- Biomedical Research (2012) Volume 23, Issue 4
Bonding of cholesterol oxides to functionalised multiwalled carbon nanotubes for in-vitro biosensor application
A. Malarvizhi1 and A. Pandurangan1,2*1Department of Chemistry,Anna University, Chennai-600 025, India
2Institute of Catalysis and Petroleum Technology, Anna University, Chennai-600 025, India
- *Corresponding Author:
- A. Pandurangan
Department of Chemistry
Anna University
Chennai-600 025, India
Accepted date: May 16 2012
Abstract
MWCNTs (multiwalled carbon nanotubes) were synthesized by chemical vapour deposition (CVD) and functionalised using Hcl and HNO3 at 80 °C. Their FTIR spectra revealed the characteristic peaks for carboxylic acid and alcoholic groups. Both unfunctionalized (MWCNTs) and functionalised multiwalled carbon nanotubes ( f-MWCNTs) were reacted with cholesterol oxidase using 1-ethyl-3-(3-dimethylaminopropyl) carbodimide and Nhydroxyl sulfo-succinimide.But bonding with cholesterol oxidase was established only with f- MWCNTs. Cholesterol oxidase bonded to f-MWCNTs was tested for cholesterol sensing using cyclic voltametry. It established interaction of cholesterol with cholesterol oxidase bonded to f- MWCNTs. Hence cholesterol oxidase bonded to f-MWCNTs could be a novel in-vitro cholesterol sensor in hyper lipidemia
Keywords
Carbon nanotubes, Functionalized CNT, Cholesterol, Cholesterol oxidase, Cyclic voltammogram.
Introduction
Carbon nanotubes (CNTs) discovered by Sumio Iijima are believed to be a tool for passage from macromolecules to nanotechnology and for the transformation of the latter from a scientific concept into reality. A high level of research activity has been concentrated on carbon nanotubes ever since the first report on the discovery of the multiwalled carbon nanotubes (MWNTs) [1]. Several methods have been employed to produce CNTs, such as arc-discharge [2], laser ablation [3] and chemical vapour deposition (CVD) [4]. CVD has been used for relatively easy control of length, diameter, high quality and probability of mass production [5,6].
The performance of CNTs has been found to be superior to other carbon electrodes and there has been a growing interest to use CNTs in biosensor platforms [7]. However, manipulation and processing of CNTs have been limited by their solubility in most common solvents, although some dissolution has recently been reported [8]. Their chemical modification might pave the way to many useful applications, including immobilization of biological molecules such as enzymes (i.e., for biosensors and electrochemical sensors). The nitric acid treatment is an oxidative method that can produce the functionalised carbon nanotubes (f-CNT) [9]. This process introduces carboxylic acid group on the surface and at the ends of the CNTs and they become sites for the covalent attachment of proteins/ enzymes [10] or other such kinds of reagents. Hence CNTs display a great deal of potential for usefulness as delivery agents and sensor, with very low toxic effect [11] probably due to their small size, stable atomic structures and non-polar nature, which make them biologically inert.
Sensitive and selective detection of a particular biomarker in a multi-component blood system is a challenging task in clinical analysis/diagnosis. Cholesterol is one such bio-marker found in blood, linked to a number of clinical and heart disorders. Its high accumulation in blood is strongly correlated with coronary heart disease, arteriosclerosis, myocardial infarction, brain thrombosis, lipid metabolism dysfunction, hypertension, [12]. Cholesterol is normally found in blood as lipoproteins, but is more commonly found in lymphatic fluid, which is the primary extra cellular fluid found in the body.
Cholesterol oxidase (CHOx) was chosen as the model enzyme in this methodology study, because of its excellent performance and bright commercial prospective in biosensor applications. Cholesterol oxidase is industrially and commercially important for application in bioconversions for clinical determination of total or free serum cholesterol [13-16]. CHOx-MWCNTs composite electrodes were explored in biosensors in detecting the cholesterol concentration. To evaluate the electrode performance in various conditions, the thermal stabilities of the fabricated CHOx-MWCNTs biosensor electrodes were monitored at room temperature and 80 °C.
