2018;2(1):8-12.
Abstract
Three hundred midstream urine samples were randomly collected from apparently healthy male and female subjects (not showing symptoms of urinary tract infection), and were examined for urinary tract infection. Samples from these subjects indicated conditions of bacteriuria, Candidiasis and Schistosomiasis. Seventy five (75) of these samples gave significant bacterial growth (Colony forming unit ≥ 105/ml). Escherichia coli was the most prevalent microorganism 25 (33.3%), followed by Staphylococcus species 16(21.3%), Staphylococcus aureus 12(16%), Enterococcus faecalis 10(13.3%), Klebsiella oxytoca 5(6.8%), Lactobacillus acidophilus 4(5.3%), Proteus mirabilis 3(4.0%). Also Candida albicans was isolated from ten (10) cases and ova of Schistosoma heamatobium were seen in two of the subjects. The bacterial isolates showed varied degrees of sensitivity to the antibiotics used against them. The quinolones and Nitrofurantoin showed nearly 100% of effectiveness against the different isolates while Ampicillin, Tetracycline and Septrin showed the least percentage antimicrobial activity.
Keywords
Carriers, Urinary tract infection, Apparently healthy individuals
Introduction
Urinary tract infection (UTI) implies both microbial multiplication and colonization of the lower or upper urinary tract, or both. It is defined by the presence of more than (105) 100,000 organisms per ml of midstream sample of urine [1]. Urinary tract infection is one of the most common types of bacterial infection encountered in clinical practice. Urinary tract infection may be categorized into two areas of involvement i.e., cystitis (bladder infections) and pyelonephritis (kidney infections) [2]. These infections are frequently encountered in both the community and hospital environment. It has been reported in the sexes of all age groups but they are most common in females [3,4]. About one third of women have urinary tract infection at some time, and the prevalence is about 3% at the age of 20, increasing by about 1% in each subsequent decade. In males, urinary tract infection is uncommon except in the first year of life. Urinary tract infection causes considerable morbidity and in some cases, renal damage and chronic renal failure [1].
The occurrence of UTIs varies with frequencies from about 1 to 10% in different age groups. In UTIs, there are two types of patients namely the bacteriuric and the non-bacteriuric [5]. But there exist two main types of UTIs which are predominant in most patients; these are the symptomatic UTI and the asymptomatic UTI. The symptomatic UTI vary with age and sex, showing increased tendency with age in both sexes [6].
Asymptomatic bacteriuria may be defined as significant bacteriuria in the absence of symptoms of urinary tract infection [7]. Urine is said to have significant bacteriuria when the bacteria count from freshly voided mid-stream urine exceeds 105 cfu/ ml (Colony forming unit per ml). Many microorganisms are known to cause UTI, but the most common causative agents are bacteria namely: Escherichia coli, Enterobacter aerogenes, Proteus mirabilis, and Staphylococcus spp [8]. Others are α-haemolytic Streptococci, Lactobacillus spp, Diphteroids and mixed Gram positive flora were categorized as non-pathogens. Urine secreted by normal kidney is sterile and remain so while it travels to the bladder, however the normal urethra has a microbial flora and any voided urine in normal persons may therefore contain thousands of bacteria per ml, derived from this normal flora.
The colony count is simply a number designed to provide the clinician with a reasonable statistical probability that the patient either have or does not have significant bacteriuria. This must however be interpreted in the light of patient's symptoms, the time of the day, the manner of the urine collection, method of quantitation and whether the patient is receiving antimicrobial therapy as well as the type of organism and purity of culture obtained.
Many factors are responsible for the relationship of this infection in both sexes; since many species of microorganisms have been known to colonize the urinary tract of both sexes. The factors are as follows:-
In males there is a greater frequency of the occurrence of UTI recorded in males after 50 years of age, when it becomes a problem. Prostatic obstruction and urethral instrumentation influence the rate of infection. In the earlier age in male, the infection is quite rare which requires careful evaluation, though in some cases, UTI may occur without an apparent infection which is referred to as non-infectious prostates which may be caused by bacteria or viruses [9].
