Research Article - International Journal of Respiratory Medicine (2023) Volume 8, Issue 5
BRONCHOPULMONARY INVOLVEMENT IN DISSEMINATED KAPOSI�S SARCOMA - ATYPICAL CLINICAL PRESENTATION IN AN AFRICAN IMMUNOCOMPETENT PATIENT: A CASE REPORT
Bashir Taiye Aminu1*, I. A. Hauwa2, M. I. Maryam2, T. Oloyede1, A. B. Hussain3, M. B. Maiyaki2, M. Babashani21Department of Internal Medicine Federal Teaching Hospital, Katsina, Nigeria
2Aminu Kano Teaching Hospital, Kano, Nigeria
3Murtala Muhammad Specialist Hospital, Kano, Nigeria
- *Corresponding Author:
- Bashir Taiye Aminu
Department of Informatics Engineering
University of Coimbra, Coimbra, Portugal
E-mail: aminbashyr85@gmail.com
Received: 30-Sep-2023, Manuscript No. AAIJRM-23-118938; Editor assigned: 02-Oct-2023, PreQC No. AAIJRM-23-118938(PQ); Reviewed: 16-Oct-2023, QC No. AAIJRM-23-118938; Revised: 16-Oct-2023, Manuscript No. AAIJRM-23-118938(R); Published: 23-Oct-2023, DOI: 10.35841/aaijrm-8.5.166
Citation: Aminu B.T. Bronchopulmonary involvement in disseminated Kaposi’s sarcoma - atypical clinical presentation in an African immunocompetent patient: A case report. Int J Respir Med. 2023; 8(5):166
Abstract
Bronchopulmonary Kaposi’s sarcoma is not common even among HIV infected patient despite KS being recognized as an AIDS-defining illness. Pulmonary involvement is even particularly rare especially in immunocompetent individuals. This is a rare case of atypical presentation of disseminated KS with florid bronchopulmonary involvement in a young immunocompetent patient who had masquerading symptoms and diagnostic challenges at initial presentation. Following diagnosis, this 40 year old immunocompetent Nigerian patient with KS responded dramatically to combination of chemotherapy. This affirms the importance of considering KS as possible diagnosis having ruled out tuberculosis, fungal lung diseases and malignancies in patients with suspicious symptoms and radiological findings.
Keywords
Bronchopulmonary Kaposi’s sarcoma, HIV Infection, Chemotherapy, Lung Diseases, Hyper-Immune Malaria Syndrome.
Introduction
Kaposi’s sarcoma (KS) is an angioproliferative disorder that was first described by Moritz Kaposi Kohn in the late 1800s as a highly vascularized tumour that is commonly associated with human herpes virus 8 (HHV-8) and human immunodeficiency virus (HIV), even though KS has been discovered and described before HIV [1,2]. Following the dramatic increase in the prevalence of KS in the late 1900s, it was found to be the most common malignancy associated with immunodeficiency disease [3]. Generally, visceral Kaposi’s sarcoma lesions are less commonamongtheHIV-negativepatientswhencomparedto theHIVinfectedindividuals[4]. Bronchopulmonary KS is not a common presentation in immunocompetent patient especially in association with diffuse cutaneous KS that is commonly seen as an AIDS defining illness [5].
Case Presentation
A 40 year old butcher from North-western Nigeria who was first seen at our facility on 11th August, 2020 with a month history of progressively increasing left flank swelling and pains, he also had intermittent high grade fever with associated easy fatigability, dizziness and progressive weight loss. Then, there was no cough, skin rashes or discolorations, no bleeding from any body parts. He has never smoked cigarette. Examinations then were notable for palor, tachycardia without lymphadenopathy, digital clubbing or oedema. Chest findings were not remarkable. Massive, firm, non-tender splenomegaly of 20cm below with left costal margin was observed with no ascites and other systems were found to be normal. Provisional diagnosis of hypersplenism likely secondary to chronic myeloid leukaemia (CML) KIV Hyper-immune malaria syndrome (HIMS) was made. Investigations done included a complete blood count with pancytopenia with haemoglobin of 8.7g/dL, WBC was 1.83, and platelet was 102. ESR was 56mm/hour, peripheral blood film showed microcytic hypochromic cells with leucopenia and neutrophilic toxic granulations. Bone marrow aspiration also showed mildly megaloblastic marrow with toxic granulation of myeloid cells. Abdominopelvic ultrasound and CT revealed features of complex splenic mass with differentials of lymphoma, organized haematoma. Patient had antibiotics, proguanil, folic acid, and haematinics. He was followed up at the medical outpatient clinic over several months with no new onset complaints. Following remarkable improvement, progressive decrease in spleen size (to about 9cm) and improved hemodynamic status, patient was lost to follow up.
