Research Article - Journal of Clinical Oncology and Cancer Research (2020) Volume 3, Issue 1
Bone marrow involvement in non-small cell lung cancer: Detection of disseminated tumor cells and cancer stem cells.
SV Chulkova1* and NN Tupitsyn2
1Department of Immunology ?aematopoiesis, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia, Russia
2Department of ?aematopoiesis Immunology, N.N. Blokhin National Medical Research Center of Oncology, Ministry of Health of Russia, Russia
- Corresponding Author:
- SV Chulkova
Department of Immunology ?aematopoiesis
N.N. Blokhin National Medical Research Center of Oncology
Ministry of Health of Russia
Russia
E-mail: chulkova@mail.ru
Accepted date: 17 July, 2020
Citation: Chulkova SV, Tupitsyn NN. Bone marrow involvement in non-small cell lung cancer: Detection of disseminated tumor cells and cancer stem cells. Allied J Clin Oncol Cancer Res 2020;3(1):40-45.
Abstract
Introduction: There is evidence that disseminated tumor cells (DOCs) in the bone marrow (BM) are
precursors of subsequent distant metastases. The detection of DOCs in non-small cell lung cancer
(NSCLC) will provide important information about the features of metastasis, as well as the
possibilities of identifying new targets for the treatment of NSCLC.
Purpose of the study: Is to evaluate the possibility of detection DOCs in BM and to identify the
frequency of BM involvement in patients with NSCLC, as well as their effect on the population of bone
marrow lymphocytes.
Materials and methods: 62 bone BM of NSCLC patients were studied by morphological and
immunological methods. Doc’s analysis was performed using flow cytometry (FACS Canto II, USA,
Kaluza Analysis v2.1 software), monoclonal antibodies to CD45, cytokeratins directly labeled with
various fluorochromes were used.
Results: DOCs (EPCAM+CD45-) in the BM were found in 43.5% of patients (threshold level: 1 cell
per 10 million myelocaricytes). The CD133 expression was found (CD133+EPCAM+CD45-) in 33.3%
(9/27) cases. The presence of DOCs did not correlate with tumor size, lymph node status and stage of
the tumor process. The highest detection rates of DOCs were observed at stages IA and IIA: 60.7%
and 58.3% respectively. BM involvement in adenocarcinoma was observed in 45% cases, in squamous
cell carcinoma - in 37% samples (p=0.501). It was found that DOCs are more often detected in more
differentiated tumors (p=0.023). Significant correlations between the presence of DOCs in the BM and
myelogram parameters have not been established. A decrease in the number of granulocyte germ cells
was observed in 4% of BM involvement (p=0.036).
Conclusion: The possibility of detecting DOCs in the BM of NSCLC patients has been established. BM
involvement was 43.5%. DOCs are detected even in the early stages of NSCLC. Relationship between
BM involvement and the degree of tumor differentiation was found. More frequent BM involvement
was observed in adenocarcinoma compared with squamous cell carcinoma of the lung.
Keywords
Disseminated tumor cells, Bone marrow, Non-small cell lung cancer, Morphology, Flow cytometry, Cancer stem cells, CD133.
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of death from malignant tumors in the most countries of the world. The latest advances in drug therapy, based on the molecular biological characteristics of the tumor, have become a major breakthrough, but the survival rate of patients with NSCLC is still quite low. Unfortunately only 18% of patients experience 5-years [1].
