Research Article - Biomedical Research (2017) Volume 28, Issue 5
Cardioprotective potential of sesamol against ischemia/reperfusion injury induced oxidative myocardial damage
Haiyan Tian and Ruiqiang Guo*Department of Ultrasound, Renmin Hospital of Wuhan University, Wuhan University, Wuhan, Hubei 430060, PR China
- *Corresponding Author:
- Ruiqiang Guo
Department of Ultrasound
Renmin Hospital of Wuhan University, PR China
Accepted on October 13, 2016
Abstract
Sesamol, an active component of sesame oil exhibits wide array of biological properties. The present was carried out to scrutinize the protective role of sesamol in myocardial ischemia reperfusion (M I/R) oxidative cardiac injury in a murine model. Pretreatment of Sesamol (50 mg/kg) was done for 7 days before the surgery period. M I/R injury were surgically induced by left anterior descending (LAD) ligation for 45 min followed by 3 h of reperfusion. The cardioprotective efficacy of sesamol was authenticated by measuring biochemical markers, protein expression of apoptotic related genes and inflammatory gene expression. In the present study, M I/R rats displayed elevated infarct size, serum cardiac markers (CK, LDH and cTnI) MDA and MPO activity. Meanwhile, the level of antioxidants (SOD, CAT and GSH) was significantly reduced in ischemic tissues due to oxidative stress. Further, serum (TNF-α, IL-1β) and mRNA (TNF-α, IL-1β and NF-?B) level of inflammatory mediators were significantly elevated M I/R rats. Protein expression of apoptotic related genes (Bcl-2, Bax and Caspase-3) were significantly altered in the event of M I/R injury. However, sesamol treatment significantly reduced the infarct size, resorted the cardiac markers, reduced the lipid peroxidation, neutrophil infiltration and increased the antioxidants level. Furthermore, sesamol administration elicited significant downregulation of inflammatory genes, Bax, Caspase-3 apoptotic proteins and upregulation of anti-apoptotic Bcl-2 protein. In conclusion, our study demonstrated that sesamol exerts cardioprotective property against M I/R injury mediated through antioxidant, anti-inflammatory and anti-apoptotic mechanism.
Keywords
Sesamol, Cardioprotective, Ischemia/reperfusion, Antioxidant, Inflammation, Myocardial apoptosis.
Introduction
Globally, cardiovascular related diseases are the prominent causes of fatality and acute myocardial infarction is the serious cardiovascular event which elicits higher rate of morbidity and mortality. Therapeutic intervention like reperfusion of ischemic myocardial tissue is vital to ameliorate the devastating consequences of an infarcted heart [1]. Albeit, the patient may recover substantially however, it leads to myocardial ischemia-reperfusion (I/R) injury. Thus, myocardial I/R injury elicit increase in the size of myocardial infarct and diminished cardiac function [2].
Reports suggest that several bio mechanisms have been implicated in the pathology of myocardial I/R injury. In the myocardial ischemic area, rampant release of reactive oxygen species (ROS) and thus directly detoriate the cell membrane leading to cell toxicity and death [3]. Further, the generated ROS triggers the signal transduction pathways to activate inflammatory cytokines network which are the mainstay in the regulation of cell viability and apoptosis [3]. Meanwhile, rampant release of inflammatory mediators such as tumor necrosis factor-a (TNF-a) and IL-1β enhances the production of ROS to cause oxidative myocardial injury [4,5]. In this scenario, therapeutic agent which can limit ROS, diminishing myocardial apoptosis, inflammation and restoring heart functions have been implicated to ameliorate myocardial damage following I/R.
Sesamol, an active phenolic compound of sesame oil exhibits potent antioxidant and free radical scavenging activity. Previous preclinical reports underscore that sesamol exhibits wide array of biological activities like radioprotective [6], antimutagenic [7], anti-ulcer [8], neuroprotective [9] and antiplatelet activity [10]. Previous reports substantiate the protective role of sesamol against the isoproterenol and doxorubicin provoked myocardial injury [11,12]. In the light of above credible evidences, the current study was undertaken to evaluate the protective role of sesamol on ischemia/reperfusion induced oxidative myocardial injury.
Materials and Methods
Animals
Sprague Dawley male rats (200-250 g) were procured from and housed in our institution’s Laboratory Animal Center, Zhejiang University, Hangzhou, China The rats were caged in a well maintained animal room with a 12 h light/dark cycle and with central air conditioning (25°C, 70% humidity). The animals were allowed free access to tap water and standard rodent diet.
Chemicals
Sesamol and sodium thiopental were purchased from Sigma- Aldrich Chemical Co. (St. Louis, MO, USA). All other chemicals used were analytical grade.