These highly stable and active enzyme-coated MWCNTs composites significantly improved the performance of biosensors. The main advantage of CNTs is their large accessible surface areas due to a high surface-to-volume ratio. This offers possibility of constructing biosensors with high sensitivity.
In this present work MWCNTs were grown over Fe- MCM-41 t (Si/Fe=25, 50, 75 and 100) by Chemical Vapour Deposition method. The MWCNTs were treated with HF for purification and further it was functionalized with HNO3 - H2SO4 interaction between f-MWCNTs and cholesterol was studied. The results indicated that the interaction with cholesterol can be explored for biosensor applications.
Experimental Techniques
Materials and Methods
Chemicals used in this work, such as sodium silicate (Na2SiO3.9H2O) ferric nitrate Fe(NO3)3.9H2O), cetyltrimethylammonium bromide (CTAB), hydrofluoric acid (HF)and concentrated sulfuric acid (H2SO4), all analytical reagent grade, were purchased from Sigma Aldrich. MWCNTs with >90% purity were synthesized by chemical Vapourdepositionmethod.1-Ethyl-3-(3-dimethylaminopropyl) carbodiimidehydrochloride, (EDAC) and Nhydroxyl- succinimide (NHS) were obtained from Himedia. Cholesterol was isolated from hypercholestermia blood samples collected from Health Centre, Anna University, Chennai. Cholesterol oxidase (CHOx) (50 U/mg) was purchased from Sigma-Aldrich. The phosphate buffer solution (0.1 M PBS) was used as supporting electrolyte. All aqueous solutions were prepared using deionized water.
Synthesis of Fe-MCM-41
Fe-MCM-41 molecular sieves were synthesised hydrothermally according to the previous work [17], using a gel composition of SiO2: 0.01 Fe2O3: 0.2 CTAB: 0.89 H2SO4. In a typical synthesis, 21.2 g of sodium metasilicate was added to ferric nitrate and dissolved in distilled water. The pH of the solution was adjusted to 10.5 using 1 M sulphuric acid and the content was stirred well to form a el. An aqueous solution of CTAB was prepared by dissolving 7.2 g of it in distilled water and it was added slowly to the gel for a period of about 30 min. The resulting mixture was then stirred for 1 h at room temperature and finally the solution was transferred to stainless steel autoclave and is kept at 145°C for 48 h in an oven. The crystallized Fe-MCM-41 was recovered by filtration, washed several times with distilled water and dried at 80°C for 3 h. The occluded surfactant was removed by calcination at 550°C in muffle furnace for 6 h to obtain Fe-MCM-41.
Growing of CNTs
About 300 mg of the catalyst, prepared by the above procedure, was uniformly spread over the quartz boat and was placed in the central position of the CVD furnace. It was then pre-activated by passing nitrogen gas (99.99%) through the chamber for 20 min at 120 ml/min flow rate. The reaction temperature was maintained at 800°C.
It was followed by the flow of acetylene (99.6%) at the optimized rate 140 ml/min for 30 min through the reaction chamber, under nitrogen flow. After the scheduled time, the furnace was cooled to room temperature. The grown sample was then collected as a black powder from the quartz boat.
Purification and Functionalization of CNTs
In order to remove the silica phase, the synthesized CNTs were treated with 40 wt% HF at ambient temperature. The obtained samples were immersed in nitric acid solution to remove the metal catalysts and amorphous carbon. In general, nitric acid breaks the carbon shell and then oxidizes the metal catalysts to the corresponding metal oxide or hydroxide. When the metal gets oxidized, the volume increases and the metal oxides crack open the carbon coatings. Furthermore, oxidants tend to initially attack MWCNTs from the ends, which have more highly strained five-membered rings.