Currently the efforts of scientists are aimed at developing various strategies to increase the effectiveness of the antitumor immune response. Following this direction, a significant proportion of scientific researchesare devoted to the study of the immuno-biological mechanisms of hematogenous metastasis [2-4]. Disseminated tumor cells (DTC) are the subject of close attention in this context, which are quite often found in the bone marrow (BM) of patient [5-8]. It should be emphasized that almost 40% of such patients have early stages. Study of BM is very importment since DTC getting into BM interact with the new microenvironment on which their fate depends. In some cases DTC remains dormant [9]. However, DTC can avoid an immune response, which further leads to the appearance of secondary tumors. They contribute to their own survival and form a metastatic niche significantly violating the strictly controlled cellular [10] and molecular mechanisms of the microenvironment [11]. BM becomes a new refuge of the DTC in which they undergo successful clonal expansion and parallel progression [2,12]. These processes lead to their acquisition of new phenotype. A recent work showed the heterogeneity of DTC: they express on their surface a diverse set of antigens [7], which distinguishes them from the primary tumor and reflects the complex architecture of the relationship between DTC and the microenvironment. There is evidence that DTC in the BM may have genomic profiles that are not associated with the primary tumor [13]. L Foulds in the sixties of the 20th century postulated that a tumor cell as a result of tumor progression, irreversibly acquires new traits necessary for its survival [14]. Thus new opportunities are created for further microevolutionary changes. They lead to increased independence of DTC growth from local, systemic or therapeutic effects. These DTC properties make them biologically closer to cancer stem cells (CSCs), a minor primary tumor subset seeming to play a leading role in the selfmaintenance and metastasis of malignancies. Compared to the dominant clone of tumor cells and normal stem cells, CSCs have dysregulated signaling pathways and aberrant phenotypes. A distinctive repertoire of cell surface markers allows identification and isolation of CSCs from a population of tumor cells.
Nowdays there are highly sensitive immunological methods and extensive experience was accumulated in DTC detection. The possibility of quantitative detection of DTCs and their phenotypic characteristics were established [7]. BM lesion and the role of DTC as an unfavorable prognosis factor are shown for breast cancer [6,15], stomach cancer [16] and melanoma. The DTC information in NSCLC is contradictory today [8].
The DTC identification and characterization allowed us to obtain important information of metastasis mechanisms which led to a better understanding of the molecular changes and profiles underlying the drug resistance of tumors [17]. In this regard DTC are considered as a possible target for drug therapy [18]. It would be more advisable to aim at the metastatic seeds before they germinate instead to wait for the appearance of metastases. Study of development regimens to prevent DTC activation and proliferation had already begun [19], especially in patients with a high risk of relapse, as well as in patients with a complete response to treatment. Preliminary clinical and laboratory data indicate the effectiveness of this strategy in gastric cancer and some endocrine-dependent tumors [19].
A number of features of hematopoiesis in cancer patients had been established [20], which can serve as the basis for a deeper understanding of the DTC behavior in BM.
Thus, the study of BM in NSCLC and the possibility of DTC detection is significant interest. Analysis of the DTC and their relationship with the clinical and morphological characteristics of the tumor will allow a deeper understand of tumor growth patterns and assess the possibilities of identifying new targets for drug therapy.
The purpose of the study
To evaluate the possibility of DTC detection in BM in patients with NSCLC and also to analyze the frequency of the lesion BM and the relationship with the clinical and morphological parameters of the tumor.
Materials and Methods
This study included 62 patients with NSCLC who received treatment under the conditions of the Federal State Budgetary Institution Scientific Research Center for Oncology named after NNBlokhinaof the Ministry of Health of Russia. This study was approved by the institutional ethical committees (Local ethical committee NNBlokhin Russian Cancer Research Center of Ministry of Health of the Russian Federation; UDC 616-006, Reg. AAAA-A16-116122210071-4, Inv. 479.) and was done with the informed consent of the patients.
The age of patients ranged from 17 to 80 years (average - 63 years). The study was dominated by men – 48 (77.4%). The number of women was 14 (22.6%). All patients were underwent a surgical manual, with the exception of one case in which a biopsy was performed. Table 1 presents the distribution of patients by stages.
| Stage | N | % |
|---|---|---|
| IA | 5 | 8,2 |
| IB | 13 | 21,0 |
| IIA | 12 | 19,5 |
| IIB | 9 | 14,5 |
| IIIA | 13 | 21,0 |
| IIIB | 4 | 6,5 |
| IV | 6 | 9,7 |
| Total | 62 | 100 |
Table 1: The distribution of patients with NSCLC in stages.