In the case of neonate males, the rate of incidence is just 25-30% who are hospitalized and 2-3% preterm new born infants. Males are more affected than females and the greater frequency of neonates UTI in males have been attributed to structural abnormalities in the urinary tract in this category. The incidence rate of UTI in men is less than their women counterpart in the same age bracket. In females UTIs occur very frequently especially in women between the age 15 and 50 years, in whom they are the most common infection requiring antibiotic treatment [10]. In pregnant women, UTIs are one of the commonest problems during pregnancy. Since it may be asymptomatic and failure to detect it may lead to other more serious pathological complications. Conversion of asymptomatic infection or bacteriuria to symptomatic bacteriuria may lead to pyelonephritis which increases the possibility of premature birth in pregnant women or even in more serious cases, abortion [11].
In pregnant females, the incidence of bacteriuria has been known to be insignificantly different from that of the nonpregnant, out-patient and healthy asymptomatic women, UTI occurred in almost 54-55% of pregnant women compared to 54.77% asymptomatic and 17% non-pregnant [8]. In the incidence of asymptomatic bacteriuria, women were considered to be at risk when they had neither asymptomatic bacteriuria nor symptomatic urinary tract infections. The rates of asymptomatic bacteriuria are similar to those described in non-pregnant and pregnant women but higher than those reported in healthy school girl [12].
Hansson et al. reported that UTIs are common during infancy and childhood but are easily overlooked because of the unspecific symptoms. The incidence of asymptomatic bacteriuria in male subjects is believed to be greatest with the new born [13]. At this period of life UTI is said to be more in males than in females [14].
Nebigil and Wilson reported a prevalence rate of 4% in new borns, 5.2% in infants, 5.8% in pre-school children and 4.5% in elementary school children [15]. Hodson and Wilson considered that the first urinary tract infection occurs at any time between 0 and 5 years. It is believed that the peak incidence is between 3 and 5 years [16].
Materials and Methods
Collection and processing of samples
Midstream urine samples were collected from subjects, after having been instructed on how to collect the urine samples. Commercially sterile universal bottles were used.
In the laboratory, the samples were inoculated onto Blood agar and CLED (Cysteine Lactose Electrolyte Deficient) agar using the filter paper dip strip method of Leigh and Williams as recommended by the World Health Organization for combined quantitative and presumptive identification [17].
After inoculation, 10 ml of each urine was poured into centrifuge tube and spun at 1,500 revolutions per minute for 5 minutes.
After spinning, 9.5 ml of the supernatant was decanted and the deposit was re-suspended and examined microscopically for blood cells and any other organized elements and parasites. The white blood cells were expressed as cells per high power field (WBC/HPF).
The inoculated plates were incubated overnight at 37°C after which there was colony count on those plates showing bacterial growth. With the help of the diagram adapted from World Health Organization (1991) the average number of colonies was converted to the number of bacteria per ml of urine.
Identification procedures were then initiated with well separated colonies of plates having significant bacterial growth of a single bacterial species, using standard methods of identification of bacteria of medical importance [18].
Isolation and identification procedures
Well separated colonies from the plates were isolated, streaked on fresh CLED agar and Nutrient agar slants; they were incubated at 37°C for 18-24 hours. The agar slant cultures were referred to as stock cultures.
Preliminary identifications of bacterial isolates were done as either lactose fermenters or non-lactose fermenters. CLED agar gives good colonial differentiation of most urinary pathogens. These colonial differentiations as given by Monica Cheesbrough were further investigated with other Biochemical tests. Preliminary identifications include; Colonia morphology, Gram staining reactions and Motility test [19].
Several biochemical tests were carried out on the different bacterial isolates for the purpose of identification are given below.
Test for sugar utilization
This test was performed to show the ability of these organisms to ferment the following sugars: glucose, maltose, mannitol, sucrose and lactose.
The peptone sugar water containing glucose has inverted Durham tubes filled with the broth. The glucose and other sugar solutions have been previously sterilized by filtration before they were added to the basal medium under aseptic conditions. They were inoculated with respective isolates and incubated for about 48 hours at 37°C. The utilization of a particular sugar results in the production of acid and causes a yellow coloration of the red broth. The production of gas was also confirmed by the space present in the Durham tube which initially was filled with the broth. Other biochemical tests done include; methyl red test, potassium cyanide test, citrate utilization test, indole test, catalase test, oxidase test, coagulase test, urea utilization test.