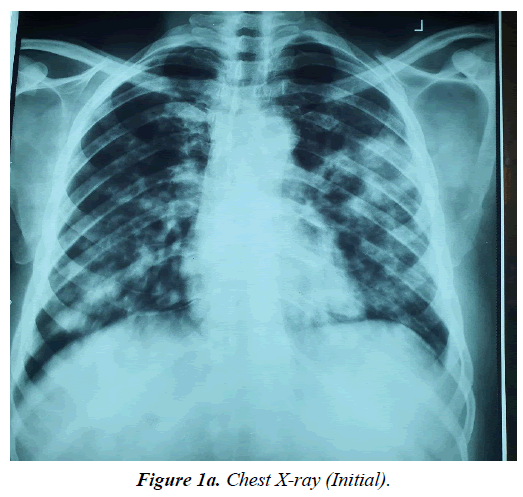
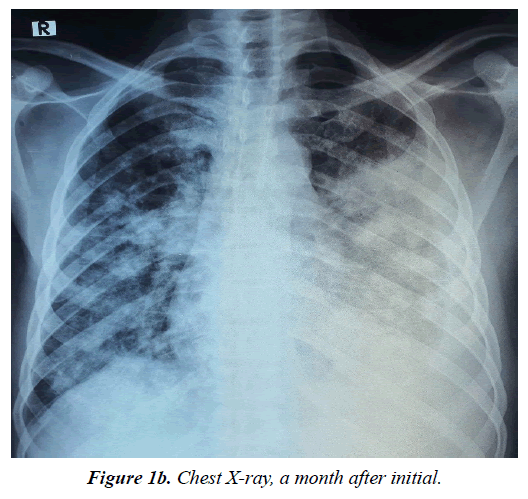
Patient represented at the facility through the emergency room (ER) on 30th July, 2023 with 4 months history of gradual onset of cough productive of yellowish non-copious sputum, associated with haemoptysis and pleuritic chest pains and progressive shortness of breath (SOB), with worsening bilateral legs swelling. He has anorexia, night sweats, some weight loss but no fever. Prior to presentation, he was said to have been seen at a peripheral facility and commenced on anti-Koch’s on account of a suspicious chest X-ray (CXR) findings (Figure 1a and 1b) as a geneXpert negative DTB.
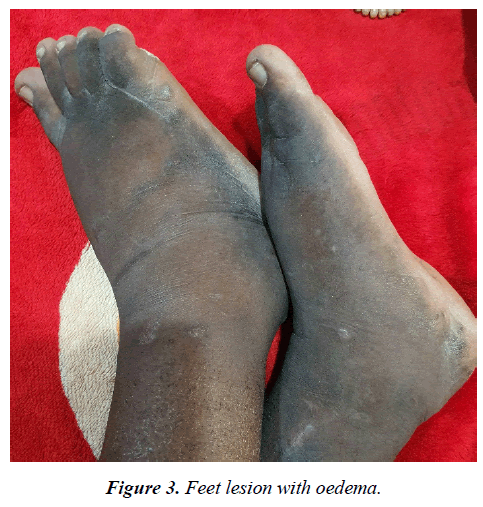
Physical examination revealed a dark-skinned young man in obvious respiratory distress, dyspnoeic, pale, not febrile, no digital clubbing. He had generalized, palpable and discrete lymph nodes in the submental, submandibular, cervical, supraclavicular, axillary and inguinal regions. Notably were violaceous skin lesions involving the palms and the feet, bilateral non-pitting, woody hard pedal oedema (figure 2 and 3) Pulse rate was 94/minute, blood pressure at 100/60mmHg. He had a respiratory rate of 36/minute and with an oxygen saturation of 78% on room air, trachea was central, with symmetrical chest. There was reduced chest expansion on the left, dull percussion note over the left mid and lower chest zone and wide spread coarse crackles on all lung fields bilaterally. Patient still had splenomegaly of 10 cm below the left costal margin.