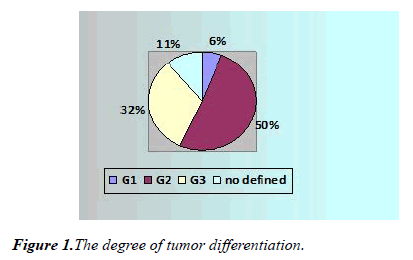
According to the pathomorphological findings, 27 patients (43.5%) were diagnosed with squamous cell carcinoma, 33 patients (53.3%) had adenocarcinoma, and in two cases (3.2%) other histological forms were diagnosed. Tumors were moderately differentiated (G2) (50%, n = 31), highly differentiated (G1) tumors were observed in 6.5% (n = 4), a low degree of differentiation (G3) was noted in 32% (n = 20) (Figure 1) In 7 cases, G is not defined.
BM is obtained by sternal puncture. The volume of BM punctate did not exceed 1.0 ml, since the probability of dilution of the sample with peripheral blood increased in larger volume. The study of BM was carried out by two methods: morphological and immunological. The morphological study was performed on 6 glasses stained by the Romanovsky method. Myelogram counting and tumor cell search were carried out simultaneously by two morphologists. Monoclonal antibodies to cytokeratins EPCAM (Becton Dickinson, USA) and KL-1 (Immunotech, France), CD133 (Becton Dickinson, USA), CD45 (Becton Dickinson, USA), directly labeled with various fluorochromes: FITC, PE, V500, V450, PerCP, were used for immunological assessment of the DOC presence in BM. Cell collection was carried out on a FACS Canto II flow cytometer, USA. The results were analyzed using the Kaluza Analysis v2.1 software (Beckman Coulter, USA). An estimated 20 million myelocaryocytes (or all cells of the sample). DOC was detected by the lack of expression of the total leukocyte antigen CD45 in combination with expression of EPCAM or KL-1. Statistical data processing was performed using the IBM-SPSS Statistics v package.
Results and Discussion
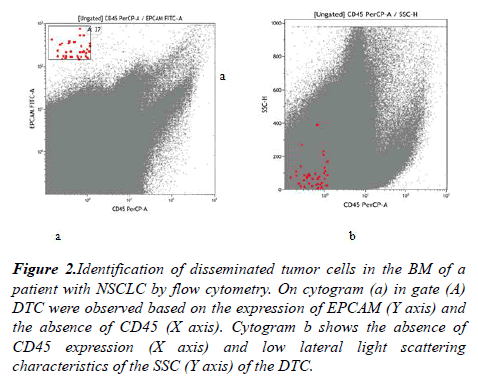
The morphological analysis of BM included the calculation of myelograms and the search for tumor cells. Morphologically DTC in BM was detected in only 1 of 62 cases. In an immunological assessment of the DTC presence 1 tumor cell per 10 million myelokaryocytes was taken as a threshold level. DTC (CD45-EPCAM +) were detected in 43.5% of the BM samples (n = 27) (Figure 2).
Figure 2: Identification of disseminated tumor cells in the BM of a patient with NSCLC by flow cytometry. On cytogram (a) in gate (A) DTC were observed based on the expression of EPCAM (Y axis) and the absence of CD45 (X axis). Cytogram b shows the absence of CD45 expression (X axis) and low lateral light scattering characteristics of the SSC (Y axis) of the DTC.
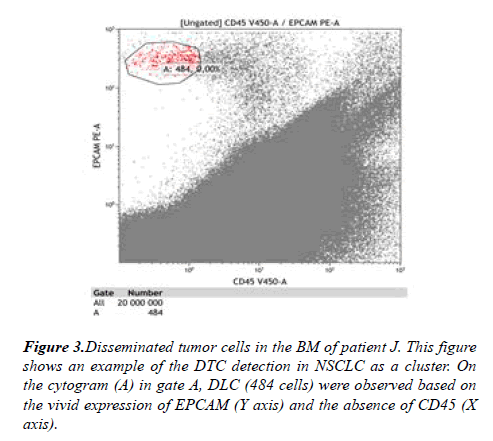
One epithelial (tumor) cell among 10 million myelocaryocytes in BM samples was detected in 9 (14%) patients. In other cases, the number of tumor cells ranged from 2 to 242. A clear cluster of tumor cells (micrometastasis) was diagnosed in 2 (3.2%) of 62 patients with NSCLC. The highest number of DTC in BM was observed in a patient with stage IIA (T2aN1M0), squamous cell lung cancer (Figure 3). Micrometastases had not been established in these 2 patients by morphological method.
