Research Article - Journal of Pharmacology and Therapeutic Research (2018) Volume 2, Issue 1
Biological markers of oxidative stress and allopurinol therapy: A meta-analysis of randomized controlled trials
Manal M. Alem*
Department of Pharmacology, College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
- *Corresponding Author:
- Dr. Manal M. Alem
Department of Pharmacology
College of Medicine
Alfaisal University
Takhasusi Road, P.O. Box 50927
Riyadh 11533, Kingdom of Saudi Arabia
E-mail: malem@alfaisal.edu
Accepted on January 15, 2018
Citation: Alem. Biological markers of oxidative stress and allopurinol therapy: A meta-analysis of randomized controlled trials.. J Pharmacol Ther Res 2018;2(1):7-16.
DOI: 10.35841/pharmacology.2.1.7-16
Visit for more related articles at Journal of Pharmacology and Therapeutic ResearchAbstract
Aim: Oxidative stress contributes to the development of atherosclerosis via production of reactive oxygen species (ROS). Xanthine oxidase is an important enzyme that generates ROS and mediates oxidative modification of low-density lipoprotein (LDL) which is a key step in fatty streak and subsequent atheroma formation. Allopurinol is a xanthine oxidase inhibitor that has been shown to reduce the concentrations of the biological markers of oxidative stress. This meta-analysis was designed to summarize the effect of allopurinol treatment on markers of oxidative stress.
Methods: Medline, PubMed, Pro Quest Health & Medical Complete, Clinical Key, Wiley Online Library, and Cochrane Central Register of Controlled Trials were searched till 29th July, 2017. Meta-analysis was planned for randomized controlled trials (RCTs) that investigated allopurinol effects on oxidative stress. A random-effect model was used to calculate the raw mean difference or the standardized mean difference (with 95% confidence intervals: CI) as an estimate of effect size. Heterogeneity was quantified by four types of information; Q-statistic, I2 statistic, T2, and T.
Results: 37 eligible studies were identified; 14 were included in the final analysis and subdivided between two oxidative stress markers: there were 8 Malondialdehyde (MDA)-based studies (198 subjects) and 6 oxidized Low-density lipoprotein (Ox-LDL)-based studies (347 subjects). Allopurinol treatment was associated with a statistically significant reduction in mean MDA serum concentration, by 0.403 nmol/ml (95% CI; -0.618, -0.189) (P<0.001) and a non-significant reduction in Ox-LDL serum concentration assessed by the standardized mean difference, by 0.409 (95% CI; -1.332, 0.515) (P=0.386).
Conclusion: The antioxidant potential of allopurinol has been confirmed by a significant reduction of the serum concentration of MDA in a heterogeneous group of patients and healthy adults. The non-significant reduction in Ox-LDL may reflect methodological differences. To confirm whether or not allopurinol has clinically relevant antioxidant properties, future studies require reproducible measurement techniques and clearer characterization and quantification in different patient groups.
Keywords
Allopurinol, Xanthine oxidase inhibitor, Xanthine oxidase, Oxidative stress, Malondialdehyde, Oxidized LDL
Introduction
Atherosclerosis is a classical pathological process that results in significant cardiovascular complications, leading to death and major disabilities. Oxidative stress is a well-established contributor to the development of atherosclerosis via several enzyme systems involved in the production of reactive oxygen species (ROS). Such enzyme systems include (but are not limited to) xanthine oxidase (XO), NADPH oxidases and NO synthase [1]. ROS produced in the vascular system initiate several processes involved in atherosclerosis, including adhesion molecule expression, vascular smooth muscle proliferation, apoptosis in the endothelium, activation of matrix metalloproteinases, altered vasomotor activity, and oxidative modification of lipids, most importantly, low density-lipoproteins (LDL). Such oxidative modification is more likely to affect LDL that is retained in the arterial wall, leading to formation of oxidized LDL (Ox-LDL) [1]. The importance of the oxidative modification of LDL has been studied in vitro, and it was found that this phenomenon varies from minimal modification, by which LDL particles can still be recognized by LDL receptors, to extensive modification to particles that are not bound by the LDL receptor, but by scavenger receptors expressed on macrophages and smooth muscle cells [2]. Previous studies have demonstrated that Ox-LDL in the arterial wall could account for the generation of foam cells and fatty streak formation beneath the endothelium as a precursor to atheroma formation [3,4]. This process also involves inflammatory and immune cells which all play a part in fatty streak formation [2].
There has been a considerable amount of research into exploring the entity of Ox-LDL, its properties and its generation. Oxidative modification of lipoproteins is not unique to LDL: it is a complex process that involves several oxidation mechanisms mediated by copper-, ceruloplasmin-, iron-, lipoxygenase-, peroxidase- and by superoxide generators such as xanthine oxidase and NADPH oxidase [5]. Oxidation of LDL is a process via which proteins and lipids undergo oxidative modification to generate complex products such as fatty acid oxidation products: the most widely investigated have been aldehydes (such as malondialdehyde MDA) and prostaglandin-like products (such as isoprostanes) [5]. In addition to these markers, lipid derived products, and protein oxidation products are generated during LDL oxidation. In the literature, Ox-LDL, aldehydes, and prostaglandin-like products are commonly investigated as markers of oxidative stress, in different patient’s populations.
A natural extension of the available knowledge is the search for an antioxidant that could interrupt this vicious circle. Following unsuccessful attempts with vitamin E [6-8], the xanthine oxidase inhibitor, allopurinol, has emerged as a candidate for several, small scale clinical trials. Not only is xanthine oxidase an important oxidative enzyme circulating in the plasma and binding to the endothelial cells but it also (in addition to its role in uric acid generation from xanthine) has the unique property of reducing oxygen to form superoxide (O2−) and hydrogen peroxide (H2O2), thereby contributing to the ROS pool [9]. Xanthine oxidase accordingly contributes to endothelial injury and dysfunction [10], and to LDL oxidation together with metal ion co-factors [11,12]. Thus, allopurinol as a xanthine oxidase inhibitor has been investigated in short-term clinical trials and found to possess an antioxidant properties that have improved endothelial function and reduced the concentrations of biological markers of oxidative stress in different patient populations [11-15]. However, the published studies have differed with respect to the characteristics of the study population, the outcome assessed (oxidative stress markers of interest), the intervention dose and duration of therapy, and they have demonstrated variable results. This meta-analysis aims to summarize the effect of allopurinol treatment on two particular biological markers of oxidative stress that have been assessed in different patient populations and in healthy adults.
Methods
This review has been performed according to the PRISMA statement (Preferred reporting items for systematic reviews and meta-analysis) [16] and has been registered on the PROSPERO register with registration number CRD42016046468.
Search strategy
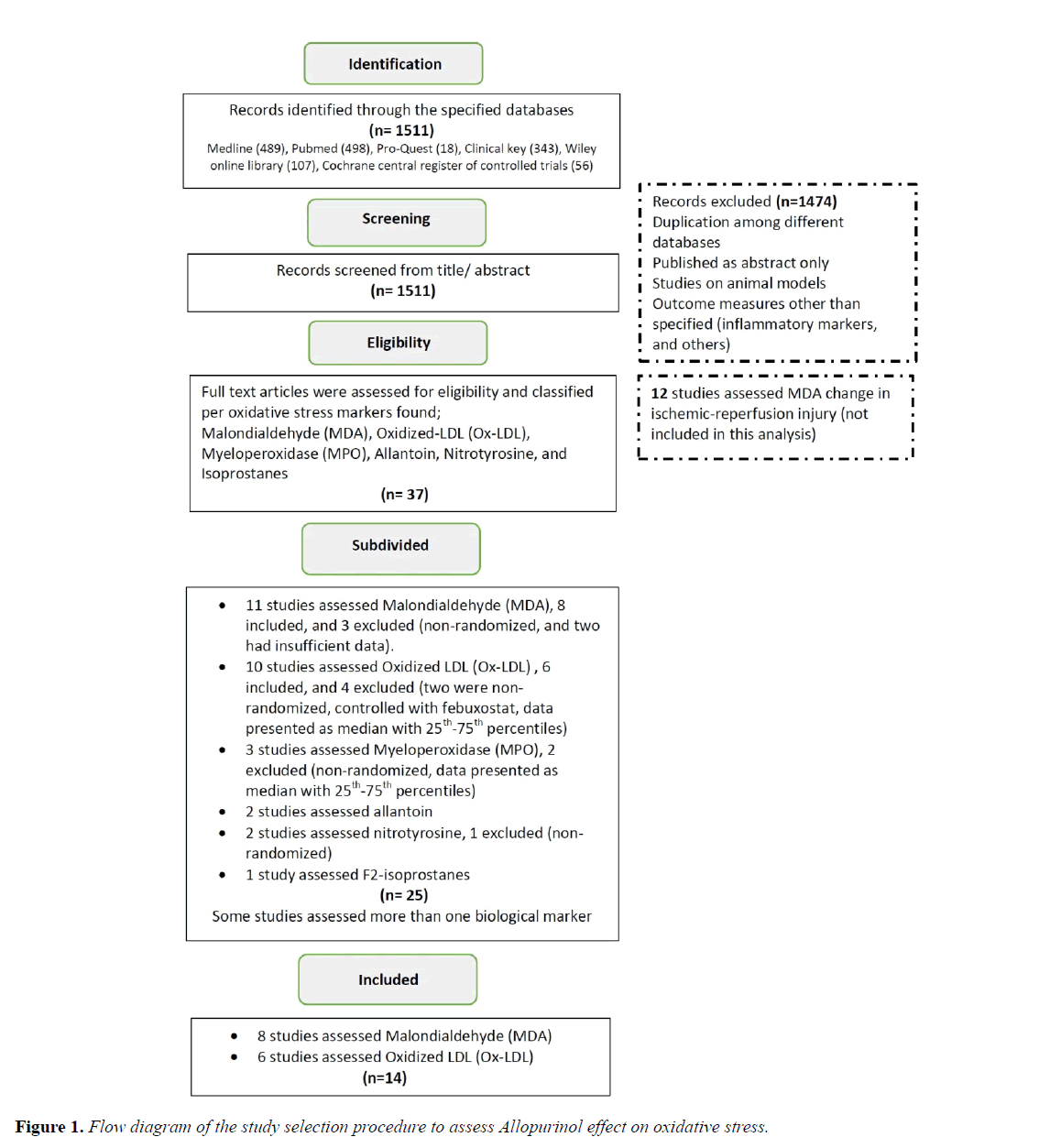
Medline, PubMed, ProQuest Health & Medical Complete, Clinical Key, Wiley Online Library, and Cochrane Central Register of Controlled Trials were searched using the search items in titles and abstracts, in combination with MESH terms; (allopurinol AND oxidative stress OR allopurinol AND malondialdehyde/oxidized-LDL). The literature was searched till 29th July, 2017. Search strategy and articles included in the final analysis are displayed in Figure 1.
Study selection
Randomized controlled trials (RCT) were included if they met the following inclusion criteria;
Published as full-text article
Reported with either parallel or cross-over design
Recruited human subjects randomized to allopurinol therapy or to control group (no treatment/ or placebo)
Assessed any marker of oxidative stress as primary or secondary endpoints
Data reported as mean ± SD/SEM for each group after treatment or reported a change or % change from baseline.
After excluding studies on animal models, review articles, and duplicate publications, eligible studies were subdivided according to the biological marker of oxidative stress reported, such as; malondialdehyde, Ox-LDL, myeloperoxidase, allantoin etc. (Figure 1). Final analysis requires a minimum of 3 studies per oxidative stress marker [17]. Studies assessed oxidative stress markers in the setting of ischemia-reperfusion injury/acute surgical settings were excluded.
Data extraction and quality assessment
The following data including first author name, year of publication, study design, participant’s disease status, number of participants in the allopurinol group and control group, age, sex, and uric acid level in the study participants were extracted from eligible full-text articles. Intervention strategies and outcomes; dose and duration of allopurinol therapy, oxidative stress marker assessed, method of detection, and status of participants at the time of assessment (at rest vs. post-exercise). The seven domains of the Cochrane risk of bias tool was used to evaluate the quality of the included studies (Table 1).
| Ref | Random sequence generation | Allocation concealment | Blinding of participant and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | |
|---|---|---|---|---|---|---|---|---|
| Butler et al. 2000 | 15 | L | U | L | L | L | L | L |
| Desco et al. 2002 | 20 | L | L | L | L | L | L | L |
| El Solh et al. | 12 | L | L | L | L | L | L | L |
| Farquharson et al. 2002 | 11 | L | U | L | L | L | L | L |
| Greig et al. 2011 | 21 | L | L | L | L | L | L | L |
| Heunks et al. 1999 | 22 | H | H | L | L | L | L | L |
| Sanchis et al. 2015 | 23 | U | H | U | L | L | L | L |
| Yiginer et al. 2008 | 24 | U | U | L | L | L | L | L |
| Eskurza et al. 2006 | 26 | U | L | L | L | L | L | L |
| Jalal et al. 2016 | 13 | L | L | L | L | L | L | L |
| Kao et al. 2011 | 14 | L | L | L | L | L | L | L |
| Rajendra et al. 2011 | 27 | L | L | L | L | L | L | L |
| Robertson et al. 2016 | 28 | L | L | L | L | L | L | L |
| Szwejkowski et al. 2013 | 29 | L | L | L | L | L | L | L |
Table 1: Risk of bias assessment in the included studies using Cochrane criteria H, high risk of bias; L, low risk of bias; U, unclear risk of bias.
Quantitative data analysis
Meta-analysis was conducted using Comprehensive Meta- Analysis (CMA) V3 software (Biostat, Englewood, New Jersey, USA). If all studies in the analysis estimated the parameter of interest with the same methodology and reported it with the same scale or unit, raw mean difference is used in the statistical analysis. Standardized mean difference (Hedges’s g) can be used also as an estimate of effect size in certain conditions; such as; reporting the mean change or percentage change in the oxidative stress marker without reporting the absolute figures post treatment, and if the included studies have used more than one method of detection of the oxidative stress marker, each could report it with different units. Accordingly the software divides the mean difference in the parameter of interest in each study by that study’s standard deviation to create an index (the standardized mean difference) that would be comparable across studies. Where some studies used SEM, SD was estimated using the following formula;
SD= SEM × sqrt (n)
Where, n is the number of participants. Subgroup analysis was done where it was possible to check if allopurinol had tendency for different effect size based on the status of participants at the time of assessment (at rest vs post-exercise), or based on baseline uric acid (above or below 7 mg/dl).
Heterogeneity of the effect size
The observed effect size varies from one study to another, but a certain amount of variation is expected due to sampling error. CMA software can quantify heterogeneity by providing four types of information; Q-statistic, I2 statistic, T2, and T. The Q-statistic provides a test of the null hypothesis that all studies included in the analysis share a common effect size. In such a case, it is expected that the value of Q would be equal to the degrees of freedom df (the number of studies minus 1). I2 statistic tells us what proportion of the observed variance reflects differences in true effect sizes, rather than sampling error. Tau-squared (T2) is the estimate of the variance in true effect sizes (in log units). Tau (T) is the estimate of standard deviation of true effect sizes (in log units). All statistics are displayed in the footer section of forest-plots.
Publication bias
One concern of publication bias is that some non-significant studies are missing from the analysis, and these studies if included, would nullify the observed effect. For that reason, classic fail-safe N was calculated by CMA software. This statistic measure can be defined as the number of new, unpublished/unretrieved studies with non-significant results that would be required to make the results of this meta-analysis non-significant or that would bring p-value > alpha (0.05) [18,19]. Funnel plot was not done because of the small number of included studies in the analysis.
Results
Search results
A total of 1511 records were identified through the database searched and 1474 records were excluded based on title/ abstract screening. The remaining 37 full-text articles were assessed for eligibility, out of which 12 were excluded (Figure 1). Thus, the final analysis incorporated a total of 14 studies which met the inclusion criteria, divided between 2 markers of oxidative stress: Malondialdehyde (8 studies, 198 subjects) and Ox-LDL (6 studies, 347 subjects).
Study Characteristics
Malondialdehyde-based studies
Seven of the 8 RCTs [11,12,15,20-24] which assessed the allopurinol effect on malondialdehyde concentrations in the serum of different patient populations were placebo-controlled but one study did not administer placebo to the control group [22].
The first 6 studies assessed the change in MDA concentration at rest, in patients with; chronic heart failure (functional class II-III) [11,21], type I diabetes mellitus [20], type II diabetes mellitus [15], metabolic syndrome [24], and obstructive sleep apnoea [12]. Mean age of those patients was 47-67 years and they were predominantly males (58-91%) in 4 studies [11,12,15,21]. One study was predominantly females (72%) [24], and another did not provide details about age, or gender of the patients [20]. Allopurinol was administered to all with a daily dose of 300 mg for a period of 2 weeks to 1 month. Serum uric acid varied from 4.6 to 7.4 mg/dl in 4 studies [11,12,21,24] which also reported a significant drop in serum uric acid with allopurinol treatment that varied from -15% to -60%. The other 2 studies did not provide data on uric acid concentration. No severe adverse events were reported.
The remaining two studies [22,23] assessed the change in MDA concentration post-exercise in, respectively, patients with chronic obstructive pulmonary disease (mean age 63 years) and professional soccer players (mean age 25 years). Male gender constituted 81-100 %. Allopurinol was administered before exercise with doses of 600 mg and 300 mg, respectively. No data were provided from these two studies on serum uric acid and no severe adverse events were reported. In the included studies, baseline serum MDA level varied from 0.34 to 1.61 nmol/ml in all studies, with the exception of one that reported a higher baseline concentration of 5.47 nmol/ml [24].
Method of detection of Malondialdehyde
Malondialdehyde concentrations were reported in these studies as either nmol/ml or μmol/L, and detected via the method of Wong. The principle of this method is that plasma lipoperoxides are hydrolyzed by boiling in dilute phosphoric acid. Malondialdehyde (MDA), one of the hydrolysis products, is reacted with thiobarbituric acid (TBA) to form MDA (TBA)2 adduct. Separation of the formed adduct is done by high performance liquid chromatography, followed by quantification by spectrophotometry [25].
Oxidized-LDL-based studies
There were 6 RCTs [13,14,26-29] (all placebo-controlled) which assessed the allopurinol effect on Ox-LDL concentrations in the serum of different patient populations. The populations included patients with chronic kidney disease [13,14] type 2 diabetes mellitus [29], coronary artery disease [27], peripheral arterial disease [28] and healthy adults [26]. The mean age of the participants varied from 26 to 74 years and male gender dominated with a percentage of 53-88%. Allopurinol was administered in doses of 300-600 mg for periods of 2 to 9 months, except in the study by Eskurza et al. which was designed to test the effect of a single dose of 600 mg in 2 groups of healthy adults with different age ranges [26]. Serum uric acid varied from 5.7 to 9.2 mg/dl, and they reported a significant drop in serum uric acid with allopurinol treatment that varied from -10% to -56%. No severe adverse events were reported. Baseline Ox-LDL concentration in these studies varied from 28.5 to 58.3 U/L.
Method of detection of Oxidized LDL
Ox-LDL measurements were performed by immunological techniques (enzyme-linked immunosorbent assay-ELISA) with a monoclonal antibody (anti-Ox-LDL mAb). This technique followed the identification and cloning of scavenger receptors that bind modified LDL (including Ox-LDL). Currently, there are four procedures available for Ox-LDL measurement; Itabe’s procedure, Kyowa Medex “MX” kit, Witztum’s procedure, and Holvoet’s procedure (Mercodia kit). These procedures use 3 different mAbs; DLH3, E06, and 4E6 [30]. The studies included in this meta-analysis used two kits for Ox-LDL estimation (Mercodia and ALPCO) and two studies did not specify the procedure/ kit used [14,29]
Outcome Results
Malondialdehyde serum concentration
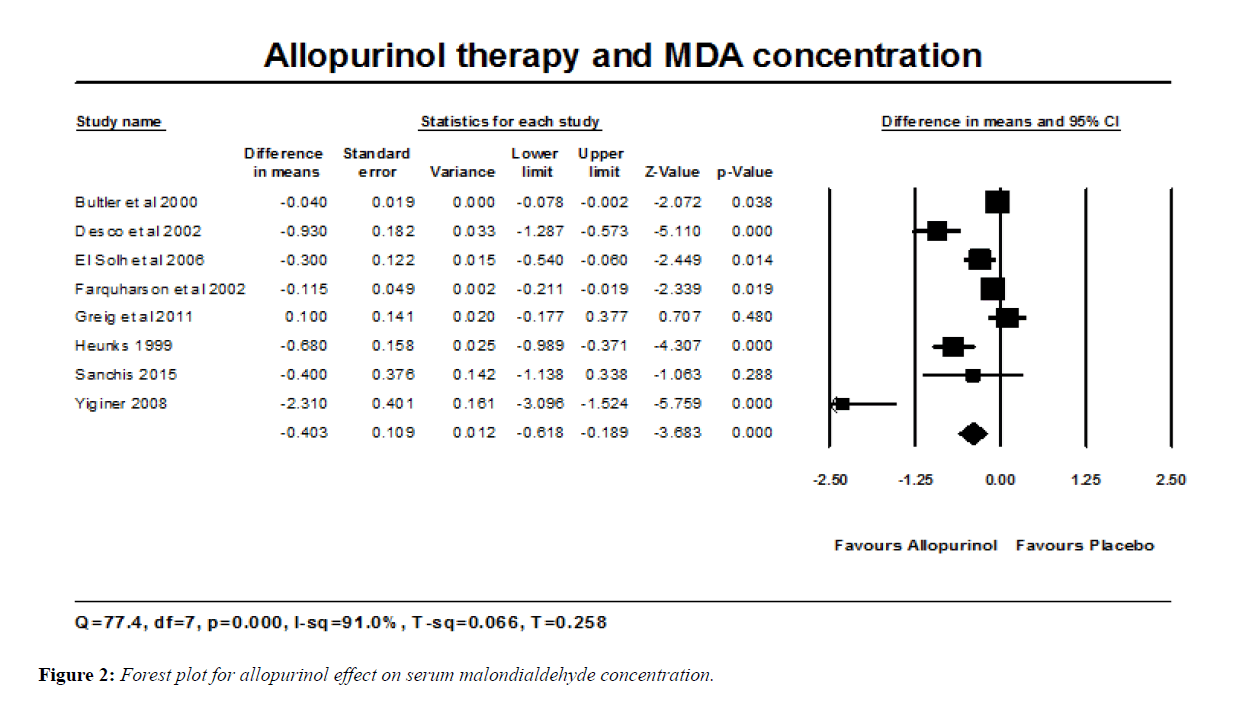
Allopurinol treatment reduced the concentrations of MDA in a total number of 198 individuals of different patient groups (and including 12 healthy subjects). The estimated effect size was the raw mean difference, as all studies used a common method of detection of MDA and it was estimated with one common unit (nmol/ml). The reduction in MDA was 0.403 nmol/ml with 95% confidence interval (-0.618, -0.189). This change was statistically significant (P<0.001) (Figure 2). Heterogeneity of the effect size was estimated with Q-statistic; Q-value is 77.4, df=7 and P=0.000; indicating that there are different effect sizes in different types of populations. I2 statistic states that 91% of the observed variance reflects differences in true effect size rather than sampling error.
For such high value of I2 statistic, subgroup/ moderator analysis was done. It was considered if the status at which the assessment was done (at rest vs. post-exercise) might explain such heterogeneity. Therefore the analysis was subdivided to two groups; group 1; MDA assessment at rest; 6 studies with 170 patients [11,12,15,20,21,24]. Group 2; MDA assessment post-exercise; 2 studies with 28 patients and athletes [22,23]. MDA was reduced by 0.343 nmol/ml; 95% CI (-0.566, -0.120) (P=0.003) in the former and by 0.598 nmol/ml; 95% CI (-1.070, -0.127) (P=0.013) in the latter. This subgroup analysis shows that reduction in MDA post-exercise was almost double that seen at rest, but such difference did not reach statistical significance (P=0.337).
Classic Fail-safe N was 123, and that means we need to retrieve 123 non-significant studies in order to make the result of this meta-analysis non-significant.
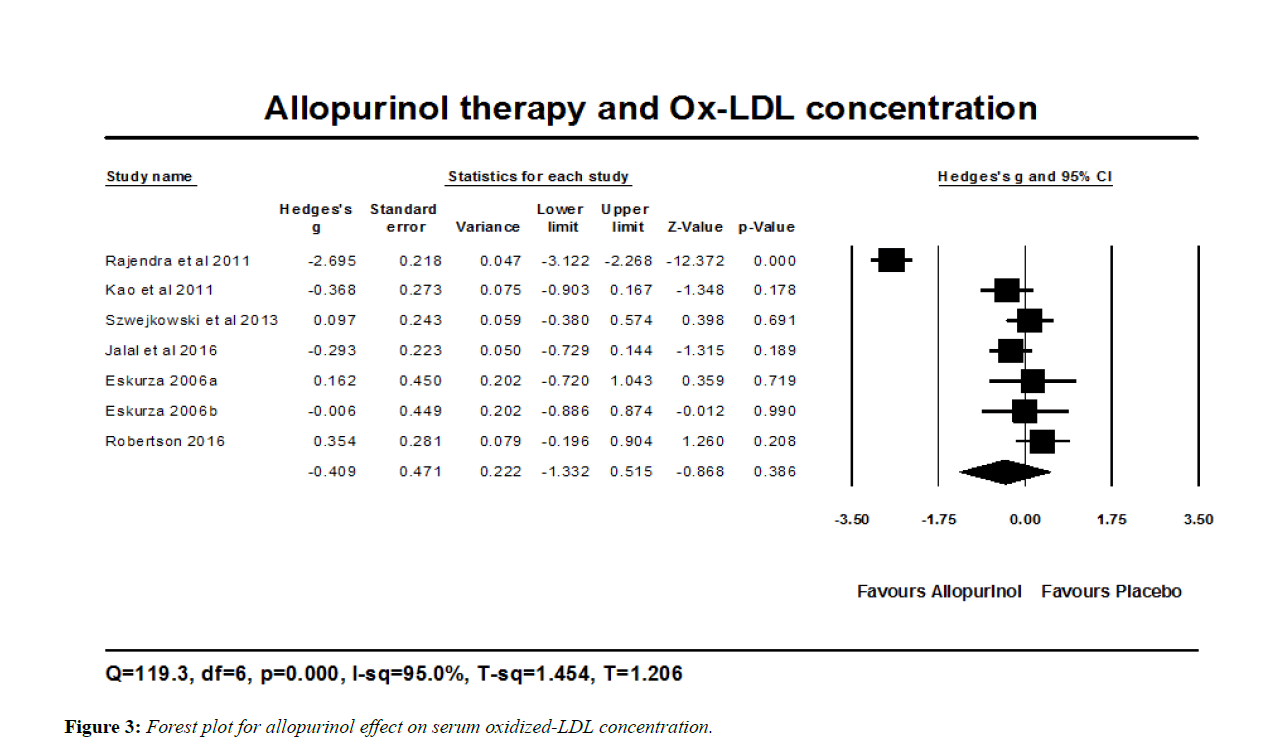
Oxidized-LDL serum concentration
Allopurinol treatment reduced the concentration of Ox-LDL in 347 individuals included with different disease states (and including 18 healthy subjects). The estimated effect size was the standardized mean difference (Hedges’s g), which was reduced by 0.409. Therefore individuals treated with allopurinol might have an Ox-LDL level that is 0.409 a standard deviation lower than those treated with placebo. 95% confidence interval (-1.332, 0.515). Such change did not reach statistical significance (P=0.386) (Figure 3). Heterogeneity of the effect size was quantified by Q-statistic, I2 statistic, T2, and T demonstrated at the footer section of the forest plot (Figure 3). Subgroup analysis was done based on the baseline uric acid level; if above or below 7 mg/dl, because the range of uric acid of the participants was wide. Ox-LDL was reduced by 0.188; 95% CI (-1.588, 1.213) (P=0.793) in the three studies where baseline serum uric acid was above 7 mg/dl, and by 0.582; 95% CI (-1.821, 0.658) (P=0.358) in the four studies with uric acid below that value. This subgroup analysis shows that reduction in Ox-LDL with allopurinol treatment is likely to be higher in those with serum uric acid within normal values, but such difference did not reach statistical significance (P=0.680). Another subgroup analysis was done based on age of participants (if above or below 50 years of age), but age was not a significant moderator for the reduction in serum Ox-LDL (P=0.642).
Discussion
Biological markers of oxidative stress include many molecules: these markers are derived from lipids, proteins, free amino acids and DNA and modified upon exposure to ROS. The literature has a heterogeneous collection of different biomarkers assessed in different disease states, with different methodologies. Accordingly, the results and conclusions of those studies require a careful interpretation of the significance of a specific marker, its prognostic ability and, in particular, its method of assessment. Lipid molecules and the products of polyunsaturated fatty acid peroxidation (particularly MDA) are the most widely studied. The significance of MDA is attributed to its genotoxic activity that might lead to mutations [31] and its toxic effects which stiffen the collagen of the cardiovascular system [32]. In terms of its prognostic significance, MDA has been shown to be a strong and independent predictor of cardiovascular events in patients with coronary artery disease [33] and to be one of the strongest predictors of carotid atherosclerosis progression [34]. Hence, reducing its concentration via pharmacological therapy might suggest a future therapeutic target.
Considering the uniformity, sensitivity, and reproducibility of method of detection of MDA in the included studies [35], the results of this meta-analysis demonstrate a beneficial and significant effect of allopurinol treatment on serum levels of MDA. Heterogeneity of the results, however, was not explained by the status at which the assessment was made, whether single-dose administration (post-exercise) or short-term administration (at rest). It is likely that having different patients groups with different underlying pathologies might be the reason, as oxidative stress is likely to differ among different disease states.
Ox-LDL on the other hand, does not define a specific molecule or a family of molecules. It is a heterogeneous mixture of modified lipoprotein particles from which, during their oxidative modification, aldehydes (MDA) and prostaglandin-like molecules (isoprostanes) are produced. Ox-LDL has been studied extensively in different disease states and its significance is supported by the fact it was found to be increased in hypertension, coronary artery disease, chronic heart failure, cerebrovascular disease, insulin resistance, and diabetes [36]. However, the results of studies assessing its prognostic ability have been inconsistent. It was found to be a strong predictor for acute coronary artery disease in the general population [37], and showed a significant positive correlation with the severity of acute coronary syndrome [38]. When Ox- LDL concentrations were reduced by treatment with an antioxidant (vitamin E), however, there was no benefit on the progression of carotid artery intima-media thickness over a follow up period of 3 years [39].
In the background, there are important considerations regarding the methods for measuring Ox-LDL concentrations. Firstly, it is an enzyme-linked immunosorbent assay (ELISA) which involves 3 different monoclonal antibodies. Thus the result of one kit might not be comparable with another, and they might not correlate with each other [30,40], so it is important to recognize the differences and limitations of the different ELISA procedures before drawing any biological or clinical inference. Secondly, the assay method might detect native LDL. Thus, the results of this meta-analysis with regard to Ox-LDL might be compromised.
A further consideration was identified in the study by Holvoet et al. which demonstrated that the levels of both MDA and Ox- LDL were significantly higher in patients with acute coronary syndrome than in those with stable CAD. However, the MDA (but not Ox-LDL) level was associated with increased level of troponin I and C-reactive protein, suggesting that MDA might be a marker of plaque instability [41]. In view of this study result, MDA reduction by a pharmacological agent might be a more worthwhile target (rather than Ox-LDL), especially if the less well-defined properties of Ox-LDL as an oxidative stress marker (and the methodological issues) are taken into consideration.
This meta-analysis is derived from studies in the published literature and summarizes the results for two well-recognized markers of oxidative stress (Malondialdehyde and Ox-LDL). Allopurinol treatment has thus been shown to reduce these markers of oxidative stress and this is consistent with the results of other studies investigating alternative markers. For example, two studies assessed the allopurinol effect on the serum concentration of allantoin and showed a significant reduction by 20% in patients with chronic heart failure [42,43]. Another study demonstrated a significant reduction of 20% in serum myeloperoxidase in patients with metabolic syndrome [24] and a further study demonstrated a non-significant reduction in serum F2-Isoprostanes in patients with coronary artery disease [27]. Overall, however, it remains unclear which patient populations are likely to benefit most from the reductions in the different markers of oxidative stress and further, targeted studies are required.
Limitations
The results of this meta-analysis show that allopurinol has an antioxidant potential, but definitive conclusions cannot be drawn with respect to identifying which patient populations are likely to benefit most from allopurinol treatment. There are also concerns about assay reliability. For example, despite the sensitivity and reproducibility of the method described to measure MDA, it lacks specificity, as other aldehydes can react with TBA to produce compounds that absorb in the same range [44]. ELISA quantification of MDA by antibodies has been validated and found to be more specific [45], but was not used by any of the studies included in this analysis.
Conclusions
The contribution of oxidative stress to the development of atherosclerosis is mediated by several enzyme systems, one of them is xanthine oxidase. Allopurinol, as a xanthine oxidase inhibitor, administered as a short-term treatment, has been found to possess a genuine antioxidant property, evidenced by its ability to reduce the level of MDA in the sera of a heterogeneous group of subjects. As discussed, the lack of a significant effect on Ox-LDL in this analysis might have resulted from the limitations of Ox-LDL assessment methodology.
The following recommendations for further studies investigating the antioxidant properties of a particular drug appear obvious. The biological marker should have proven prognostic significance; the assessment methods should be validated and standardized; and defined patient populations and treatment regimens should be specifically and systematically examined. It is definitely worthwhile identifying novel pharmacological agents with antioxidant properties especially if we can prove that these properties directly contribute to a better prognosis for a particular patient population.
Conflict of Interest
This research holds no conflict of interest and is not funded through any source.
References
- Harrison D, Griendling KK, Landmesser U, et al. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91(3):7-11.
- Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104(4):503-516.
- Quinn MT, Parthasarathy S, Fong LG, et al. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987;84(9):2995-2998.
- Steinbrecher UP, Parthasarathy S, Leake DS, et al. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984;81(12):3883-3887.
- Parthasarathy S, Raghavamenon A, Garelnabi MO, et al. Oxidized low-density lipoprotein. Methods Mol Biol. 2010;610:403-417.
- Rapola JM, Virtamo J, Ripatti S, et al. Randomised trial of alpha-tocopherol and beta-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet. 1997;349(9067):1715-1720.
- Eidelman RS, Hollar D, Hebert PR, et al. Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease. Arch Intern Med. 2004;164(14):1552-1556.
- Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293(11):1338-1347.
- White CR, Darley-Usmar V, Berrington WR, et al. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc Natl Acad Sci U S A. 1996;93(16):8745-8749.
- Houston M, Estevez A, Chumley P, et al. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem. 1999;274(8):4985-4994.
- Farquharson CA, Butler R, Hill A, et al. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106(2):221-226.
- El Solh AA, Saliba R, Bosinski T, et al. Allopurinol improves endothelial function in sleep apnoea: a randomised controlled study. Eur Respir J. 2006;27(5):997-1002.
- Jalal DI, Decker E, Perrenoud L, et al. Vascular Function and Uric Acid-Lowering in Stage 3 CKD. J Am Soc Nephrol. 2016;28(3):943-952.
- Kao MP, Ang DS, Gandy SJ, et al. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22(7):1382-1389.
- Butler R, Morris AD, Belch JJ, et al. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35(3):746-751.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
- Davey J, Turner RM, Clarke MJ, et al. Characteristics of meta-analyses and their component studies in the Cochrane Database of Systematic Reviews: a cross-sectional, descriptive analysis. BMC Med Res Methodol. 2011;11(1):160.
- Borestein M, Hedges LV, Higgins JP, et al. Publication Bias. Introcuction to Meta-Analysis. 2009:277-292.
- Persaud R. Misleading meta-analysis. "Fail safe N" is a useful mathematical measure of the stability of results. BMJ. 1996;312(7023):125.
- Desco MC, Asensi M, Marquez R, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002;51(4):1118-1124.
- Greig D, Alcaino H, Castro PF, et al. Xanthine-oxidase inhibitors and statins in chronic heart failure: effects on vascular and functional parameters. J Heart Lung Transplant. 2011;30(4):408-413.
- Heunks LM, Vina J, van Herwaarden CL, et al. Xanthine oxidase is involved in exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Physiol. 1999;277(6):R1697-R1704.
- Sanchis-Gomar F, Pareja-Galeano H, Gomez-Cabrera MC, et al. Allopurinol prevents cardiac and skeletal muscle damage in professional soccer players. Scand J Med Sci Sports. 2015;25(1):e110-e115.
- Yiginer O, Ozcelik F, Inanc T, et al. Allopurinol improves endothelial function and reduces oxidant-inflammatory enzyme of myeloperoxidase in metabolic syndrome. Clin Res Cardiol. 2008;97(5):334-340.
- Wong SH, Knight JA, Hopfer SM, et al. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem. 1987;33:214-220.
- Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol. 2006;571(3):661-668.
- Rajendra NS, Ireland S, George J, et al. Mechanistic insights into the therapeutic use of high-dose allopurinol in angina pectoris. J Am Coll Cardiol. 2011;58(8):820-828.
- Robertson AJ, Struthers AD. A Randomized Controlled Trial of Allopurinol in Patients With Peripheral Arterial Disease. Can J Cardiol. 2016;32(2):190-196.
- Szwejkowski BR, Gandy SJ, Rekhraj S, et al. Allopurinol reduces left ventricular mass in patients with type 2 diabetes and left ventricular hypertrophy. J Am Coll Cardiol. 2013;62(24):2284-2293.
- Itabe H, Ueda M. Measurement of plasma oxidized low-density lipoprotein and its clinical implications. J Atheroscler Thromb. 2007;14(1):1-11.
- Del RD, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15(4):316-328.
- Slatter DA, Bolton CH, Bailey AJ. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia. 2000;43(5):550-557.
- Walter MF, Jacob RF, Jeffers B, et al. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: a longitudinal analysis of the PREVENT study. J Am Coll Cardiol. 2004;44(10):1996-2002.
- Salonen JT, Nyyssonen K, Salonen R, et al. Lipoprotein oxidation and progression of carotid atherosclerosis. Circulation. 1997;95(4):840-845.
- Breusing N, Grune T, Andrisic L et al. An inter-laboratory validation of methods of lipid peroxidation measurement in UVA-treated human plasma samples. Free Radic Res. 2010;44(10):1203-1215.
- Trpkovic A, Resanovic I, Stanimirovic J, et al. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2015;52(2):70-85.
- Meisinger C, Baumert J, Khuseyinova N, et al. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112(5):651-657.
- Ehara S, Ueda M, Naruko T, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103(15):1955-1960.
- Hodis HN, Mack WJ, LaBree L et al. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS). Circulation. 2002;106(12):1453-1459.
- Frijhoff J, Winyard PG, Zarkovic N, et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid Redox Signal. 2015;23(14):1144-1170.
- Holvoet P, Vanhaecke J, Janssens S, et al. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98(15):1487-1494.
- Doehner W, Schoene N, Rauchhaus M, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105(22):2619-2624.
- von HS, Bode-Boger SM, Martens-Lobenhoffer J, et al. Elevated levels of asymmetric dimethylarginine in chronic heart failure: a pathophysiologic link between oxygen radical load and impaired vasodilator capacity and the therapeutic effect of allopurinol. Clin Pharmacol Ther. 2010;88(4):506-512.
- Meagher EA, FitzGerald GA. Indices of lipid peroxidation in vivo: strengths and limitations. Free Radic Biol Med. 2000;28(12):1745-1750.
- Bevan RJ, Durand MF, Hickenbotham PT, et al. Validation of a novel ELISA for measurement of MDA-LDL in human plasma. Free Radic Biol Med. 2003;35(5):517-527.