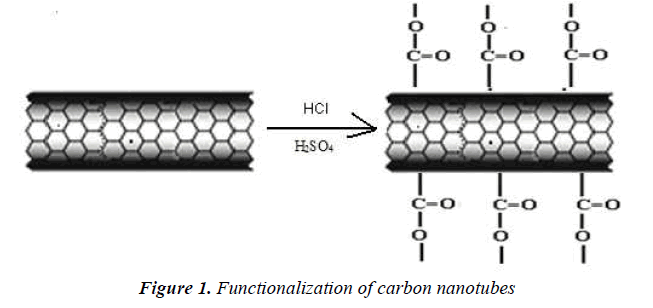
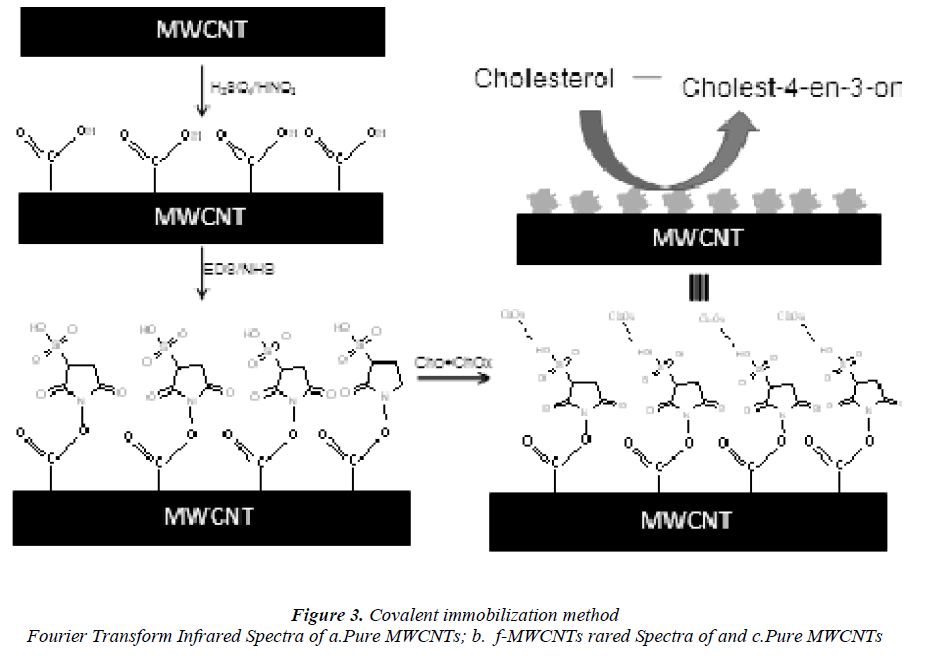
They were functionalized by an acid treatment, 1mg of MWCNTs was added to an aqua regia solution (3:1 ratio of hydrochloric acid and nitric acid respectively) and functionalization process was carried out in an ultra sonicator. The beaker was kept in an ice bath at 65 0C for 18 h .After functionalization, the dispersed MWCNTs were filtered and dried. It is well known that this treatment caused segmentation and generation of–COOH groups at the terminus and sidewalls of MWCNTs [18] scheme 1.
Isolation & Estimation of Cholesterol:
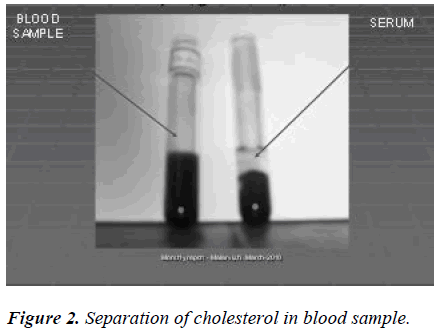
The blood samples were collected and serum was separated by centrifugation. To 2 ml of the serum supernatants, 1.0 ml of perchloric acid (2 mol/L) was added and the mixture was allowed to stand at room temperure for longer than 1 h. After centrifugation (3000 rpm, 10 min) the supernatant was discarded and the precipitate was used for cholesterol analyses. Scheme 2.