DTC was detected in 4 (6.65%) BM samples using the threshold level of 1 cell per 1 million myelokaryocytes. It should be noted stage II of NSCLC was observed in 2 cases, equally adenocarcinoma and squamous cell carcinoma. The presence of DTC in BM was established in 1 case using the threshold level of 1 cell per 100 thousand myelokaryocytes in a patient with stage IIA (T2aN1M0).
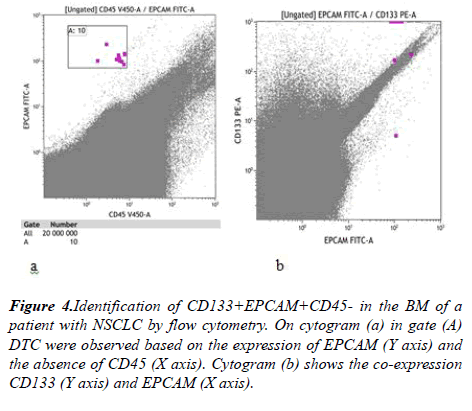
It is known that CSCs with maximum resistance to conventional anticancer therapies plays a special role in metastasis. They are characterized by aberrant activation of signaling pathways, which leads to uncontrolled cell proliferation, and also maintains their plasticity. We identified CSCs among NSCLC DTCs by CD133 expression. CD133 expression was analyzed in 27 BM samples. There were 9 (33,3%) sample containing CD133+EPCAM+CD45- cells. The amount of DTC expressing CD133 in the samples was small from 1-5 cells (Figure 4).
We have not established a reliable relationship between the presence of DTC in BM (1 cell/10 million myelocaryocytes) and age, sex of patients. The relationship of BM lesion and clinical and morphological characteristics of the primary tumor was analyzed. The frequency of DTC detection in BM depending on the tumor stage is presented in Table 2. Tumor cells are present among myelokaryocytes even in the initial stages. This fact indicates an early process of hematogenousmicrometastasis. The highest detection rate of DTC was observed at stages IA and IIA and amounted to 60.7% and 58.3%, respectively.
| Stage | DTC | Amount | ||
|---|---|---|---|---|
| Presence | Absence | |||
| I A | n | 2 | 3 | 5 |
| % | 40,0% | 60,0% | 100,0% | |
| I B | n | 8 | 5 | 13 |
| % | 61,5% | 38,5% | 100,0% | |
| II A | n | 5 | 7 | 12 |
| % | 41,7% | 58,3% | 100,0% | |
| II B | n | 5 | 4 | 9 |
| % | 55,6% | 44,4% | 100,0% | |
| III A | n | 9 | 4 | 13 |
| % | 69,2% | 30,8% | 100,0% | |
| III B | n | 3 | 1 | 4 |
| % | 75,0% | 25,0% | 100,0% | |
| IV | n | 3 | 3 | 6 |
| % | 50,0% | 50,0% | 100,0% | |
| amount | n | 35 | 27 | 62 |
| % | 56,5% | 43,5% | 100,0% |
р=0,475
Table 2: Bone marrow lesion in patients with NSCLC depending on the tumor stage.