Antibiotic sensitivity testing
Sensitivity agar (Lab M) plates aseptically prepared were used and also commercially prepared antibiotic disc. The plates were flooded with 3ml suspension of the organism in Normal saline; excess inoculum was drained off and was allowed to stand for 20 minutes for the organisms to be fully absorbed into the agar. Commercially prepared sterile antibiotic multidisc were impregnated into the agar surface and left to stand for about 15 minutes to ensure absorption and incubated at 37°C for 24 hours. Sensitivity pattern of isolates were recorded by measuring the zones of inhibition. Antibiotic which caused inhibition with minimum diameter of 10mm was taken to be sensitive and those below 10mm were taken to be resistant.
Results
Clean voided midstream urine samples were collected from a total of 300 male and female subjects of ages between 3 months and 60 years in Ikare-Akoko. Among the 300 samples, 75(25.0%) showed significant growth, 60 (20.0%) had insignificant growth while 165(55%) of the urine samples showed no growth. Wet preparation was carried out on centrifuged samples to determine the presence of non-bacterial organisms; 2(0.7%) of the samples showed the presence of Schistosomes.
The incidence of UTI was surprisingly high (31.4%) among the age group of 31 years to 40 years (Table 1), and also high among females (30%) than in males (Table 2).
Table 1. Carrier rate of urinary tract infection by age distribution in Ikare Akoko.
| Age (Years) | Number examined | Number infected with UTI | Age-Specific infection rate |
|---|---|---|---|
0-10 |
10 |
1 |
10% |
Table 2. Carrier rate of urinary tract infection by sex distribution in Ikare Akoko.
| SEX | No. Examined | No. Infected | Percentage sex specific |
|---|---|---|---|
MALE FEMALE TOTAL |
100 200 300 |
15 60 75 |
15% 30% 25% |
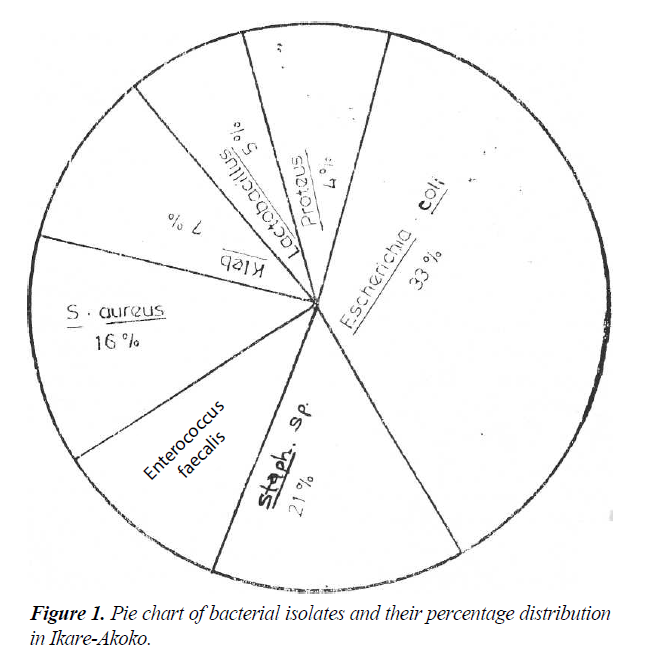
A total of six different genera of bacteria were isolated from the urine samples of these subjects that had significant bacteriuria as reported in Figure 1, the organism isolated with the highest frequency was Escherichia coli: 33.3% followed by Staphylococcus: spp 21.3%, Staphylococcus aureus: 16.0% Enterococcus faecalis: 13.3%, Klebsiella oxytoca: 6.8% Lactobacillus spp: 5.3% and Proteus mirabilis had the least frequency of 4.0%. The non-bacterial isolates shows frequency of Candida albicans 3.3%, Schistosoma heamatobium 0.7% and no case of Trichomoniasis was seen.
The antibiotic sensitivity pattern of the isolates was equally investigated. These organisms exhibited varying susceptibility or resistance to some of the antimicrobial agents.