Chemical adsorption of cholesterol onto f-MWCNTs
The redox mediator thionin (TH) was attached to the broken tips containing carboxylic acid groups of the CNT using water-soluble coupling agents 1-ethyl-3-(3- dimethylaminoproyl) carbodiimide (EDAC) and Nhydroxyl sulfo-succinimide (sulfo-NHS) by forming carboxylic linkages between the amine residue of TH and carboxylic acid groups on the CNTs [19]. About 100 mg of f -MWCNTs was added to a freshly prepared 10 ml aqueous solution of EDAC (10 mg/ml) 300 mg of sulfo- NHS was added to it under continuous stirring. The pH of the solution was adjusted to 7 and then 100 mg of cholesterol was added to that solution. The reaction was maintained at room temperature for 2 h. The mixture was centrifuged to remove any insoluble materials and the supernatant liquid was washed with sufficient amount of water to remove buffer components and unbound cholesterol molecules. Finally, the chemically adsorbed cholesterol with f -MWCNTs were washed thoroughly with distilled water, filtered and dried at room temperature.
Covalent attachment of cholesterol oxides
The covalent attachment of cholesterol oxidase (CHOx) to the MWCNTs surface was achieved by mixing the 2.5 ml suspension of functionalized MWCNTs (1.5mg/ml) with 1 ml of CHOx (10 mg/ml), which was then allowed to react for one hour under shaking condition (600 rpm) at room temperature. The covalent attachment-CHOx sample was prepared by adding 2 ml of sodium phosphate buffer (pH 7.0, 100 mM), followed by incubation at 4 °C overnight.
Preparation of the modified electrodes
Electrodes were made from the mixing of 90% of active material and 10% Polyvinyldene difluride (PVdF) binder using agitate mortar. The mixer was pressurized by hydraulic press with the maximum pressure of 5 MPa on to Aluminium foil of thickness 1 mm. Finally the electrode was dried at 120° C under vacuum for 24 h. Cyclic Voltammetry studies were carried out by Potentiostat CHI7001C equipped with PC ( CHI Instruments Inc. USA) using the above prepared electrode as a working electrode and platinum wire, Ag/AgCl, as the counter and reference electrode respectively. 0.1 M phosphate buffer solution of PH 7 was used as the potential electrolyte
Analytical procedures
The powder XRD patterns of the CNTs were obtained with a stereo scan diffracto meter using nickel-filtered Cu Kα radiation with wavelength (λ = 1.5418 Å) and a liquid nitrogen cooled germanium solid-state detector. To analyse the microstructures of CNTs the resulting carbon nanotubes samples were characterised by scanning electron microscopy (SEM) using JEOL JSM 840 Å. Transmission electron microcopy (TEM) was carried out to characterize CNTs. For this purpose, a Philips CM 200 microscope operating at 200 kV was used. The growth morphology and crystallinity of the tubular structures were analysed by transmission electron microscopy (TEM); Fourier transformed infrared spectroscopy (FTIR) obtained in the range from 500 to 3500 cm-1 which was recorded in Shimadzu FT-IR 8300 spectrophotometer at a resolution of 4 cm-1 Cyclic voltammetry (CV) measurements were performed in a CHI 7001C electrochemical workstation (CH Instruments, USA) with a conventional three-electrode cell. A platinum wire was used as a counter electrode (CE) and Ag/AgCl with 3 M/KCl solution as a reference electrode (RE).
Results and Discussion
Characterization of catalyst and multiwall carbon nanotubes
Structure of Catalyst. The XRD pattern of the calcined mesoporous Fe-MCM-41(100) catalyst is shown in (Fig.1) . The pattern showed an intense diffraction peak between 1.8° and 2.2° (2θ) on [100], confirming the hexagonal mesophase of the material.
Thermal Analysis
Thermal stability of the purified MWCNTs and f- MWCNTs was investigated using a Thermo gravimetric analysis under nitrogen flow in the temperature range between 40 to 1000 °C. The observed thermograms of MWCNTs and f-MWCNTs are shown in (Fig.2a,b) .The TGA profile of MWCNTs showed a gradual weight loss from 200 to 500° thus evidentially the thermal stability of the pure MWCNTs. Beyond 500 °C the major mass decrease appeared which mainly due to the bond breaking of MWCNTs.