Next we analyzed the relationship between the size of the primary tumor, as well as the number of affected lymph nodes, and the presence of DTC in the BM (Table 3). The majority of DTC -positive cases (71.4%, 5\7) were observed at stage T1. The incidence of BM lesions was within 43.2% in stage T2. DTC was detected less frequently with large tumor sizes: 36.4 and 28.6%, respectively, in stages T3 and T4 (p=0.130). Reliable dependence of the DTC status on tumor size was not found.
| DTC | Amount | ||||
|---|---|---|---|---|---|
| Presence | Absence | ||||
| T | Т1 | n | 2 | 5 | 7 |
| % | 28,6% | 71,4% | 100,0% | ||
| Т2 | n | 21 | 16 | 37 | |
| % | 56,8% | 43,2% | 100,0% | ||
| Т3 | n | 7 | 4 | 11 | |
| % | 63,6% | 36,4% | 100,0% | ||
| Т4 | n | 5 | 2 | 7 | |
| % | 71,4% | 28,6% | 100,0% | ||
| Amount | n | n | 27 | 62 | |
| % | % | 43,5% | 100,0% | ||
Table 3: Frequency of DTC depending on the size of the primary tumor.
DTC was detected when regional lymph nodes were intact: in 46.4% (13\28) of BM samples,. BM lesion was observed in 42.1% (8 \ 19) patients with N1, and in 33.3% (4 \12) and 100% (2 \ 2) cases, respectively in N2 and N3 status while the differences were not statistically significant. It should be noted that the study was dominated by patients with lymph nodes (53%). Remote metastases (M1) in the study were established in 6 cases. BM lesion was observed in 50% (3 \6) patients: the number of detected tumor cells averaged 5 per 10 million myelocaryocytes.
Evaluation of the DTC presence in BM depending on the tumor histological type revealed that bone marrow lesion was more often observed with adenocarcinoma (45.5%) (Table 4). In cases of squamous cell carcinoma DTC were detected in 37% of the samples. The differences are not significant: p=0,501.
| DTC | Amount | ||||
|---|---|---|---|---|---|
| Presence | Presence | ||||
| Histological type | Squamous cell carcinoma | n | 17 | 10 | 27 |
| % | 63,0% | 37,0% | 100,0% | ||
| Adenocarcinoma | n | 18 | 15 | 33 | |
| % | 54,5% | 45,5% | 100,0% | ||
| Amount | n | 35 | 25 | 60 | |
| 58,3% | 41,7% | 100,0% | |||
р= 0,501
Table 4: Bone marrow lesion depending on the histological type of NSCLC.
Next step we analyzed the frequency of BM lesions depending on the degree of tumor differentiation, the connection was established, p=0.023 (Table 5). The highest detection rate of DTC was noted in highly differentiated cancer (G1). DTC was detected in 8 out of 20 patients with low differentiation (G3) which amounted to 40%. Similar data were obtained in a study. DTC was significantly more often detected (32%) in BM with more differentiated tumors (G1-G2) than with tumors with low differentiation (4%) G3-G4, p=0.03 [21].
| DTC | Amount | ||||
|---|---|---|---|---|---|
| Presence | Presence | ||||
| G | G3 | n | 12 | 8 | 20 |
| % | 60,0% | 40% | 100,0% | ||
| G2 | n | 20 | 11 | 31 | |
| % | 64,5% | 35,5% | 100,0% | ||
| G1 | n | 0 | 4 | 4 | |
| % | 0,0% | 100,0% | 100,0% | ||
| Amount | n | 32 | 23 | 55 | |
| % | 60,4% | 39,6% | 100,0% | ||
р=0,023
Table 5: Frequency of DTC detection depending on the degree of tumor differentiation.
Thus, according to the results of the analysis of the relationship between DTC status and morphological characteristics of the tumor, it turned out that BM lesion is mainly observed in more differentiated tumors. The fact is very interesting and requires a deeper study in a larger cohort. It is possible that NSCLC patients with similar types of tumors will need a different treatment regimen.
According to the available data on the peculiarities of granulocyte and erythropoiesis in cancer patients in whom DTC was detected, we also evaluated the myelogram parameters of patients with NSCLC depending on the presence of BM lesion, as well as on the number of tumor cells detected in it. Analysis the mean values of significant differences between the groups with the presence and absence of DTC in BM was not revealed. However, lower cellularity in case of BM lesion is noteworthy. In the study of Ryabchikov D.A. et al. with breast cancer it was shown the content of myelokaryocytes is [1,6] times significantly lower with a DTC -positive status than in DTC-negative status [6]. It should also be noted that the leuko-erythronormoblastic ratio (LER) is higher in the presence of BM lesion, although the differences are not statistically significant. A similar fact, but with a significant difference was noted in the study of BM with melanoma [7].