The diagnosis of Lymphoproliferative disorder with metastasis to the lungs was made provisionally with differentials of CML, ALL, CLL. Repeat CXR (Figure 1b) had done revealed left middle and lower lobe opacity, loss of costophrenic and cardiophrenic angles with patchy deposits on the entire right lung. Repeated ELISA HIV infection tests done were negative, Haemoglobin was 10g/dL, WBC were 1.93 x 109/L, and ESR was 72mm/hour. ECG and ECHO were essentially normal. Sputum work-ups for both GeneXpert and Alcohol Acid Fast Bacilli were negative for TB. Chest CT (Figure 4 and 5) reveal left middle lobe peripheral mass with wide spread nodular hyper-densities and hilar lymph nodes. No bone involvement or liver metastasis. Doppler ultrasound of both lower limbs showed normal vascular studies.
Patient had bronchoscopy which revealed hyperaemic and inflamed mucosa in left lower bronchus with contact bleeding (Figure 6 and 7), biopsies were taken for histology, and bronchoalveolar lavage (BAL) was also taken for cytology, microbiology and geneXpert. All BAL analysis came out negative for malignancy and infection, while the histology report was reported to be difficult to differentiate from a well differentiated carcinoma, lung biopsy was advised. Cervical lymph node biopsy was taken for histological studies and result revealed silt-like to jagged vascular channels surrounded by cell with hyper-chromatic nuclei focally forming fascicles in keeping with Kaposi’s sarcoma (still found to be same following histology review). S100, CD34 and HHSV8 immunochemistry is advised by the pathologist.
The patient was co-managed by the pulmonology and clinical haematology units. He had Meropenen based on Sputum culture and sensitivity for E.cloi, antifungal with oxygen supplementation to maintain the saturation. Closed thoracostomy tube drainage (CTTD) was inserted to drain the left sided haemorrhagic effusion. Recombinant Human Granulocyte colony stimulating Factor was given to improve the Cytopoenias. He was then placed on Allopurinol followed by chemotherapy with Adriamycin, Bleomycin and Vincristine (ABV).
His condition improved remarkably and was discharged subsequently to be followed up.
Discussion
Kaposi’s sarcoma is the most frequent cancer connected to HIV infection [1]. It is an angio-proliferative condition that is frequently linked to human herpes virus 8 [1]. Endothelial cells of lymphatic or blood arteries are the source of KS. It was regarded as a rare disease until the AIDS epidemic in the late 1900s, but over the past three decades, KS has become more well-known [6]. It is extremely rare for visceral involvement to occur without skin signs, despite the fact that a small number of instances have been recorded as solitary visceral manifestations of KS [2]. Lungs, liver, spleen, gastrointestinal tract, and lungs can all be involved by visceral Kaposi [2]. The most common manifestations of pulmonary KS include increasing dyspnoea, dry cough, and low-grade fever. Pulmonary KS can however show a wide range of signs and symptoms [7]. The diagnosis of KS lesions in the lungs depends heavily on imaging, especially CT [2]. However, it is imperative to carry out a bronchoscopy in order to confirm pulmonary involvement [2]. Since pulmonary involvement is linked to a worse prognosis and higher mortality than other systems, it is especially crucial [1].
There are four primary subtypes of KS, each with a variety of clinical manifestations: immunocompromised, endemic or African, classic or sporadic, and AIDS-related or epidemic [8]. The index patient likely has the endemic subtype. The KS variant of AIDS has primarily been associated with homosexual men. Less case has been documented among women and heterosexuals [8]. Classical Kaposi's sarcoma is rare and more frequently affects males over 60 who are of Mediterranean or Ashkenazi Jewish ancestry [9]. Immunocompromised individuals undergoing immunosuppressive therapy for organ transplants or those with haematological malignancies have a higher risk of developing Kaposi's sarcoma, as is widely known [4].
Clinical and radiologic criteria frequently fail to discriminate between opportunistic pneumonia and Kaposi's sarcoma of the lung in HIV-infected, even though both conditions may exist in the same patient, same in those that are HIVnegative. Dyspnoea, cough, and rarely haemoptysis and stridor are among the most prevalent clinical signs of Kaposi's sarcoma of the lung [3]. The patient presented had fever and haemoptysis but no stridor. To distinguish between pulmonary Kaposi's sarcoma and opportunistic pneumonia, fiberoptic bronchoscopy and broncho-alveolar lavage and/or biopsy continue to be the standard investigations [10]. Following the findings by Daley et al and a similar study in Rwanda where KS was found to account for a very minute proportion of flexible bronchoscopic findings, it was concluded that in developing countries where tuberculosis is widespread, that bronchoscopy with bronchoalveolar lavage added little to the diagnosis and should not be utilised as part of normal work-up [3].