In the case of f-MWCNTs the TGA showed major weight loss just about 200°C established functionalization. In addition, the thermal stability of MWCNTs was also reduced considerably by means of the functionalization. It was confirmed by the absence of characteristic decomposition of MWCNTs close to 500°C.
Microstructure Analysis
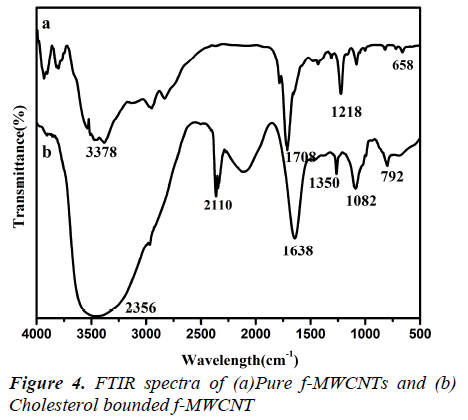
FTIR studies
The microstructure of the MWCNTs and f-MWCNTs was also verified using FTIR and Raman spectroscopy (Fig3.a, b) shows the FTIR spectra of MWCNTs before and after oxidation with concentrated acids. The oxidation of the multiwalled carbon nanotubes with the HNO3 –
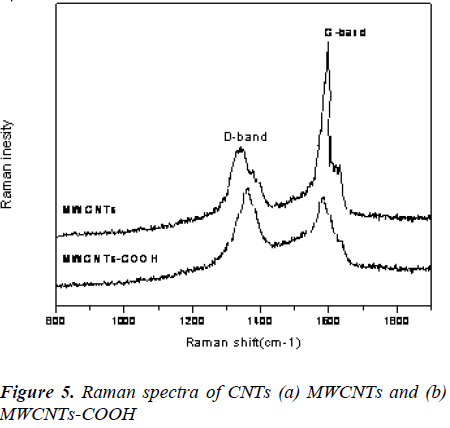
Raman Studies
The Raman spectroscopy is a powerful tool used to provide structural information about MWCNTs before and after functionalization. As shown in Fig. 3, the D and G bands of the MWCNTs at 1348 and 1578 cm-1 were due to defects, disorder-induced peaks and tangential-mode peaks [22]. Additionally, the intensity ratio (ID/IG) of the D and G bands for f-MWCNTs-COOH was lower than that of MWCNTs. The decrease of peak intensity may be attributed to the breakage of some bonds and insertion of chemical groups in the MWCNTs during oxidation.
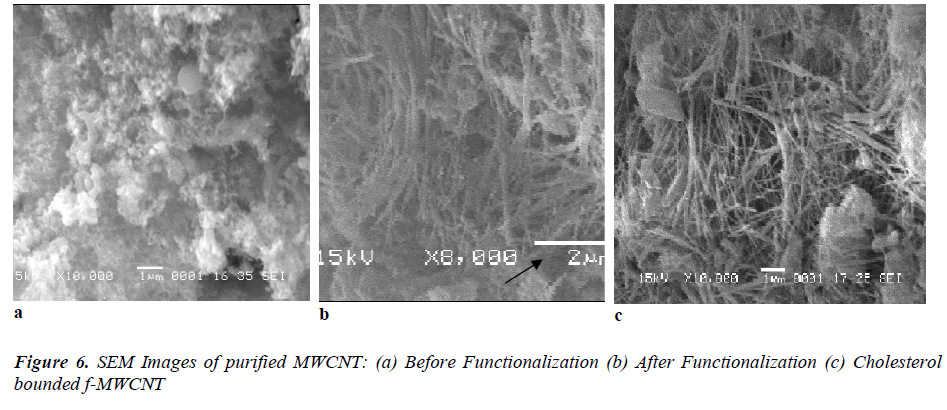
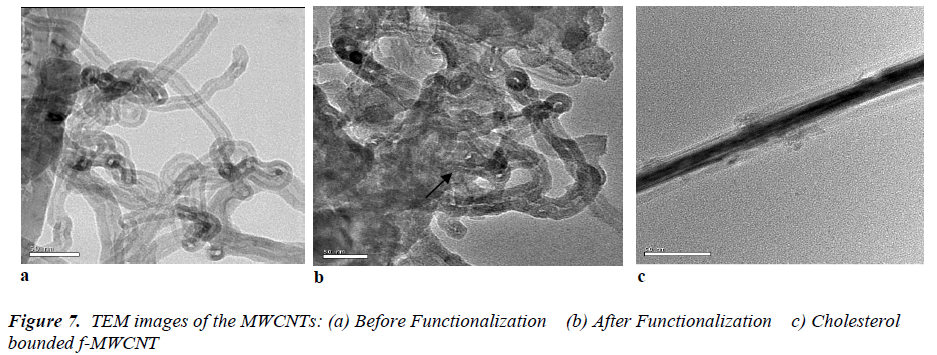
Scanning electron microscopy and Transmission electron microscopy
Fig 6 (a, b) displays the SEM images of purified MWCNTs and f-MWCNTs. The SEM pictures clearly reflected the agglomerated CNTs with nanotubular morphology. In (Fig.6 a) shows the group of MWCNTs with large agglomeration. After the functionalization many walls were broken and shown as a single tube which can be seen in (Fig.6 b). It is observed from the SEM, after the functionalization the diameter of the CNTs ranged only from 