Induction of myocardial I/R injury
The myocardial I/R injury was experimentally done by left anterior descending (LAD) ligation for 45 min followed by 3 h of reperfusion [13]. Briefly, the overnight fasted rats were anesthetized with sodium pentobarbital (40 mg/kg; i.p). Once the mechanical ventilation was done, the heart rate and rhythm was measured using limb lead II of the ECG apparatus. The trachea was removed and the rats were ventilated with room air by rodent pressure ventilator (Crompton Parkinson Ltd., England) by maintaining the followings conditions (50 strokes/ min; tidal volume of 15 ml/kg). A left thoracic incision was done exposing the pericardium and then using 4-0 sterilized silk thread a slip knot at the distal 1/3 of the LAD origin was performed. The animals were monitored for myocardial ischemia associated cardiac functions after 5-15 min of ischemia and then underwent 45 min of ischemia. After the ischemic period, the slip knot was removed gently and the myocardium was reperfused for 3 h. After the surgery period, the chest cavity was closed and post-operative analgesic buprenorphine (0.1 mg/kg s.c.) was administered.
Experimental design
The rats were randomly categorized into three groups (n=10) as follows,
Group 1: Sham operated group (Sham): The silk thread was inserted underneath the LAD but the ligation was not performed.
Group 2: Myocardial ischemic group (M I/R): The LAD was ligated 45 min followed by 3 h reperfusion and treated with vehicle (0.9% NaCl; i.v).
Group 3: M I/R + Sesamol group: The rats received sesamol (50 mg/kg b.wt; p.o) daily for 7 days.
Evaluation of myocardial infarct area
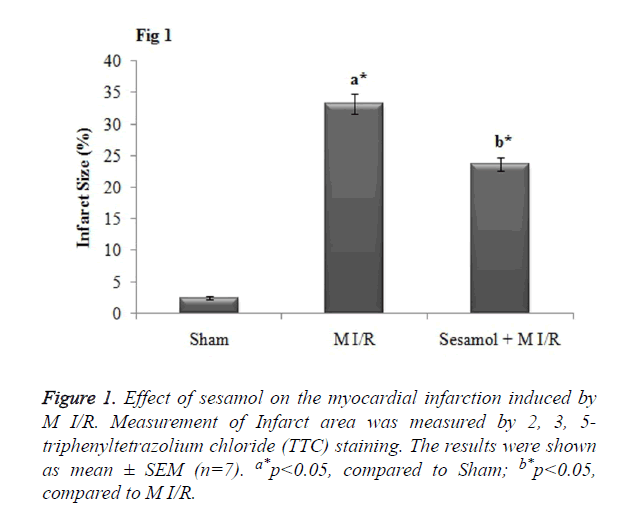
Twenty four hour after the I/R period, the whole heart was carefully removed, dissected into transverse slices of 2 mm thick sections and incubated in a 1% solution 2,3,5- triphenyltetrazolium chloride (TTC; Sigma, USA) for 15 min at 37°C. The infarct areas shows (white) and the ischemic area remain stained (red). The infarct size was measured in terms of as the ratio of the infarct area to the whole ventricular.
Estimation serum clinical markers of myocardial injury
After the reperfusion period, the blood was withdrawn by cardiac puncture, kept at 37°C for 30 min, and centrifuged at 4°C, 3000 g for 10 min. Then the separated serum was stored at -80°C for various biochemical analyses. The severity of cardiac injury was assessed by the estimation of lactate dehydrogenase (LDH), creatine kinase (CK) and cardiac troponin I (cTnI) in serum. LDH and CK levels were analysed by spectrophotometric methods using commercially available diagnostic kits (Biosino Bio-Technology and Science Inc., Beijing, China). ELISA technique was used for measuring the serum cTnI levels as per the manufacturer step wise instruction (Nanjing Jiancheng Bio-Engineering Institute, Nanjing, China).
Evaluation of antioxidants
After reperfusion, the myocardial tissue samples were homogenized (IKA, Staufen, Germany) in 10 ml of phosphate buffer at 48°C. Further, the homogenate was centrifuged at 300 g for 15 min. The level of antioxidants, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), reduced glutathione (GSH) in the supernatant was measured by spectrophotometer using commercially available kits. The protein content was measured using the Bradford method [14].
Evaluation of lipid peroxidation and Myeloperoxidase
The myocardial tissue level of malondialdehyde (MDA), an effective marker of lipid peroxidation and myeloperoxidase (MPO) activity were estimated using standard kits (Nanjing Jiancheng Bio-Engineering Institute, Nanjing, China).
Serum TNF-α and IL-1β concentrations
The serum level of proinflammatory cytokines (TNF-α and IL-1β) were detected by ELISA technique based on the protocol instructed by the manufactures (Huijia Biotechnology Company, Xiamen, China).
Detection of apoptotic proteins markers by western blotting
The ischemic myocardial tissue were isolated and homogenized in ice chilled RIPA buffer conditions (Applygen Technologies Inc., China). Then 20 μg of protein samples were resolved by 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) for Bcl-2, Bax and Caspase-3. The primary antibodies used in the study were as follows, Bcl-2, 1:750; Bax, 1:1,000; Caspase-3, 1:1,000 (Abcam, USA). The blot was thoroughly washed and incubated with HRP conjugated goat anti-mouse IgG (Abcam, USA). β-actin (1:5,000, Abcam, USA) was served as control. The binding of antibody was detected by ECL detection kit (Applygen Technologies Inc, China) and the protein bands were visualized using Gel Doc XR system (Bio-Rad, USA).
RT-PCR detection of inflammatory markers
From the frozen rat ischemic myocardial sample the RNA was isolated and purified using RNAase (Sigma, USA) according to the manufacturer’s protocol. The cDNAs was synthesized from 1 μg of the purified RNA sample. The oligonucleotide primers used in the study were shown in Table 1. The reaction was done for 30 cycles at using the following steps: 30 s denaturing at 95°C followed by a 30 s annealing step at 57°C. Finally the extension step was carried out for 1 min at 72°C. The densities were analyzed with computer based image analysis (Motic Images Advanced 3.2).
| S.No | Gene | Primer Sequence |
|---|---|---|
| 1 | NF-ƙB | Forward: 5'- CCTAGCTTTCTCTGAACTGCAAA-3' |
| Reverse: 5'- GGGTCAGAGGCCAATAGAGA-3' | ||
| 2 | IL-1ß | Forward: 5'- GAGGCTGACAGACCCCAAAAGAT -3' |
| Reverse: 5'- GCACGAGGCATTTTTGTTGTTCA-3' | ||
| 3 | TNF-a | Forward: 5'-ATGAGCACGGAAAGCATGATCCGA-3' |
| Reverse: 5'-CCAAAGTAGACCTGCCCGGACTC-3' | ||
| 4 | ß-actin | Forward: 5'GCACCACACCTTCTACAATG-3' |
| Reverse: 5'-TGCTTGCTGATCCACATCTG-3' |
Table 1. List of primers used in the RT-PCR analysis.
Statistics
The biochemical data are shown as mean ± SEM. The data were analysed using SPSS software v 17. The differences between the measured parameters among groups were done by one-way ANOVA. The Tukey’s test was applied for multiple comparisons. P values<0.05 were considered as statistically significant.
Results
Effect of sesamol on serum clinical markers of myocardial ischemia
The M I/R animals displayed marked elevation of LDH, CK and cardiac troponin I levels in the serum (p<0.05). By contrast, sesamol treatment significantly decreases the clinical markers level in the serum, compared with that of the M I/R group (p<0.05). The results were shown in Table 2.
| Groups | LDH (IU/L) | CK (IU/L) | cTnI (µg/ml) |
|---|---|---|---|
| Sham | 145.34 ± 5.43 | 103.76 ± 4.32 | 1.23 ± 0.23 |
| MI/R | 567.98 ± 10.76a* | 456.32 ± 9.76a* | 3.43 ± 0.56a* |
| Sesamol + MI/R | 176.65 ± 4.67b* | 112.43 ± 6.78b* | 1.41 ± 0.35b* |
| All values expressed as mean ± SEM (n=7). a* p<0.05, compared to Sham; b* p<0.05, compared to MI/R. LDH, Lactate Dehydrogenase; CK, creatine kinase; cTnI, Cardiac Troponin T. | |||
Table 2. Effect of Sesamol and myocardial I/R injury on serum cardiac markers.
Treatment with sesamol reduced the myocardial infarct size
As when compared to the sham rats, the infarct size was significantly (p<0.05) increased in the M I/R group. Meanwhile, sesamol pretreatment significantly (p<0.05) reduced the infarct size percentage (Figure 1).
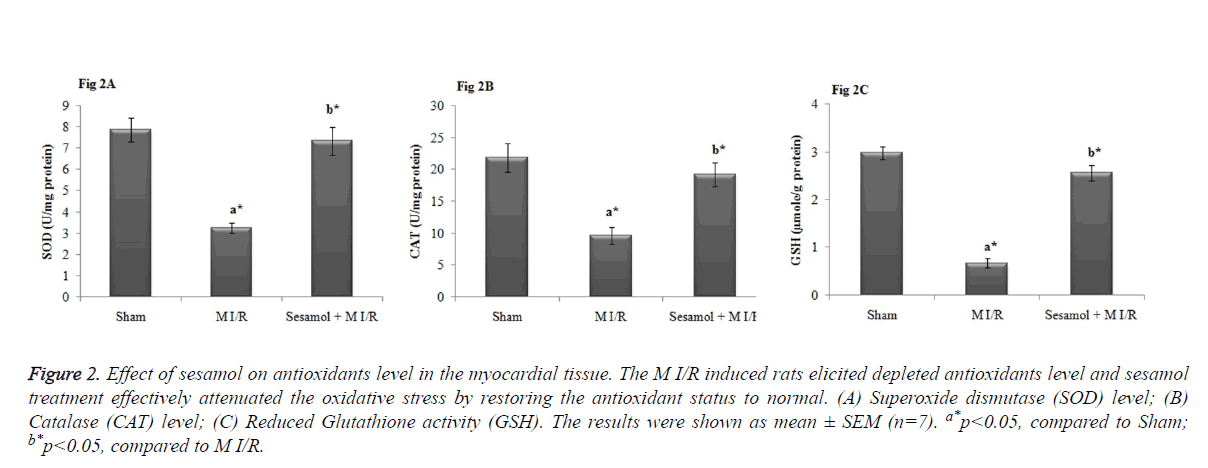
Effect of sesamol treatment on antioxidant status
As shown in Figure 2, myocardial levels of SOD, GPx and GSH were significantly elevated in the M I/R group, compared to sham operated group (P<0.05). Whilst, the inhibited antioxidant levels were significantly restored by sesamol treatment (P<0.05).
Figure 2. Effect of sesamol on antioxidants level in the myocardial tissue. The M I/R induced rats elicited depleted antioxidants level and sesamol treatment effectively attenuated the oxidative stress by restoring the antioxidant status to normal. (A) Superoxide dismutase (SOD) level; (B) Catalase (CAT) level; (C) Reduced Glutathione activity (GSH). The results were shown as mean ± SEM (n=7). a*p<0.05, compared to Sham; b*p<0.05, compared to M I/R.
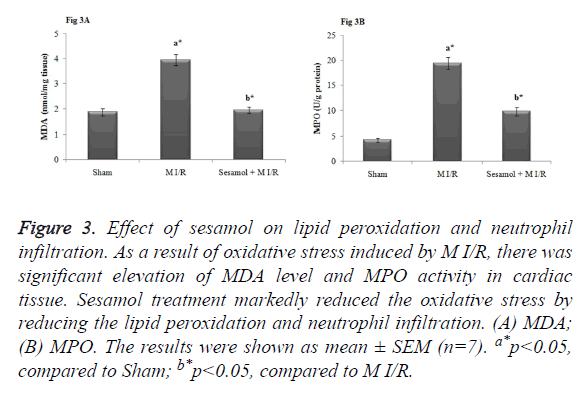
Sesamol inhibited the oxidative stress during myocardial I/R injury
In our study, M I/R rats displayed significant MDA, a lipid peroxidation marker and MPO, an index of neutrophil infiltration in myocardial tissue (p<0.05). In contrary, sesamol treatment significantly (p<0.05) attenuated the elevated MDA and MPO activity and thus inhibited the oxidative stress (Figure 3).
Figure 3. Effect of sesamol on lipid peroxidation and neutrophil infiltration. As a result of oxidative stress induced by M I/R, there was significant elevation of MDA level and MPO activity in cardiac tissue. Sesamol treatment markedly reduced the oxidative stress by reducing the lipid peroxidation and neutrophil infiltration. (A) MDA; (B) MPO. The results were shown as mean ± SEM (n=7). a*p<0.05, compared to Sham; b*p<0.05, compared to M I/R.
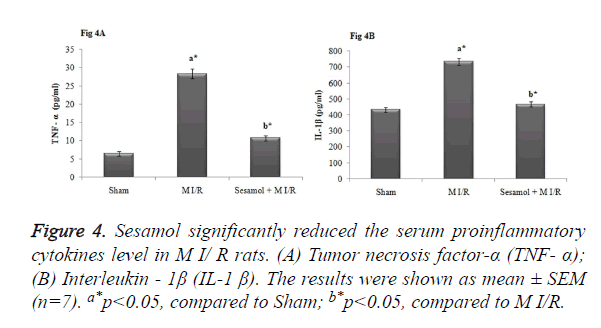
Sesamol reduced the serum level of TNF-α and IL-1β
As elicited in Figure 4, the level proinflammatory cytokines (TNF-α and IL-1β) were significantly elevated in the serum of M I/R rats (p<0.05). However, sesamol administered rats elicited substantial reduction of serum inflammatory markers to normal status (p<0.05).
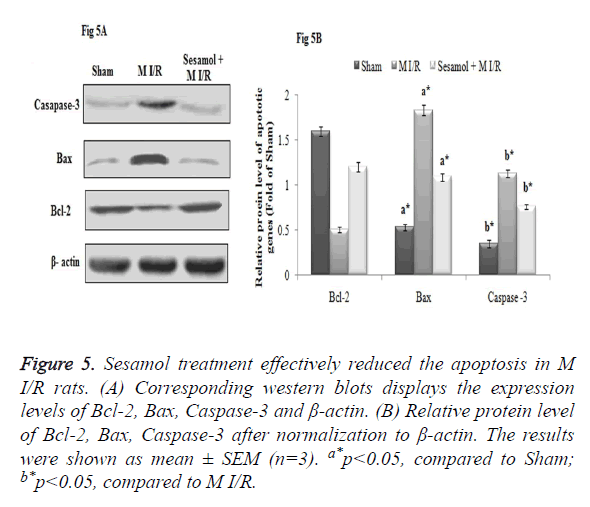
Effect of sesamol on myocardial apoptosis
In our study, myocardial I/R injury markedly affected the apoptosis process, which was evident from the increased protein expression of Caspase-3, Bax and decreased expression of Bcl-2 expression in myocardial tissue. Meanwhile, sesamol treatment downregulated the Caspase-3, Bax protein expression and upregulated the Bcl-2 protein expression effectively and thus inhibited the apoptosis (Figure 5A). The Relative protein level of Caspase-3, Bax protein expression was significantly higher and Bcl-2 was decreased in the M I/R group, compared to the sham rats (p<0.05). Compared to the M I/R group sesamol treated rats displayed significant (p<0.05) reduction of Caspase-3, Bax protein expression and elevation of Bcl-2 protein levels (Figure 5B).
Figure 5. Sesamol treatment effectively reduced the apoptosis in M I/R rats. (A) Corresponding western blots displays the expression levels of Bcl-2, Bax, Caspase-3 and β-actin. (B) Relative protein level of Bcl-2, Bax, Caspase-3 after normalization to β-actin. The results were shown as mean ± SEM (n=3). a*p<0.05, compared to Sham; b*p<0.05, compared to M I/R.
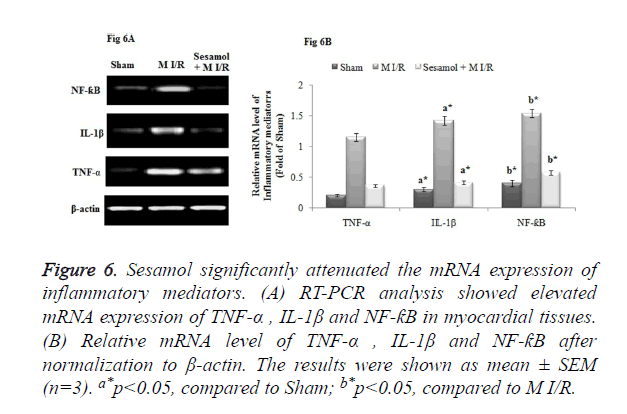
Sesamol down-regulated inflammatory markers mRNA expression profile in M I/R group
The RT-PCR analysis elicited the up-regulated mRNA gene expression of inflammatory markers, TNF-α, IL-1β and NF-ƙB in the ischemic myocardial tissue. Whilst, administration of sesamol downregulated the mRNA gene expression of TNF-α, IL-1β and NF-ƙB (Figure 6A). The Relative mRNA expression of TNF-α, IL-1β and NF-ƙB was significantly higher in the M I/R group, compared to the sham rats (p<0.05). Compared to the M I/R group, sesamol treated rats displayed significant (p<0.05) reduction of TNF-α, IL-1β and NF-ƙB mRNA expression levels (Figure 6B).
Figure 6. Sesamol significantly attenuated the mRNA expression of inflammatory mediators. (A) RT-PCR analysis showed elevated mRNA expression of TNF-α , IL-1β and NF-ƙB in myocardial tissues. (B) Relative mRNA level of TNF-α , IL-1β and NF-ƙB after normalization to β-actin. The results were shown as mean ± SEM (n=3). a*p<0.05, compared to Sham; b*p<0.05, compared to M I/R.
Discussion
The present study reveals the protective role of phenolic antioxidant, sesamol in myocardial I/R injury induced oxidative myocardial damage. Preclinical reports substantiates that oxidative stress is a prime factor in the etiology of myocardial I/R insult. In the event of reperfusion of ischemic heart, rampant generation of free radicals occurs which overture to form oxidative myocardial injury [15]. The ROS generated during reoxygenation of ischemic heart lead to the turbulence in the myocardial membrane, thus alters the integrity which results in the leakage of cardiac marker enzymes LDH and CK in the serum. Elevated cardiac marker enzymes in the serum are a severe indicator of myocardial membrane integrity [16]. On the other hand, cardiac troponins are the proteins molecule, which mediate calcium dependent actin and myosin interaction. Thus, cardiac Troponin-1 (cTnI) serves as a reliable diagnostic marker to assess myocardial infarction [17]. Sesamol treatment effectively restricted the leakage of cardiac markers in serum and thus preserves the myocardial membrane integrity [12]. TTC staining is a reliable technique to assess the infarct size, which reflects the degree of myocardial necrosis in the event of M I/R. During TTC staining, it interacts with LDH in the viable myocardial tissue to form red formazan precipitate, whereas the infracted area stains white with TTC [18]. In the current study, M I/R rats displayed elevated myocardial infarct size with lack of TTC stain absorbing effect, thus reflecting a LDH leakage from the heart. Meanwhile, sesamol treatment effectively reduced the infarct size by preventing the LDH leakage [12].
