Case Report - Current Trends in Cardiology (2021) Volume 5, Issue 4
Cardiac magnetic resonance depiction of left ventricular cardiomyopathy secondary to roundworm infection
Onkar B Auti1, Arul Narayanan2, Vimal Raj3*
1Department of Radiology, Ruby Hall Clinic, Pune, India
2Department of Pediatrics, Narayana Hrudayalaya, Bangalore, India
3Department of Radiology, Narayana Hrudayalaya, Bangalore, India
- Corresponding Author:
- Vimal Raj
Department of Radiology
Narayana Hrudayalaya
Bangalore, India
Tel: +91 9538659316
E-mail: drvimalraj@gmail.com
Accepted date: 04 June, 2021
Citation: Auti OB, Narayanan A, Raj V. Cardiac magnetic resonance depiction of left ventricular cardiomyopathy secondary to roundworm infection. Curr Trend Cardiol. 2021;5(4):40-42.
Abstract
Background: Cardiac manifestations of hyper-eosinophilia can have high morbidity with about 40-50% of patients having signs and symptoms of cardiac diseases. We present a case of Hyper- Eosinophilic Cardiomyopathy (HEC) due to Strongyloide Stercoralis (S. Stercoralis) infection with its characteristic Cardiac Magnetic Resonance (CMR) appearances.
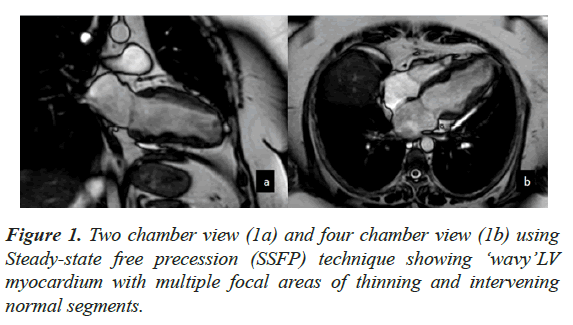
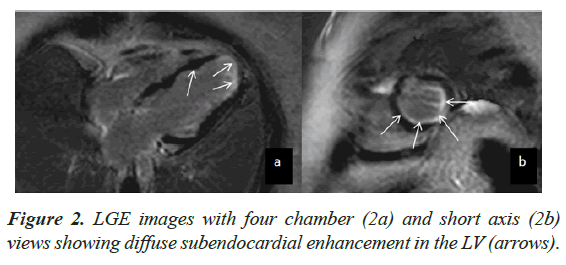
Case presentation: An adult female underwent CMR for evaluation of LV dysfunction. At the time of examination, she had peripheral eosinophilia and had a positive history of Strongyloide infection. CMR showed moderate LV dysfunction with LVEF of 43%. The myocardium demonstrated a ‘wavy’ appearance with multiple focal areas of thinning and intervening normal segments. There was diffuse subendocardial enhancement on Late Gadolinium Enhancement, which did not fit any vascular territory.
Conclusion: CMR imaging is a useful tool in assessment of HEC by providing early diagnosis. HEC can also occur due to parasitic infection such as Strongyloide (roundworm) and may demonstrate unique/characteristic appearances of the myocardium on CMR.
Keywords
Hyper-eosinophilic cardiomyopathy, Restrictive cardiomyopathy, Cardiac MRI, Peripheral eosinophilia, Loffler endocarditis.
Background
Cardiac manifestations due to hyper-eosinophilia can cause high morbidity with dire prognosis [1]. Literature shows about 40%- 50% of hyper-eosinophilic syndrome patients will have signs and symptoms of cardiac disease [2]. However, Hyper-Eosinophilic Cardiomyopathy (HEC) due to Strongyloide stercoralis (S. Stercoralis) or roundworm infection is a rare presentation [3].
CMR is an excellent modality in evaluation of patients with cardiomyopathy. In this report, we present the characteristic CMR features of HEC due to roundworm infection.
Case Presentation
A 39-year-old female presented to the cardiologist with a history of recent onset of acute chest pain. Electrocardiography (ECG) showed normal sinus rhythm with no features of ischemia or infarction. Echocardiography showed LV systolic dysfunction with global hypo kinesis. There was no LV hypertrophy or significant valvular incompetency. CMR was planned to look for the cause of LV dysfunction.
Standard CMR sequences were performed without and with contrast (1.5 Tesla, Achieva System, Philips, Best, and The Netherlands). These included: Black blood spin echo static images to establish anatomy and assess the pericardium, Cine imaging using Steady-state Free Precession (SSFP) sequences in standard cardiac planes to establish cardiac function. Edema sequences and post contrast Late Gadolinium Enhancement (LGE) imaging was performed to assess the myocardial morphology.
CMR findings included a non-dilated LV with systolic dysfunction and an LV Ejection Fraction (EF) of 43%. The myocardium demonstrated a ‘wavy’ appearance with multiple focal areas of thinning and intervening normal segments associated with hypo kinesis (Figure 1). There was also restricted vertical lengthening of the LV, suggestive of underlying diastolic dysfunction. No LV hypertrophy was seen. There was further intraventricular dyskinesis in the apical cavity. Increased trabeculations with sluggish flow was seen in the LV apex suspicious of early thrombosis. The Right Ventricle (RV) was normal in appearance with preserved systolic function. Left atrial dilatation was noted without significant mitral regurgitation. No myocardial edema was seen. On LGE imaging, there was diffuse sub endocardial enhancement which did not fit any vascular territory (Figure 2).
Overall CMR appearances were suggestive of restrictive LV cardiomyopathy with focal areas of myocardial fibrosis and early LV thrombosis. On review of patient’s history and laboratory records, it was seen that the patient had peripheral eosinophilia and was positive for Strongyloide infection. Patient was also on long term steroid therapy for bronchial asthma. She was started on anti-parasitic treatment for roundworm and showed significant symptomatic improvement on follow up visits. The LV function also became normal on echo.
Based on the CMR and other investigations the diagnosis of hyper- eosinophilia related endocarditis was made with roundworm infection being the main cause.
Discussion
Hyper Eosinophilic Syndrome (HES) is defined by persistent hyper eosinophilia (>1500 eosinophils/mm3) for more than 6 months [2]. There are multiple causes of peripheral eosinophilia including idiopathic, parasitic infection, allergy, malignancies, Loffler syndrome, Churg-strauss syndrome, allergic bronchopulmonary aspergillosis and connective tissue disorders [4-6]. Detailed clinical history and examination is needed to look for the cause of peripheral eosinophilia. Cardiac manifestations of hyper-eosinophilia include congestive heart failure and restrictive cardiomyopathy [7]. Restrictive cardiomyopathy pattern in hyper eosinophilia is due to toxic damages produced by activated eosinophils, which provoke endo myocardial fibrosis with obliteration of the right and left ventricles. It is characterized by three stages a) an acute necrotic stage, where direct endo myocardial infiltration and damage occurs, b) an intermediate phase, in which thrombi form along damaged endocardium especially in areas of relative stasis such as the ventricular apices and c) a fibrotic stage, which is characterized by endo myocardial fibrosis [2,7]. Involvement of RV is more common than LV [8].
CMR imaging is an excellent tool for the evaluation of cardiac disease in hyper eosinophilia. LGE imaging is capable of detecting myocardial fibrosis and inflammation. Specific pattern of LGE in hyper eosinophilia is due to presence of fibrotic tissue only in the sub endocardium which is unrelated to any particular vascular territory [3]. ‘Eosinophilic thrombus’ an early pathology of Loffler endocarditis can also be clearly demonstrated with LGE in CMR which differentiates apical thrombus from endocardial fibrosis [9]. Lack of myocardial wall thinning and transmural enhancement differentiates the endocarditis from infarction [7]. Other features of restrictive cardiomyopathy like obliterated bi-ventricular apices, bi atrial dilatation, mitral and tricuspid regurgitation can be easily detected on CMR [8]. CMR can also provide guidance regarding the optimal anatomic site for biopsy as well as evaluation of treatment response [10].
This case was different from the classical description as the RV was spared and there was no obliteration of the ventricular cavities. The ‘wavy’ pattern seen in this case is also not a well described entity and could represent a specific feature of roundworm infestation. Allergic causes of HEC are more frequent in developed countries and parasitic infections remain major cause in tropical and developing countries. Our case showed features of endocarditis (restrictive physiology with diffuse endocardial enhancement and early thrombosis) with history of Strongyloide infection and hyper- eosinophilia. This case also highlights the importance of picking up these pathologies early and starting appropriate therapy.
Conclusion
CMR imaging is a useful tool in assessment of HEC by providing early diagnosis. HEC can also occur due to parasitic infection such as Strongyloide and may demonstrate unique/characteristic appearances of the myocardium on CMR.
Declarations
Consent for publication
Written and informed consent from the patient was obtained to publish the clinical details and CMR images.
Written and informed consent from all contributing authors was obtained for submission of manuscript to the journal.
Conflicts of interests
No conflicts of interests.
Author contributions
All authors have contributed significantly in this case report.
Acknowledgements
None.
Funding
Not applicable.
Ethics approval
As above.
References
- Genée O, Fichet J, Alison D. Cardiac magnetic resonance imaging and eosinophilic endomyocardial fibrosis. Circulation. 2008;118(23):710-1.
- Ogbogu PU, Rosing DR, Horne III MK. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27(3):457-75.
- Alizadeh-Sani Z, Vakili-Zarch A, Kiavar M, et al. Eosinophilic endomyocardial fibrosis and strongyloides stercoralis: a case report. Res Cardiovasc Med. 2013;2(2):104-5.
- Seifert M, Gerth J, Gajda M, et al. Eosinophilia--a challenging differential diagnosis. Medizinische Klinik (Munich, Germany: 1983). 2008;103(8):591-7.
- Helbig G, Wieczorkiewicz A, Dziaczkowska-Suszek J, et al. T-cell abnormalities are present at high frequencies in patients with hypereosinophilic syndrome. haematologica. 2009;94(9):1236-41.
- Cincin AA, Ozben B, Tanrikulu MA, et al. Large apical thrombus in a patient with persistent heart failure and hypereosinophilia: Löffler endocarditis. J Gen Intern Med. 2008;23(10):1713-8.
- Cummings KW, Bhalla S, Javidan-Nejad C, et al. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics. 2009;29(1):89-103.
- Jagia P, Gulati GS, Sharma S. Cardiac magnetic resonance in the assessment of cardiomyopathies. Indian J Radiol Imaging. 2007;17(2):109.
- Chang SA, Kim HK, Park EA, et al. Loeffler endocarditis mimicking apical hypertrophic cardiomyopathy. Circulation. 2009;120(1):82-5.
- Hilty KC, A Koonce J, W Ston R, et al. The role of cardiac MRI in the diagnosis and management of Loeffler’s endocarditis: a case report with clinical and pathologic correlation. Open Cardiovasc Imaging J. 2010;2(1):10-13