50 to 100 nm. In addition, some tubes were also observed in the SEM. In order to further confirmations the TEM observation were taken for both the MWCNTs and f-MWCNTs. The obtained TEM metaphors are shown in (Fig.7 a, b). Isolated or small bundles of CNTs with clean tube walls were observed in the image. It can be seen that the purified CNTs appeared with multiwall nanostructure. The obtained TEM images of our CNTs were consistent with earlier reported results [23] and the presence of CNTs was confirmed. From (Fig.7 b) we can seen that the tips of many MWCNTs were open, which indicates broken C-C bonds along the graphene layer of the co-axial tubes which is in accordance with the previous report [24]. In addition, with the bond breakage the side walls of the tubes were also affected by the functionalization process. In general, the side walls get oxidized and damaged the π-conjugation present along the sidewalls of CNTs there by introducing –COOH group on the side wall and at the ends.
Analysis of cholesterol binding with f-MWNTs
With addition to the morphological analysis, the SEM and TEM micrographs further confirmed the adsorption of cholesterol on the surface of f-MWCNT which is shown in (Fig. 6c) and (Fig. 7c). The adsorbed cholesterol mole cules on the surface of the functionalized CNT are marked with the arrow on the obtained SEM image, which represents the cholesterol molecules were agglomerated on the f-MWCNT surfaces. The strong covalent attachment of CHO on f-MWCNTs surface is shown in fig 6(c) it confirmed the used linkers (N-ethyl-N’-(3- dimethylaminopropyl) carbodimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (NHS)) were fully mixed with CHO and made strong covalent bond with f- MWCNT surface. These results are having the good agreement with FTIR analysis.