Evidences highlight that ischemic tissue generates excessive free radicals and other noxious products which adversely affects the vital biomolecules like lipids, proteins, carbohydrates and DNA, overture to oxidative myocardial damage [19]. The myocardial tissue is highly prone to oxidative attack due to the deprived state of antioxidants like SOD and CAT. This may lead to lipid peroxidation (LPO), distortion of membrane integrity and finally prelude to necrosis and cell death [20]. MDA is a toxic adduct formed during the LPO process and also serves as a reliable clinical indicator to asses LPO. Similarly in our study the myocardial MDA level was increased significantly in I/R rats. Whilst, the elevated MDA level was significantly attenuated by sesamol treatment mediated through its free radial scavenging ability [12].
During the initial period of reperfusion, rampant generation of superoxide and hydroxyl radicals are triggered in cardiac tissue. Whilst, in I/R conditions the antioxidants level are decreased which prone the myocardium to accelerated oxidative free radical attack [21,22]. The ROS orchestrate a predominate role in the myocardial I/R injury by substantially decreasing the body’s natural antioxidant molecules like SOD, GPx and GSH [22]. Mounting evidences elicit that inhibition of ROS may be a viable clinical option to attenuate oxidative stress conditions [23]. Antioxidant protection during stress conditions, encompasses a complicated network in which each antioxidant are interlinked and thus exhibits synergistic action. In the current study, sesamol intervention displayed significant elevation of antioxidants SOD, GPx and GSH in myocardium, mediated through free radical quenching and anti-lipid peroxidative effect. Array of preclinical reports underscore the antioxidant activity of sesamol in oxidative cardiac assault conditions [24,25].
Inflammation mediates a cardinal factor in the progression of myocardial I/R injury [26]. Increased release of pro inflammatory cytokines, inflammatory cells aggregation and infiltration are the noxious process in the inflammatory reactions [27]. Chemokines mediated neutrophils attraction towards the infarct zone after 6 h of reperfusion is the hallmark reaction during myocardial I/R injury. The attracted neutrophils overture to cause vascular spasm and further aggravate the release of ROS, and other toxic enzymes [28]. TNF-α is a key inflammatory cytokine secreted during inflammation, which kindle the inflammatory cascade by elevating the level of other inflammatory molecules like Interleukin-1β (IL-1β) and Interleukin-6 (IL-6) [29]. TNF-α, is the major cytokine involved in the initiation and progression of myocardial I/R injury and also worsen the conditions by upregulation the expression of cell adhesion factor. Furthermore, TNF-α blocks the cardiac contractility through negative inotropic effect and also elicits hypotensive activity. On the other hand, TNF-α induces myocardial apoptosis and involved in the cardiac remodelling [30]. Results of the earlier research studies show a significant interlink betwixt the neutrophil and I/R injury. So, mitigation of neutrophil activity may reduce the adverse events during myocardial I/R injury [31,32]. In the present study, myocardial I/R rats displayed elevated serum level of TNF-α, IL-1 β and tissue activity of MPO, a marker for neutrophil infiltration. However, sesamol pretreated M I/R rats displayed reduced level of TNF-α, IL-1 β and MPO as a result of significant anti-inflammatory effect. Mounting experimental evidences portrays the anti-inflammatory potential of sesamol in various pathological conditions.
Previous reports suggest that NF-κB act as an upstream signal transduction molecule and regulate the expression of genes involved in the apoptosis [33]. Thus, NF-κB plays a predominant role in early inflammatory process during myocardial I/R injury [34]. Further, activation of NF-κB leads to upregulated expression of TNF-α and other inflammatory mediators, neutrophil infiltration, loss of myocardial contractility, and activation of myocardial apoptosis leading to I/R injury [35,36]. In our study, NF-κB expression was significantly downregulated by sesamol treatment when compared myocardial I/R group. Previous reports suggest that sesamol effectively inhibits NF-κB activation in various pathological conditions [37,38].
Bcl-2 is associated with the Bcl family anti apoptotic proteins, which is majorly in the r impediment of cell death and escalation of cell generation and growth [39]. Bcl-2 in sync with Bax proteins orchestrates equilibrium between cell development and cell death [40]. Reports highlights that the Bcl-2 and Bax level have a predominant role in the protection or triggering of myocardial apoptosis in the event of I/R injury [41]. Caspase-3 is the cardinal death protease in the apoptotic process since it acts downstream molecule for other caspases and death-initiating proteins involved in the apoptotic cascade. The apoptotic effect of Caspase-3 is mainly rendered by chromatin condensation chromatin in nucleus and by DNA fragmentation process [42,43]. Thus, Caspase-3 serves as an effective indicator to study the degree cellular apoptosis. In this study, myocardial I/R rats showed upregulated expression of Bax, Caspase-3 and downregulated expression of Bcl-2. Meanwhile, sesamol treated rats displayed significant anti-apoptotic effect by reducing the expression of Bax, Casapase-3 and increasing the expression of Bcl-2. The anti-apoptotic effect of sesamol is widely reported in array of experimental studies [44-47].
In conclusion, the current research study exemplifies that sesamol mitigates myocardial ischemia/reperfusion injury. The protective mechanism is highly attributed due to infarct size reduction, prevention of myocardial membrane damage, blockade of lipid peroxidation and elevation of antioxidants. Further, the efficacy of sesamol may be due to inhibition of inflammation, neutrophil infiltration and apoptosis. However, further molecular and mechanistic perspective studies are warranted for the clinical utility of sesamol in ischemic conditions.
References
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 2013; 123: 92-100.
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007; 357: 1121-1135.
- Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res 2009; 81: 457-464.
- Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 2011; 23: 594-604.
- Woo CH, Eom YW, Yoo MH, You HJ, Han HJ, Song WK. Tumor necrosis factor-generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J Biol Chem 2000; 275: 32357-32362.
- Prasad NR, Menon VP, Vasudev V, Pugalendi KV. Radioprotective effect of sesamol on gamma-radiation induced DNA damage, lipid peroxidation and antioxidants levels in cultured human lymphocytes. Toxicology 2005; 209: 225-235.
- Kaur IP, Saini A. Sesamol exhibits anti-mutagenic activity against oxygen species mediated mutagenicity. Mutat Res 2000; 470: 71-76.
- Hsu D, Chu P, Chandrasekaran VRM, Liu M. Sesame lignan sesamol protects against aspirin-induced gastric mucosal damage in rats. J Funct Foods 2009; 1: 349-355.
- Narasimhan R, Vaithiyanathan M, Janardanam V. Neuroprotective effect of sesamol in glioma induced in rats. Biomed Int 2011; 2: 22-27.
- Chang CC, Lu WJ, Chiang CW, Jayakumar T, Ong ET, Hsiao G Potent anti-platelet activity of sesamol in an in vitro and in vivo model: pivotal roles of cyclic AMP and p38 mitogen-activated protein kinase. J Nutr Biochem 2010; 21: 1214-1221.
- Periasamy S, Chen SY, Hsu DZ, Liu MY. Comments on Vennila and Pugalendi. Vennila L, Pugalendi KV. Protective effect of sesamol against myocardial infarction caused by isoproterenol in Wistar rats. Redox Rep 2010; 15: 36-42. Redox Rep 2010; 15: 288-289.
- Chennuru A, Saleem MT. Antioxidant, Lipid Lowering, and Membrane Stabilization Effect of Sesamol against Doxorubicin-Induced Cardiomyopathy in Experimental Rats. Biomed Res Int 2013; 934239.
- Cao F, Sun D, Li C, Narsinh K, Zhao L, Li X. Long-term myocardial functional improvement after autologous bone marrow mononuclear cells transplantation in patients with ST-segment elevation myocardial infarction: 4 years follow-up. Eur Heart J 2009; 30:1986-1994.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248-254.
- Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem 1988; 263: 1353-1357.
- Rossoni G, Manfredi B, Civelli M, Berti F, Razzetti R. Combined simvastatin manidipine protect against ischemia-reperfusion injury in isolated hearts from normocholesterolemic rats. Eur J Pharmacol 2008; 587: 224-230.
- Jaffe AS, Landt Y, Parvin CA, Abendschein DR, Geltman EM, Ladenson JH. Comparative sensitivity of cardiac troponin I and lactate dehydrogenase isoenzymes for diagnosing acute myocardial infarction. Clin Chem 1996; 42: 1770-1776.
- Donnelly TJ, Sievers RE, Vissern FL, Welch WJ, Wolfe CL. Heat shock protein induction in rat hearts. A role for improved myocardial salvage after ischemia and reperfusion? Circulation 1992; 85: 769-778.
- Panda VS, Naik SR. Cardioprotective activity of Ginkgo biloba Phytosomes in isoproterenol-induced myocardial necrosis in rats: A biochemical and histoarchitectural evaluation. Exp Toxicol Pathol 2008; 60: 397-404.
- Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol 2005; 100: 179-190.
- Massoudy P, Becker BF, Seligmann C, Gerlach E. Preischaemic as well as postischaemic application of a calcium antagonist affords cardioprotection in the isolated guinea pig heart. Cardiovasc Res 1995; 29: 577-582.
- Hamilton KL. Antioxidants and cardioprotection. Med Sci Sports Exerc 2007; 39: 1544-1553.
- Zhang N, Andresen BT, Zhang C. Inflammation and reactive oxygen species in cardiovascular disease. World J Cardiol 2010; 2: 408-410.
- Vennila L, Pugalendi KV. Efficacy of sesamol on plasma and tissue lipids in isoproterenol-induced cardiotoxicity in Wistar rats. Arch Pharm Res 2012; 35: 1465-1470.
- Nayak PG, Paul P, Bansal P, Kutty NG, Pai KS. Sesamol prevents doxorubicin-induced oxidative damage and toxicity on H9c2 cardiomyoblasts. J Pharm Pharmacol 2013; 65: 1083-1093.
- Xiong J, Xue FS, Yuan YJ, Wang Q, Liao X. Cholinergic anti-inflammatory pathway: a possible approach to protect against myocardial ischemia reperfusion injury. Chin Med J 2010; 123: 2720-2726.
- Speyer CL, Ward PA. Role of endothelial chemokines and their receptors during inflammation. J Invest Surg 2011; 24: 18-27.
- Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res 2004; 61: 481-497.
- Khimenko PL, Bagby GJ, Fuseler J, Taylor AE. Tumor necrosis factor-alpha in ischemia and reperfusion injury in rat lungs. J Appl Physiol (1985) 1998; 85: 2005-2011.
- Zhu J, Liu M, Kennedy RH, Liu SJ. TNF-alpha-induced impairment of mitochondrial integrity and apoptosis mediated by caspase-8 in adult ventricular myocytes. Cytokine 2006; 34: 96-105.
- Ma XL, Lefer DJ, Lefer AM, Rothlein R. Coronary endothelial and cardiac protective effects of a monoclonal antibody to intercellular adhesion molecule-1 in myocardial ischemia and reperfusion. Circulation 1992; 86: 937-946.
- Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation 2001; 103: 2296-2302.
- Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell 2004; 6: 203-208.
- Moss NC, Stansfield WE, Willis MS, Tang RH, Selzman CH. IKKbeta inhibition attenuates myocardial injury and dysfunction following acute ischemia-reperfusion injury. Am J Physiol Hear Circ Physiol 2007; 293: H2248-2253.
- Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ Res 2011; 108: 1122-1132.
- Yang J, Jiang H, Yang J, Ding JW, Chen LH, Li S. Valsartan preconditioning protects against myocardial ischemia-reperfusion injury through TLR4/NF-kappaB signaling pathway. Mol Cell Biochem 2009; 330: 39-46.
- Wu XL, Liou CJ, Li ZY, Lai XY, Fang LW. Sesamol suppresses the inflammatory response by inhibiting NF-κB/MAPK activation and upregulating AMP kinase signaling in RAW 264.7 macrophages. Inflamm Res 2015; 64: 577-588.
- Chu PY, Hsu DZ, Hsu PY, Liu MY. Sesamol down-regulates the lipopolysaccharide-induced inflammatory response by inhibiting nuclear factor-kappa B activation. Innate Immun 2010; 16: 333-339.
- Deng X. Bcl2 Family Functions as Signaling Target in Nicotine-/NNK-Induced Survival of Human Lung Cancer Cells. Scientifica (Cairo) 2014; 2014: 215426.
- Safaeian L, Abed A, Vaseghi G. The role of Bcl-2 family proteins in pulmonary fibrosis. Eur J Pharmacol 2014; 741: 281-289.
- Holly TA, Drincic A, Byun Y, Nakamura S, Harris K. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J Mol Cell Cardiol 1999; 31: 1709-1715.
- Thapaliya S, Wree A, Povero D, Inzaugarat ME, Berk M. Caspase-3 inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced NASH model. Dig Dis Sci 2014; 59: 1197-1206.
- Porter AG, Jänicke RU. Emerging roles of Caspase-3 in apoptosis. Cell Death Differ 1999; 6: 99-104.
- Khan S, Kumar A, Adhikari JS, Rizvi MA. Protective effect of sesamol against 60Co γ-ray-induced hematopoietic and gastrointestinal injury in C57BL/6 male mice. Free Radic Res 2015; 49: 1344-1361.
- Hemalatha G, Pugalendi KV, Saravanan R. Modulatory effect of sesamol on DOCA-salt-induced oxidative stress in uninephrectomized hypertensive rats. Mol Cell Biochem 2013; 379: 255-265.
- Hemshekhar M, Thushara RM, Jnaneshwari S, Devaraja S, Kemparaju K, Girish KS. Attenuation of adjuvant-induced arthritis by dietary sesamol via modulation of inflammatory mediators, extracellular matrix degrading enzymes and antioxidant status. Eur J Nutr 2013; 52:1787-1799.
- Kondamudi PK, Kovelamudi H, Mathew G, Nayak PG, Rao MC, Shenoy RR. Investigation of Sesamol on Myeloperoxidase and Colon Morphology in Acetic Acid-Induced Inflammatory Bowel Disorder in Albino Rats. The Scientific World Journal. 2014; 2014:802701.