Analysis according to the tables of conjugacy of characters noted that in the group of NSCLC patients who had BM lesions only in 4% of cases there was a decrease in the sum of granulocyte germ cells (p=0.036) compared with the group without DTC (12.5%). Similar results were obtained in a study of melanoma, but in a larger percentage of cases [7]. It is possible that the revealed patterns relate to the later stages, since in our study patients with early stages predominated. Promyelocytes were reduced in 72% of patients with NSCLC in the presence of DTC, and in the absence - in 84.4%. Myelocyte reduction was observed only in 8% of cases with BM lesion versus 18.8%. The granulocyte maturation index was reduced in the majority of patients (68.0%) who were DTC positive.
As for the erythroid sprout a decrease in polychromatophilic forms was noted in 44% of DTC positive cases in patients with NSCLC, compared with negative cases of DTC (56.3%). Oxyphilicnormoblasts were increased in 72% of BM samples in which DTC was present and in negative cases - in 58.1%. At the same time in 24% DTC positive cases the amount of erythrocyte germ cells was reduced, and DTC was increased in more than half of the patients: 14 (56.0%) of 25.
We also separately analyzed myelogram indices in those cases in which DTC were detected per 1 million myelokaryocytes. There were no statistically significant differences with DTC negative status. However, it should be noted that the cellularity in 3 (75%) patients who had a BM lesion was normal, all of them had an increase in the number of blast cells, myelocytes were normal in 3 cases. An increase in the oxyphilic form was noted in 3 patients (63.5%), an increase in the erythrocyte maturation index - in 75% versus 66%. LER in samples with BM lesion was increased in 50% of cases (against 60%).
Summarizing up the analysis of the presence of the relationship between granulocyte and erythrocytopoiesis it should be noted that there is a tendency to rearrange the BM of the microenvironment of the DTC. However not all the described observations were reliable. Nevertheless, the facts obtained should be taken into account and further studied, since in our study patients with early stage NSCLC predominated and the number of detected DTC in most patients did not exceed 10 per 20 million myelokaryocytes. It is possible that changes in the BM of the DTC take a long time and also depend on the amount of the DTC and their activity. DTC in BM can remain inactive acquiring a resting phenotype, and thus do not affect the microenvironment. Separate molecular genetic mechanisms that provide such a phenotype of DTC in BM had already been studied [22]. It should also be taken into account that the heterogeneity of the DTC population can also determine the degree of hematopoiesis changes. Data on changes in hematopoiesis of BM, obtained in other studies, undoubtedly indicate that myelo- and erythropoiesis are involved in the tumor process, and their restructuring is likely to contribute to the long-term persistence of DTC in BM.
Conclusion
Identification of DTC and the possibility of their characterization provide important information on the mechanisms of metastasis and better understanding of the changes underlying the drug resistance of the tumor. The analysis of DTC in BM, the interactions between DTC and immune cells are becoming the subject of intensive research given the growing role of NSCLC immunotherapy.
In this work, the possibility of detecting DTC in patients with NSCLC was established. The detection of BM lesion was based on the lack of expression of the total leukocyte antigen CD45 in combination with the expression of EPCAM or KL-1. The detection rate of DTC in patients with NSCLC was 43.5%, which is comparable with the results of detection of DTC in other tumors. Moreover, it was higher in the early stages of the tumor process. This fact indicates that the process of hematogenousmicrometastasis is an early event in the tumor progression of NSCLC.
The findings of CD133 expression are evidence of DTCs heterogeneity and the complex hierarchical relations between the primary and the DTCs.
The incidence of DTC in BM patients withadenocarcinoma was higher. The connection of BM lesion with the degree of tumor differentiation was established. The facts presented are interesting and require further study in order to assess the prognostic value of DTC in NSCLC and to analyze the choice of immunotherapy resources.
The proven relationship of BM lesion with an unfavorable prognosis in a number of tumors, namely, with poor overall and relapse-free survival, leads scientists to develop strategies preventing the activation and proliferation of DTC. Thus DTC are considered as a promising target for drug therapy and NSCLC is no exception.
ORCID of Authors
SVChulkova 0000-0003-4412-5019
NN Tupitsyn 0000-0003-3966-128Х
Authors’ Contributions
SVChulkova: writing manuscript text, data analysis.
NNTupitsyn: research design, data analysis, manuscript analysis.
Conflict of Interest
The authors declare no conflict of interest.
Funding
The study was performed without external funding.
References
- American Cancer Society. Cancer Fact and Figures 2018.
- Sai B, Xiang J. Disseminated tumour cells in bone marrow are the source of cancer relapse after therapy. J Cell Mol Med. 2018; 22(12): 5776-86.
- Pein M, Insua-Rodríguez J, Hongu T, et al. Metastasis-initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs. Nat Commun. 2020;11(1): 1-8.
- Markina IG, Tupitsyn NN, Mikhailova IN, et al. Hematogenous tumor metastasis: key points and evolving paradigms. Hematopoiesis Immunology.
- Tupitsyn NN. Bone marrow of a cancer patient: staging of tumors, hematopoiesis, immune system. 2018; 16(2): 10-54.
- Ryabchikov DA, Beznos OA, Dudina IA, et al. Disseminated tumor cells in patients with luminal breast cancer. Russian Biotherapeutic Journal. 2018;17(1): 53-7.
- Chernysheva O, Markina I, Demidov L, et al. Bone marrow involvement in melanoma. Potentials for detection of disseminated tumor cells and characterization of their subsets by flow cytometry. Cells 2019; 8: 627.
- Pantel K, Alix-Panabieres C. Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. Bonekey Rep.2014; 3: 584.
- Dasgupta A, Lim AR, Ghajar CM. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Molecular Oncology 2017; 11: 40–61.
- Zarrer J, Haider MT, Smit DJ, et al. Pathological Crosstalk between Metastatic Breast Cancer Cells and the Bone Microenvironment. Biomolecules. 2020; 10(2): 337.
- Price TT, Burness ML, Sivan A, et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. SciTransl Med. 2016; 8(340): 340-73.
- Klein CA Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009; 9(4): 302-12.
- Demeulemeester J, Kumar P, Moller EK, et al. Tracing the origin of disseminated tumor cells in breast cancer using single‐cell sequencing. Genome Biol. 2016; 17(1): 250.
- Foulds L. (1958). The natural history of cancer. J. Chron. Dis.1958; 8(1): 2-37.
- Tupitsyn NN. Circulating and disseminated tumor cells in breast cancer and ovarian cancer. Oncogynecology 2013;8(1): 12-8.
- Besova NS, Obarevich ES, Davydov MM, et al. The prognostic value of disseminated tumor cells in the bone marrow of patients with disseminated gastric cancer before the start of antitumor therapy. 2017; 17(350): 62-6
- Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells — mechanisms of immune surveillance and escape. Nat Rev ClinOncol 2017; 14(3): 155–67.
- Ghajar CM. Metastasis prevention by targeting the dormant niche. Nat Rev Cancer. 2015;15(4): 238-47.
- Nicolini A, Rossi G, Ferrari P, et al. Minimal residual disease in advanced or metastatic solid cancers: the G0-G1 state and immunotherapy are key to unwinding cancer complexity. Semin Cancer Biol. 2020; 579(20): 30075-30074.
- Tupitsyn N.N., Mkrtchan V.A., Palladina A.D. et al. Bone marrow lymphocyte populations of innate immunity in breast cancer patients. Glob J Med Res. 2020; 20(2): 21-7.
- Sienel W, Mecklenburg I, Dango S, et al. Detection of MAGE-A Transcripts in Bone Marrow Is an Independent Prognostic Factor in Operable Non–Small-Cell Lung Cancer. Clin Cancer Res. 2007 Jul 1;13(13): 3840-47.
- Johnson RW, Finger EC, Olcina MM, et al. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat Cell Biol. 2016; 18(10): 1078–89.