Cyclic voltammogram
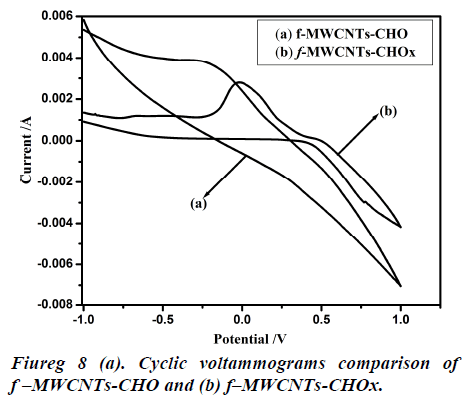
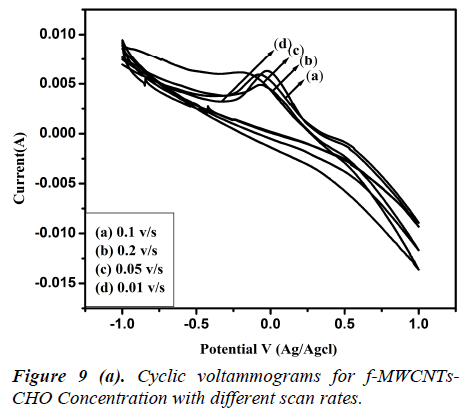
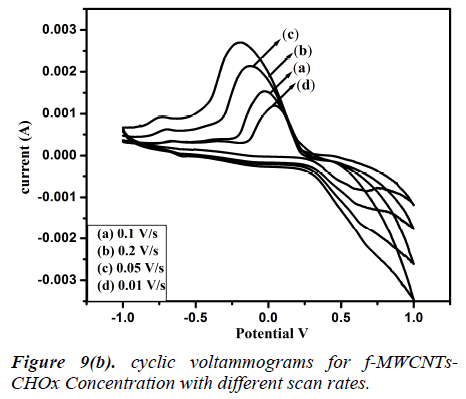
Fig 8 (a, b) represents the cyclic voltammograms of cholesterol bonded f-MWCNTs derived at different scan rate of 20 to 200 mV/s. The obtained CV profiles clearly evidences that the sensing nature of CHO and CHOx incorporated of f-MWCNTs.The rate dependence of the electrode material was observed by the variation of redox current on the CV curve which confirms the potential dependent sensing of the material. Comparison of the maximum sensitivity of the both CHO and CHOx incorporated f-MWCNTs is shown in Fig 9 (a, b). Both the electrodes showing the maximum sensitivity at 0.2 V/s which indicates that the addition of CHO and CHOx enhance the sensing ability of the MWCNTs considerably. The observed CV profile clearly reflects the two different kinds of reversible redox waves correspond to the CHO and CHOx thus ascribed the clear sensing effect of the electrode material. Neverthless, the addition of CHOx to bring about a significant increase in the reduction peak current and almost complete disappearance of the oxidation peak current. Comparison of the voltammograms with the addition of CHO and CHOx evidently showed that CHOx incorporated f-MWCNT significantly enhanced the charge transfer between electrodes through CHOx, but in the case of CHO added f-MWCNT system only a small enhancement observed. Which demonstrated that the direct electron transfer rate of CHOx incorporated f-MWCNT based sensor was very high compared to the CHO incorporated system. Alternatively, the CHOx mediated f-MWCNTs showed three times better sensitivity than the CHO added one which may be attributed due to the bioelectrocatalytic reduction of CHOx enzymes.
Conclusion
The f-MWCNTs–Cholesterol oxidase nanobiocomposite film could emerge as a novel sensor for selective detection of cholesterol in a variety of biological fluids. As the cholesterol oxidase is bonded to CNTs, it can function for a long period without leaching .Hence this study can become a model for the immobilization of other enzymes to CNTs for sensor applications. Therefore, there is large scope for enzyme immobilization on carbon nanotubes. This study encourages forecasting the application of f- MWCNTs in biomedical application especially in drug delivery system. These promising results need further “In Vitro” studies and clinical trials.
Acknowledgement
One of the authors, A. Malarvizhi is thankful to University Grand Commission, India, New Delhi, for the award of Rajiv Gandhi Fellowship.
References
- Iijima S. Helical microtubules of graphitic carbon. Nature 1991; 354: 56-58.
- Guo P, Nikolaev A, Thess DT. Catalytic growth of single-walled nanotubes by laser vaporization. Colbert,Chem Phys Lett 1995; 243: 49.
- Ebbesen TW, Ajayan PM. Large-scale synthesis of carbon nanotubes. Nature 1992; 358: 220.
- Ivanov V, Nagy JB, Lambin P, Lucas A. The study of carbon nanotubules produced by catalytic method.Chem Phys Lett 1994; 223: 329.
- Dai H, Rinzler AG, Nikolaev P, Thess A, Colbert DT, Smalley EH. Single-wall nanotubes produced by metalcatalyzed disproportionation of carbon monoxide Chem Phys Lett 1996; 260: 471.
- Pan ZW, Xie SS, Chang BH, Sun LF, Zhou WY, Wang. Director growth of align Ed open carbon nanotubes by chemical vapor deposition. Chem Phys Lett 1999; 299: 97.
- Wang J, Musameh M, Lin Y. Solubilization of carbon nanotubes by Nafion toward the Preparation of amperometric biosensors J Amer Chem Soc 2003b; 125: 2408.
- P´enicaud A, Valat L, Derr´e A, .Poulin P, Zakri C, .Roubeau O, .Maugey M, Miaudet P, Anglaret E, Petit P, Loiseau A, Enouz, Compos Sci Technol 2007; 67:795.
- Dillon AC, Gennet T, Jones KM, Alleman JL, Parilla PA, Heben MJ. A simple and Complete Purification of Single-walled Carbon Nanotube Materials Adv Mater 1999; 11: 1354.
- Shim M, Kam NWS, Chen RJ, Li Y, Dai H, Functionalization of carbon nanotubes for biocompatibility and biomolecular recognition. Nano Lett 2002; 285-288.
- Fan S, Chapin MG, Franklin NR, Tombler TW, Cassell AM, Dai H. Self oriented regular arrays of carbon nanotubes and their field emission properties. Science 1999; 283: 512.
- Fredrickson Levy R, Stanbury JB, Wyngaarden JB,Fredrickson (Eds.) DS. The metabolic Basis of Inherited Disease, McGraw-Hill Book. New York 1972;p545.
- Pollegioni L, Wels G, Pilone MS, Ghisla S. Kinetic mechanism of cholesterol oxidase from Streptomyces hygroscopicus and Brevibacterium sterolicum Eur J Biochem 1999; 264: 140.
- Lario IP, Sampson N, Vrielink AA. Sub-atomic resolution crystal structure of Cholesterol oxidase, whatatomic resolution crystallography reveals about enzyme mechanism and the role of the FAD cofactor in redox activity. J Mol Biol 2003; 326: 1635.
- Purcell JP, Greenplate JT, Jennings MG, Ryerse JS, Pershing JC, Sims SR, Prinsen MJ, Corbin DR, Tran M, Douglas S R. Stonard R. Cholesterol oxidase: a potent insecticidal protein active against boll weevil larvae J. Biochim Biophys Res Commun 1993; 196: 1406.
- Vasudevan PT, Zhou T. Kinetics of cholesterol oxidation by cholesterol oxidase Appl Biochem Biotech 1996; 60-63.
- Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmit KD, et al. A new family of mesoporous molecular-sieves prepared with liquid-crystal templates. J Am Chem Soc 1992; 114: 10834-10843.
- Wang Y, Iqbal Z, Malhotra SV. Functionalised of carbon nanotubes with amines and enzymes. chem physlett 2005; 402: 96.
- Shobha Jeykumari DR, Narayanan SS. Amperomeric determination of hydrogen peroxide by functionalized carbon nanotubes through EDC/NHS coupling chemistry J Nanosci Nanotechnol 2007; 1824: 1830.
- Luo HX, Shi ZJ, Li NQ, Gu Z, Zhuang QK. Investigation of the electrochemical and electrocatalytic behavior of single-wall carbon nanotubes film on a glassy carbon electrode Anal Chem 2001; 73: 915.
- Byrne EW, Chua-anusorn W, St. Pierre TG, Webb J, Ramsay A, Rintoul L. A spectroscopic study of thalassemic gallstones, Biospectroscopy. 1997; 3: 409-416.
- Hirsch A, Functionalization of single-walled carbon nanotubes. Angew Chem Int Ed Eng 2002; 4: 853-859.
- Perez-Cabero M, Rodriguez-Ramos I, Guerrero-Ruiz A. Characterization of carbon nanotubes and carbon nanofibers prepared by decomposition of acetylene in a fluidized bed reactor J Catal 2003; 215: 305.
- Pierard N, Fonseca A, Konya Z, Willems I, Van- Tendeloo G, Nagy JB. Production of short carbon nanotubes with op C. R en tips by ball milling Chem Phys Lett 2001; 335: 1-8.