The antimicrobial agents used are Ofloxacin (10 μg) Gentamicin (10 μg), Nalidixic acid (30 μg), Nitrofurantoin (200 μg), Chloramphenicol (25 μg), Septrin (25 μg), Streptomycin (25 μg) Colistin sulphate (25 μg), Tetracycline (30 μg), Ciprofloxacin (10 μg), Ampicllin (25 μg), Penicillin (1 unit), Erythromycin (5 μg).
Discussion and Conclusion
Asymptomatic bacteriuria has been reported to occur in both sexes of all age groups, but they are most common in females [4,20]. Some researchers believed that urinary tract infections are common during infancy and childhood but are easily overlooked because of the unspecific symptoms [21]. But the incidence of asymptomatic bacteriuria in male subjects is believed to be greatest in Newborn [13,14].
Of the 300 subjects studied, 75 had significant bacteriuria. This result is higher than the results of some previous scholars in this field. Ojiegbe et al. worked in Enugu, the Eastern part of Nigeria, and of 800 subjects of age 0-15, only 9(1.12%) showed significant bacteriuria. But however agrees with the work done in the Western part of Nigeria [8,22], from the results Escherichia coli was found to have the highest percentage of occurrence (33.3%) this is consistent with earlier report from Ado Ekiti [8,22] but however differs from the works of Omoregie et al. done in Benin and Oyewale, 2015 done in Iree, Osun State, where they both reported Staphylococcus aureus as the most prevalent bacteria [23].
Other bacteria species isolated have been incriminated in most cases of urinary tract infection with the exception of the Lactobacillus spp believed to be normal flora of the vagina tract in women [24]. The in vitro susceptibility pattern showed that the 4-quinolones are the best antibacterial agents for treating infections due to several pathogens. They are regarded as the most potent and have very high pharmacokinetic profiles.
This work agrees with the view of Oronsaye (2002) that antibacterial agents like Ampicillin, Cotrimoxazole and Tetracycline are passing out of use in treatment of urinary tract infections [25]. Gentamycin and Streptomycin, though still found potent are not usually convenient due to their mode of administration and the toxicity that is associated with their prolonged therapy. Also nitrofurantoin has some undesirable side effect, though very effective.
To routinely do microscopy, culture and sensitivity as part of screening test for healthy individuals may be time consuming and laborious. Asymptomatic infection can be routinely screened for by wet preparation of centrifuged samples of clean voided midstream urine. It was observed that samples with white blood cells count per high field of above 5 gave bacterial growth of 105 cfu/ml and above. This is consistent with the work of Ojiegbe et al. who suggested that as part of medical examination for students urinary microscopy should be done to detect asymptomatic bacteriuria so as to prevent a situation that can lead to chronic pyelonephritis.
Urinary tract infections are important complications of pregnancy, diabetes mellitus, polycystic renal disease, and renal transplantation, structural and neurological conditions which interfere with normal urine flow [9].
Urinary hydrodynamics appears to be a good protection mechanism in humans. Since urine is a good culture medium for bacteria, Diuresis does not only dilute the bacteria load in the bladder but when it is coupled with voiding it tends to rid the bladder of bacteria.
Many microorganisms can cause urinary tract infections but the most common etiologic agents are found in the gastrointestinal tract. This suggests that females which are the worst hit should be educated on ways of maintaining personal hygiene, good toilet habits and other precautionary measures [26].
Urinary tract infections are frequently encountered in both the community and hospital environment. It has been discovered to affect a good percentage of supposedly healthy individuals in Ikare - Akoko.
Urinalysis and microscopy should be part of routine medical examination for people in this area, both young and old ones.
Also, the widespread of resistance to Uro bactericides, as seen here calls for antibiotic usage policy that would be made applicable to different tiers of our health care providers. In addition, there should be an increase and sustained enlightenment and media publicity, focusing attention on the dangers of high incidence of bacterial resistance to antibacterial agents in general, and the consequences of therapeutic failures if the trend is not halted or reversed. The need for prescribed guidelines, as effective tools for rational antibacterial prescribing for urinary tract infections cannot be over emphasized.
References
- Davison AM, Cumming AD, Swainson CP, et al. Diseases of the kidney and urinary system. In: Davidson’s Principles and Practise of Medicine. Churchill Livingstone, Edinburgh. 2000; 1175.
- Mabeck CE, Vejlsgaard R. Treatment of urinary tract infection in general practice with sulfamethiazole, trimethoprim or Cotrimazole (Sulphadiazine trimethoprim). J Antimicrob Chemother. 1980;6(6):701-08.
- Azubuike CN, Nuamadu OJ, Oji RU, et al. Prevalence of urinary tract infection among school children in a Nigerian Rural Community. West African J Med. 1994;13(1):48-52.
- Oyewale MO. Urinary tract infections among students of Osun State Polytechnic, Iree. International Journal of Scientific Innovation and Sustainable Development. 2015;5(2):57-64.
- Karaoni RM, Hanna A. An epidemiological study of urinary tract infection in Benghazi, Libya. J Hyg Epidemiol Microbiol Immunol. 1981;25(3):277-285.
- Rodgers K, Nicolle LE, Mcintyre M, et al. Urinary tract infection in institutionalized elderly subjects. Canadian J Inf Dis. 1991;2:142-146.
- Anderson JR. Muir’s Textbook of Pathology. Edward Arnold Publishers, London.
- Famurewa O. Prevalence of urinary tract infection in women in Ado-Ekiti, Nigeria. L’igiene Mordema. 1992;97:580-591.
- Kunin CM. Sexual intercourse and urinary tract infection. N Eng J Med. 1978;298:366-367.
- Norrby SR. Design of clinical trials in patients with urinary tract infections. 1992;35:181-188.
- Anon. Urinary tract infection during pregnancy. Lancet. 1985;1:190-192.
- Ojiegbe GC, Nworie WC. Asymptomatic bacteriuria among school pupils in Enugu urban area, Enugu, Nigeria. J Med Lab Sci. 2000;9:42-46.
- Lincoln K, Winberg J. Studies of UTIs in infancy and childhood. II. Quantitative estimation of bacteriuria in unselected neonates with special reference to the occurrence of a symptomatic infection. Acta Paediatr. 1964;53:307-10.
- Uchling DT, Weiss R, Wirth JC. Does circumcision prevent urinary tract infection? J Urol. 1986;135(5):991-992.
- Nebigil I, Wilson S. Asymptomatic urinary tract infection in children. Eur J Pedatr. 1965;151:308-309.
- Hodson CJ, Wilson S. Natural history of chronic pyelonephritis scarring. Brit Med J. 1965;2:191-194.
- Leigh DA, Williams JD. Method for the detection of significant bacteriuria groups in large groups of patients. J Clin Pathol. 1964;17:498-503.
- Cowan ST. Cowan and Steel’s Manual for the identification of Medical Bacteria. 2nd edn. Cambridge University Press, London. 1994;238.
- Monica C. District laboratory practice in tropical countries Part 2. Cambridge University Press, London. 2000;434.
- No authors listed. Asymptomatic bacteriuria in schoolchildren in Newcastle upon Tyne. Arch Dis Child. 1975;50(2):90-102.
- Hansson S, Martinell J, Stokland E, et al. The natural history of bacteriuria in children. Infect Dis Clin North Am. 1997;11(3):499-512.
- Laleye SA, Esan CO, Famurewa O. Pattern of urinary tract infection in Ado-Ekiti, Nigeria. African Journal of Science. 1998.
- Omoregie R, Erebor JO, Akhonkhai II, et al. Observed changes in the prevalence of uropathogens in Benin City, Nigeria. Nigeria J Med Lab Sci. 2008;62:29-31.
- Baker FJ, Breach MR. Medical microbiology techniques. Butterworths, London. 1980;546.
- Oronsaye FE. Comparative in vitro activity of the new 4-quionolones and other antimicrobial agents in use for the treatment of Urinary tract infection in Benin City, Nigeria. J Med Lab Sci. 2002;11(1):56-62.
- Vandepitte J, Engbeak K, Piot P, et al. Basic laboratory procedures in clinical bacteriology. World Health Organisation, Geneva.