The lesions in HIV-negative patients differed from those in HIV-positive patients in relation to the rapid progression of the lesions to more advanced stage lesions, despite previous reports that the histopathological features seen in the KS lesions in HIV-positive and HIV-negative patients were similar at each stage of development [4]. Recent research found no discernible difference between HIV-positive and HIVnegative patients in the presence of plasma cells, protuberant blood vessels, hyaline globules, red cell extravasation, and hemosiderin in the lesions [4].
Bilateral involvement, ill-defined patchy opacities, hilar lymph node enlargement, and pleural effusion are typical radiographic characteristics of pulmonary Kaposi's sarcoma (Figure 1a and Figure 1b). Index patient presented with the prototypic CXR findings and CT scan (Figure 4 and Figure 5) that showed similar left middle lobe peripheral mass with wide spread nodular opacities and hilar lymph nodes.
Although neutropenia may restrict its efficacy, systemic chemotherapy is thought to be the most effective treatment for pulmonary Kaposi's sarcoma, [3] this necessitated the administration of Recombinant Human Granulocyte colony stimulating Factor in our patient that already had pancytopenia. The most often used chemotherapy drugs include Paclitaxel, Adriamycin, Bleomycin, Vincristine, Liposomal Daunorubicin, and Doxorubicin. [3] It has been discovered that combination chemotherapy significantly improves clinical and functional outcomes compared to a single drug. Our patient had combination chemotherapy with good response and tolerance as there were gradual reduction in need for oxygen supplementation, dyspnoea, reduction in the sizes of the bilateral lower limb lymphedema and no more report of haemoptysis. He was subsequently discharged from the hospital and continued follow-up and chemotherapy on out-patient basis.
Pulmonary Kaposi’s sarcoma although rare in the immunocompetent individuals should be considered in the differential diagnosis of any patient with haemoptysis and pulmonary opacities on imaging especially after exclusion of the TB, Aspergilla, and other malignancies like Bronchogenic carcinoma and lymphoma.
References
- Diaz R, Almeida P, Morgan D. Rare presentation of bronchopulmonary Kaposi sarcoma. BMJ Case Rep. 2019;12(8):e229436.
- Bashar N, Innes N, Orrell J. Bronchopulmonary Kaposi's sarcoma. Respir Med Case Rep. 2015;15:45-7.
- Ussiri EV, Lema LE, Mbembati NA, et al. Kaposi's Sarcoma Of The Lung: A Case Report. S Afr J Surg. 2004;9(2):49-53.
- Hong A, Lee CS. Kaposi’s sarcoma: clinico-pathological analysis of human immunodeficiency virus (HIV) and non-HIV associated cases. Pathol Oncol Res. 2002;8:31-5.
- Garay SM, Belenko M, Fazzini E, et al. Pulmonary manifestations of Kaposi’s sarcoma. Chest. 1987;91(1):39-43.
- Yoo DJ, Lee KH, Munderi P, et al. Clinical and bronchoscopic findings in Ugandans with pulmonary Kaposi's sarcoma. Korean J Intern Med. 2005;20(4):290.
- Kumar T, Epstein M, Markovskaya Y, et al. Bronchoscopy and endobronchial disease in patients with human immunodeficiency virus infection. Indian J Chest Dis Allied Sci. 2011;53(2):99.
- Shiels RA. A history of Kaposi's sarcoma. J R Soc Med. 1986 Sep;79(9):532-4.
- DiGiovanna JJ, Safai B. Kaposi's sarcoma: Retrospective study of 90 cases with particular emphasis on the familial occurrence, ethnic background and prevalence of other diseases. The American Journal of Medicine. 1981;71(5):779-83.
- Hanson PJ, Harcourt-Webster JN, Gazzard BG, et al. Fibreoptic bronchoscopy in diagnosis of bronchopulmonary Kaposi's sarcoma. Thorax. 1987;42(4):269-71.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref