Research Article - Biomedical Research (2019) Volume 30, Issue 4
Biocompatibility of three calcium silicate based materials implanted in rat subcutaneous tissue.
Ranjdar Mahmood Talabani1*, Balkees Taha Garib2, Reza Masaeli3
1Conservative Department, College of Dentistry, University of Sulaimani, Kurdistan Region, Iraq
2Oral Diagnosis Department, College of Dentistry/ University of Sulaimani, Kurdistan Region, Iraq
3Department of Dental Biomaterials, School of Dentistry, Tehran University of Medical Sciences, Iran, Tehran
- Corresponding Author:
- Ranjdar Mahmood Talabani
Department College of Dentistry
University of Sulaimani Iraq
Accepted on June 13, 2019
DOI: 10.35841/biomedicalresearch.30-19-264
Visit for more related articles at Biomedical ResearchAbstract
Objective: The purpose of the present study was to evaluate the inflammatory response caused by three calcium silicate based cements in rat’s subcutaneous tissue. Materials and methods: Fifteen Wistar rats were divided into three groups of 1, 4, and 8 experimental weeks. Sterile silicone tubes were filled with Micro-Mega Mineral Trioxide Aggregate (MM-MTA), Biodentine (BD) and EndoSequence Root Repair Material (ESRRM) putty and implanted subcutaneously. Empty tubes were implanted as negative control. After the experimental periods, all animals were sacrificed and the specimens stained histologically with hematoxylin-eosin (H&E), Masson’s trichrome and Toluidine blue to assess the type of inflammation, intensity and extent of inflammatory cells, foreign body reaction, fibrous capsule thickness, coagulated necrosis, capillary reaction and vascular congestion, amount and the distribution of mature collagen and mast cell population. Statistical analysis was performed by the Kruskal-Wallis and Mann-Whitney U tests. The level of significance was 5% (P<0.05). Results: MM-MTA provoked severe inflammation after 1 week, which was significantly different from ESRRM putty and control (P<0.05); BD produced less biological reaction than MM-MTA and more than ESRRM and control during the initial periods. While both MM-MTA and BD had reduced inflammatory reaction with time. ESRRM showed tissue-tolerance features almost comparable to control during all experimental periods. Conclusion: ESRRM putty was significantly more biocompatible than MM MTA and BD in the 1st week of the experiment. However, there was no significant difference between the materials at the end of the 8th weeks. ESRRM putty, BD, and MM MTA can be considered suitable calcium silicate materials.
Keywords
Biocompatibility, MM-MTA, Biodentine, EndoSequence root repair materials putty, Subcutaneous tissue.
Introduction
An ideal root repair material should be biocompatible, have the ability to adhere to dentin, maintain a sufficient seal, be insoluble in tissue fluids, be dimensionally stable, nonresorbable over time, radiopaque, easily manipulated, adequate compressibility, adequate working time, quick setting time, and unaffected by blood contamination [1].
Biocompatibility of a root filling material, when used in pulp capping, perforation repair, or as a retrograde filling, may influence the viability of periradicular cells and cause cell death by apoptosis or necrosis. To promote healing and restoration of the function of the tooth, dental materials should either stimulate cellular repair or be biologically neutral [2].
Mineral Trioxide Aggregate yields good biocompatibility results; however, its handling and setting time are not ideal. MicroMega MTA (MM-MTA; MicroMega Besanchon, France), another formulation of MTA is being developed to overcome drawbacks of original MTA products in 2011. It is injectable osteo-conductive, osteo-inductive and biocompatible tricalcium silicate-based cement containing calcium carbonate, which purportedly reduces the setting time [3-6].
Biodentine (BD) (Septodont, Saint Maur de Fosses; France) has been developed as novel tricalcium silicate-based cement in 2009 [7]. It was described as a bioactive dentin substitute with apatite formation after immersion in phosphate solution [8]. BD is reported as presenting better biological properties than other tricalcium silicate cements such as MTA. Zhou et al. [2] and Laurent et al. [9] assessed the cytotoxicity of biodentine using human gingival fibroblast and pulp. Other studies compared biocompatibility and gene expression of BD and MTA using three-dimensional multicellular spheroid cultures and murine fibroblasts, they observed that BD was non-cytotoxic and non-genotoxic [10,11].
An alternative material, EndoSequence Root Repair Materials putty (ESRRM putty) was developed as a premixed, injectable material formulated using bioceramic technology. It has been released by Brasseler USA (Savannah, GA) to be used as a clinical replacement for MTA. ESRRM putty has the advantages of faster setting and superior handling properties [12]. Alanezi et al. [13] Ma et al. [14] evaluated the cytotoxicity of ESRRM putty using human ginigival fibroblast and L929 mouse fibroblasts by MTT assay and they reported similar in-vitro biocompatibility to MTA. On the other hand, Martínez-Cortés et al. [15] found that ERRM material was more biocompatible than MTA and did not exhibit cytotoxic effects on mouse and human fibroblast.
The purpose of this study was to evaluate the biocompatibility of three calcium silicate-based materials in rat’s subcutaneous tissue using different special stains at different time periods.
Materials and Methods
This study performed in accordance with the principles of laboratory animal care (NIH publication 85-23, 1985). The national laws on animal use were also observed for the present study by getting authorization from the Ethical Committee for Animal Research of the Sulaimani University (No. 9, 6.2.2017).
Fifteen male Wistar albino rats aged (4-5) months and weighted (250-350 g) were used in this study. The animals were housed in temperature-controlled rooms (18-23ºC) and were provided with water and food ad libitum. Animal care was performed according to the Farabi Comprehensive Center of Excellence in Ophthalmology, Tehran University of Medical Sciences.
Forty-five silicone tubes of (7 mm length, inner diameter 2 mm and the outer diameter of 4 mm with both open ends) were filled with different tested materials and fifteen extra silicone empty tubes were used as negative controls. Biodentine (Septodont, Saint Maur de Fosses; France) was prepared according to the manufacturer’s recommendations and inserted into the tubes with amalgam carrier (Shanghai, China) and condensed using small sized ash condenser (Shanghai, China). EndoSequence Root Repair Material putty (BC Fast Set Putty) (Brasseler USA; Savannah, GA) was available in a ready-touse and was directly inserted into the silicone tubes. Micro Mega MM-MTA was available in a capsule and it was prepared according to the manufacturer ’ s instructions and inserted into the tubes.
After administration of xylazine 2% (20 mg/kg Alfasan Woerden-Holland, Holland) and ketamine 10% (100 mg/kg Alfasan Woerden-Holland, Holland) intramuscular anesthesia, the backs of the animals were shaved, antisepsis was obtained with 5% iodine solution, and a 2.0 cm incision was formed in a head-tail orientation with #15 Bard-Parker blade (Moris, Germany), creating two pockets on each side of the incision. Three silicone tubes, containing the three tested materials, and an empty tube, as the control, were implanted in each animal in opposite directions [upper right (MM-MTA), upper left (empty tube), lower right (ESRRM putty), and lower left (BD)]. The skin was closed with a 4/0 silk suture (Lenosilk, Istanbul, Turkey).
On 7 days, 4 weeks and 8 weeks after implantation, the animals were euthanized by an anesthetic overdose and CO2. Sixty samples including silicone tubes with the surrounding tissues (20 samples for each time period) were removed and fixed in 10% buffered formalin at pH 7.0 for 48 hours.
Histological preparation and staining
In the histological examination of the prepared samples, the parameters were analyzed qualitatively and semi-quantitatively [16]. The samples processed routinely, and three serial tissue sections of 5 μm thickness were cut from each block. One section stained with hematoxylin-eosin (H&E) and used for histologic evaluation of the type and intensity of inflammation, the presence of giant cells, fibroblastic (capsule), capillary reactions, and coagulated necrosis. The remaining two sections were stained with two special stains. Masson trichrome stain was used for subjectively evaluating the amount and the distribution of mature collagen surrounding the open ends of the implanted tubes. While toluidine blue stain (Drury and Wallington, 1980) used to observe the occurrence of mast cells, their distribution and degranulation.
All slides were examined by Olympus light microscope at high power fields (40X) linked with a digital camera (ToupTek, ToupView, x86, 3.7.4183, 2014). The captured image were transferred to a monitor and analyzed by image J software (Image Processing and Analysis in Java; US National Institutes of Health). Inflammatory and mast cells at the opening of the tube were counted after dividing the field area by nine-squared grid (Figure 1).
The type of inflammatory cells (polymorphonuclear cells and mononuclear cells) were identified and counted by two observers blindly for two times and the mean was calculated.
The intensity of inflammatory reactions were scored as follows: 0: without inflammation, 1: <25 cell counts, 2: 25<cell count<50, 3: 50<cell count 75, and 4: Over 75 cell counts [17]. The number of metachromatic mast cells were analyzed quantitatively with purple violet granules was counted as average per field. Data were organized on specific excel sheet and statistically analyzed by SPSS software (version) using the Kruskal-Wallis test and Mann-Whitney U tests at a significance level of 5% (P<0.05). Fibrous capsule was considered to be either thin or thick. The presence or absence of suppuration or necrosis was recorded.
Results
The quantitative results of inflammatory and mast cells counting at 1st, 4th and 8th weeks are presented in tables 1 and 2.
| Experimental periods | N | Mean inflammatory infiltrate score | P value | |||
|---|---|---|---|---|---|---|
| Control | MM-MTA | BD | ESRRM putty | |||
| 1 week | 20 | 1 | 4 | 3 | 1 | P=0.029* |
| 4 weeks | 20 | 1 | 3 | 2 | 2 | P=0.172 |
| 8 weeks | 20 | 1 | 2.33 | 2 | 1 | P=0.129 |
Note: N=Number of Samples; MMMTA=Micro MegaMineral Trioxide Aggregate; BD=Biodentine; ESRRM=EndoSequence Root Repair Material.
Table 1. Mean of the inflammatory score and significant differences among the studies groups within different experimental periods.
| Experimental periods | N | Mean (SD) of Mast cell | P Value | |||
|---|---|---|---|---|---|---|
| Control | MM-MTA | BD | ESRRM putty | |||
| 1 week | 20 | 3.2 (0.424) | 4.333 (0.351) | 5.15 (4.981) | 5.4167(0.381) | P=0.253 |
| 4 weeks | 20 | 3(0.282) | 10.9(.0141) | 8.25(0.353) | 8.2(0.282) | P=0.106 |
| 8 weeks | 20 | 3.3(0.141) | 9.8(0.3)* | 4.5167(.500)* | 7.2(0.566) | P=0.037* |
Note: N=Number of Samples; MMMTA=Micro MegaMineral Trioxide Aggregate; BD=Biodentine; ESRRM=EndoSequence Root Repair Material and SD=Standard Deviation.
Table 2. Mean and Standard Deviation of the mast cell and significant differences among the studied groups within different experimental periods.
In the ESRRM putty group, the extent of cell infiltration was significantly less compared to the other group near to the control (P=0.029) on day 7. However, no statistical difference (p>0.05) was observed among them in the 4th and 8th week periods.
On the other hand, the number of the mast cells statistically no significant (p>0.05) among the control, MM-MTA, BD and ESRRM putty groups in the 1st and 4th week periods. In MMMTA, the number of mast cells was significantly increased (P=0.037) with other observations at 8th weeks.
Empty tube (Negative Control)
At day 7 there was edematous loose connective tissue, contain few crossing collagen fibrils (faint blue by trichrome stain) with fibroblast, few small round capillaries, and mild inflammatory reaction, at the opening of the tube (Figure 2) after 4th weeks and 8th weeks, a band of dense collagen fibers (stained lighter with trichrome than collagen fibers in the mature granulation tissue) and mature fibroblast. The adjacent granulation tissue contained elongated capillaries within a fibrillar background (increased amount of collagen fibers that stained dark blue and arranged in a mesh-like pattern) with no evidence of inflammatory cells (Figure 2). Sometimes this granulation tissue invaginated into the tube hole (Figure 2). The tissues attached to silicone tube also showed a band of a fibrous capsule with mature fibroblast (Figures 2).
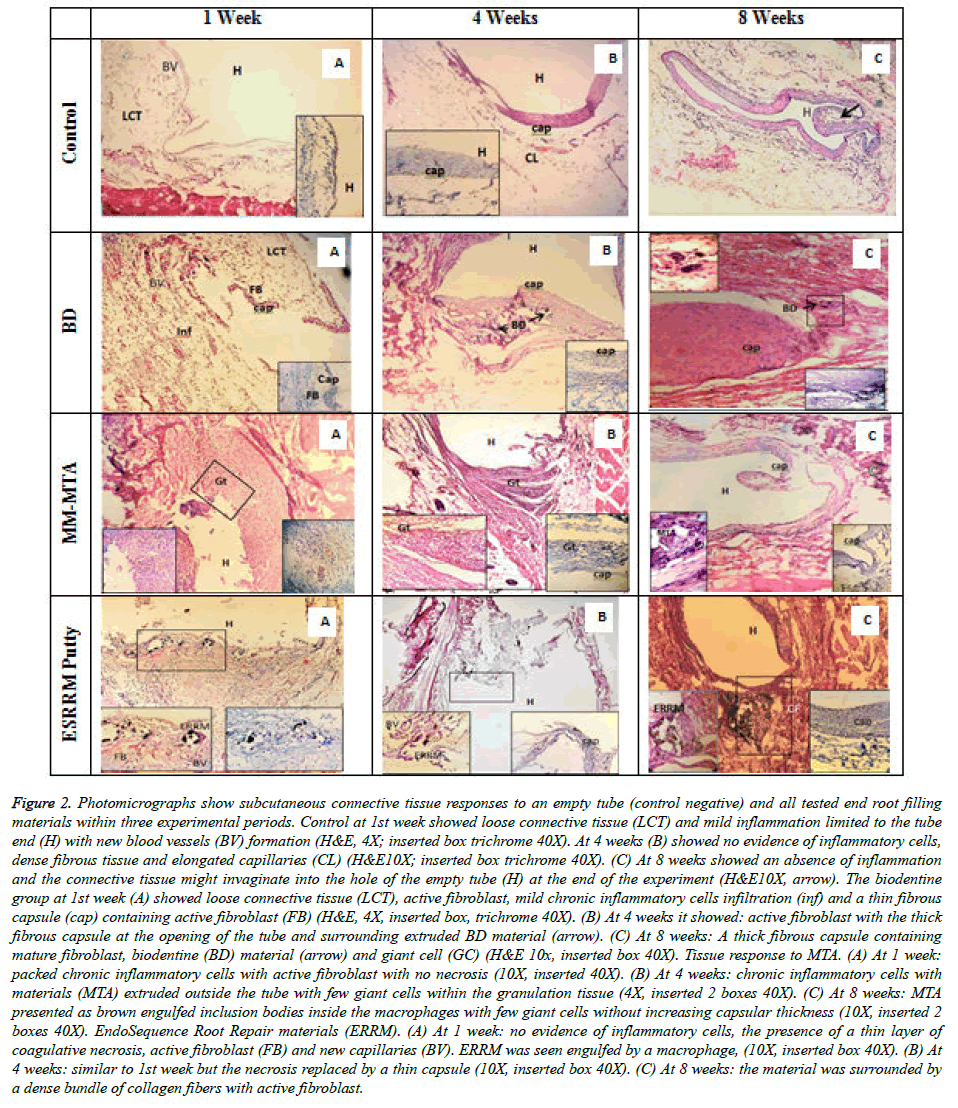
Figure 2. Photomicrographs show subcutaneous connective tissue responses to an empty tube (control negative) and all tested end root filling materials within three experimental periods. Control at 1st week showed loose connective tissue (LCT) and mild inflammation limited to the tube end (H) with new blood vessels (BV) formation (H&E, 4X; inserted box trichrome 40X). At 4 weeks (B) showed no evidence of inflammatory cells, dense fibrous tissue and elongated capillaries (CL) (H&E10X; inserted box trichrome 40X). (C) At 8 weeks showed an absence of inflammation and the connective tissue might invaginate into the hole of the empty tube (H) at the end of the experiment (H&E10X, arrow). The biodentine group at 1st week (A) showed loose connective tissue (LCT), active fibroblast, mild chronic inflammatory cells infiltration (inf) and a thin fibrous capsule (cap) containing active fibroblast (FB) (H&E, 4X, inserted box, trichrome 40X). (B) At 4 weeks it showed: active fibroblast with the thick fibrous capsule at the opening of the tube and surrounding extruded BD material (arrow). (C) At 8 weeks: A thick fibrous capsule containing mature fibroblast, biodentine (BD) material (arrow) and giant cell (GC) (H&E 10x, inserted box 40X). Tissue response to MTA. (A) At 1 week: packed chronic inflammatory cells with active fibroblast with no necrosis (10X, inserted 40X). (B) At 4 weeks: chronic inflammatory cells with materials (MTA) extruded outside the tube with few giant cells within the granulation tissue (4X, inserted 2 boxes 40X). (C) At 8 weeks: MTA presented as brown engulfed inclusion bodies inside the macrophages with few giant cells without increasing capsular thickness (10X, inserted 2 boxes 40X). EndoSequence Root Repair materials (ERRM). (A) At 1 week: no evidence of inflammatory cells, the presence of a thin layer of coagulative necrosis, active fibroblast (FB) and new capillaries (BV). ERRM was seen engulfed by a macrophage, (10X, inserted box 40X). (B) At 4 weeks: similar to 1st week but the necrosis replaced by a thin capsule (10X, inserted box 40X). (C) At 8 weeks: the material was surrounded by a dense bundle of collagen fibers with active fibroblast.
MM-MTA
At day 7, a severe packed chronic inflammatory cells aggregation was seen within unorganized fibrous connective tissue that contained many small capillaries with no evidence of necrosis (Figure 3). Trichrome stain showed blue fibers within the granulation tissue.
At 4th weeks, still the above granulation tissue contained severe chronic inflammatory cells infiltration with few giant cells. The tested material was extruded outside the tube. Collagen fibers surrounded the reaction and the collagen fibers by trichrome stain seemed to be more organized in a parallel manner.
At 8th weeks, the fibrous capsule was more organization containing fewer mature fibroblasts without increasing its thickness. However, the thick capsule stained purple-violet unlike the normal fibers in the surrounding connective tissue, which take dark violet color. Occasionally giant cells were seen. The tested material presented as inclusion bodies inside the macrophages.
Biodentine
The histological sections for the samples containing biodentine materials at 7 days showed thin layered of collagen fibrils and several active fibroblasts (swollen large nucleus and abundant cytoplasm) just ahead to the tube opening with mild inflammatory cells and loose connective tissue containing dilated large capillaries. At 4th weeks, the fibrous capsules became thicker and containing many active fibroblasts. At 8th weeks, the fibrous capsule become even thicker (nearly twice that observed in 4th weeks), and it still contained active fibroblasts. Few giant cells were also seen.
EndoSequence Root Repair materials (ESRRM) Putty
At 7th days, the interface tissue reaction showed a localized coagulative necrosis (limited) containing fragments of cells, nuclei and test material. There was no evidence of an inflammatory response. Just above this necrotic layer, there was a fibrogenic reaction with active fibroblast and small capillaries (loose granulation tissue). The amount of necrosis unrelated to the amount and size of tested material particles. The tiny particles of tested materials were engulfed by macrophages resulting in granulation tissue with minimum inflammatory cells infiltrate.
After 4th weeks, the tissue reaction was minimum and no evidence of necrosis. The connective tissue fibers were more organized with minimum capillaries and many macrophages engulfing the materials. After 8th weeks, the material was surrounded by a dense bundle of the fibrous capsule with active fibroblast and there was no inflammatory cell infiltrate.
Discussion
Due to changes in chemical composition, setting and physical properties of new bioceramic root repair materials, assessment of body response to evaluate any biological and toxic effect need to be tested carefully. Different methods are available to assess the biocompatibility and the cytotoxicity of the dental materials used in endodontics including cell lines tissue reactions in animals and clinical trials in humans [2,14,18-20]. The in-vivo subcutaneous implantation method is the most reliable and simple method that can be performed in animal model studies. The responses of subcutaneous connective tissues can be studied via histologic examinations based on selected criteria that involve inflammatory and histoimmunologic reactions, gene expression, and cytokine production. Furthermore, such a method allows for evaluating the thickness of the fibrous capsule, which is a useful marker of inflammation and assisting the biocompatibility of various materials [21].
To obtain a model resembles the root canal system and simulate clinical condition, standardization of material-tissue interface and stabilizes the material in place, a silicone tube was selected for this study which is well tolerated by tissues and easy to process histologically than polytetrafluoroethylene or polystyrene ones [22,23].
No previous histological studies had examined a combination of the three bioceramic root repair materials (MM‑MTA, BA, and ERRM putty) to determine their degrees biocompatibility or bioactivity in the animal model. Our study investigated the severity of chronic inflammation, the density of mast cells infiltration which plays a potent role in the early and late immune and inflammatory responses [24,25] and collagenous capsule.
A consequential relationship exists between the formation of the fibrous capsule and material tolerability, since it reflects an immune response which renders foreign bodies that have been recognized as inoffensive to the biological system [26].
Thus, we compare the duration of irritability and bioactivity of these tested materials when they come into contact with living tissues after (1, 4 and 8 weeks) based on based on the recommendations of the ISO to determine both short and longterm inflammatory reaction [16].
In the present study, the deposition of collagen fibers that become later a well-organized fibrous capsule in the control group and all the three tested materials increased over time agrees with Khalil et al. [27], Pinheiro et al. [28] Taha et al. [29] findings. They stated that the deposition of a fibrous capsule around the implanted material is an indication of tissue tolerance. However, it is inconsistent with the observations of other previous studies in which the authors proposed that the biocompatibility of the material and its sign of inflammation inversely related to the amount of fibrous capsule that develops around the tested material [30,31].
The thickening in the fibrous capsule was noted in all specimens although in different extent. It is attributable to enhanced collagen synthesis cytokines (Interleukin-1 [IL-1], IL-4) and growth factors (Platelet-Derived Growth Factor PDGF and Tumor Growth Factor TGF-ß) secreted by leucocytes, similar to the negative control tube indicating the tolerability of the tested materials by the tissue [32].
At the end of the experiment considering the long-term tissue response and wound healing process to significant reduction in the inflammation intensity moving towards the formation of granulation tissue and fibrous encapsulation of the implanted material observed in all tested materials suggesting a good interaction between the material and the cells from the surrounding tissue, which is a sign of good biocompatibility of MM-MTA, BD and ESRRM putty.
In the study, the empty silicone tubes used in the control group generated mild inflammatory reaction with few new capillaries formation capillaries in the subcutaneous tissue and they allowed for the formation of connective tissue at first week with no inflammatory response and thicker fibrous capsule at 4th and 8th weeks, similar to the result previously reported [33,34]. The presence of an early inflammatory response associated to the surgical trauma after tube implantation that leads to tissue disintegration and consequent infiltration of inflammatory cells.
Studies that evaluate tissue responses to new MTA-based materials are essential; since it has been shown that the biocompatibility and bioactivity of MTA can be affected by the addition of various components to the material [35,36]. The findings of the present study showed that MM-MTA elicited significantly more irritation than BD and ERRM putty at 7 days and 4 weeks, however, the severity of inflammation decrease over the time with more organization of fibrous capsule around the silicone tubes at the end of 8 weeks. These results are consistent with findings of Simesk et al. [37] who investigated the biocompatibility of MM-MTA in rat subcutaneous tissue and concluded that BD was more biocompatible than MM-MTA and Bioaggregate in the 1st week of the experiment with no significant difference between the materials at the end of the 45th day while Chang et al. [38] reported in their study that both MM-MTA and Bioaggregate exhibit similar biocompatibilities, inflammatory response, and odontoblastic differentiation, compared with ProRoot MTA in human dental pulp cells. Furthermore, Köseoğlu et al. [6] observed in their in-vitro study in which they evaluated the cytotoxicity and cell attachment of MM-MTA, BD, Endocem and MTA Angelus that MM-MTA and BD had similar biocompatibility and cytotoxicity.
High alkalinity, calcium ion release, heat release upon setting, and stimulation of inflammatory cytokines (interleukin 1 and interleukin 6) are those factors attributed to the initial powerful tissue response to the implanted MM-MTA [39-41].
Furthermore, the chemical composition of MM-MTA, which are based on Portland cement, a large amount of aluminum and trace amounts of arsenic, beryllium, cadmium and chromium were detected. Kum et al. [42] observed from their study in tracing the metal contents of MM-MTA that arsenic concentration was about 1.76 ppm which may be responsible for early severe inflammatory response in rat subcutaneous implantation with MM-MTA.
Comparing BD group with the other groups in this study, a moderate inflammatory process was observed at 7 days; however, the inflammation was found in initial periods was significantly reduced over the time. These results agree with previous studies done by Mori et al. [43] Simesk et al. [37] to assess the biocompatibility of BD after implantation in rat subcutaneous tissue. They observed a moderate inflammatory reaction that induced initially was reduced to a mild or nonsignificant level with increasing time. Thus inflammatory response in the early periods for BD may be due to the presence of calcium chloride and a hydrosoluble polymer in the liquids of BD to reduce setting time and water reducing agent which was able to reduce the viability and cell binding and produce an inflammatory reaction in dental pulps [44].
On the other hand, the differences in inflammatory reaction between BD and MM-MTA explained by the presence of radiopacifier. For MM-MTA, the radiopacifier is bismuth oxide, whereas for BD, it is zirconium oxide which had no cytotoxic effect on differentiated human cells and mouse fibroblast [45-47].
Moreover, the biocompatibility and bioactivity of calcium silicate-based cements are highly depended on the amount of calcium release. Accordingly, materials with more calcium ion release exhibit better biological properties [48]. Okiji et al. [49] compared BD and white ProRoot MTA in terms of Ca ion and Si ion uptake by adjacent root canal dentine and they observed that both materials formed tag-like structures, but dentine element uptake and Ca ion release was more prominent for BD than MTA.
In the present study, MM-MTA and BD was considered biocompatible because the injurious effect observed in the initial period was significantly reduced over time and this is supported by Silveira et al. [50] Love et al. [51] who stated that the biocompatibility of the materials depends on the reduction of inflammatory response in a reasonable amount of time to non-significant levels.
Concerning the comparison of the ESRRMs, MM‑MTA and BD with the control groups, ESRRMs showed a statistically significant reduction in inflammatory infiltration by the 7th day with no inflammatory response at the end of experiments.
Furthermore, the results of the present study agree with the finding reported by Khalil et al. [27] where grey MTA produced more extensive inflammatory reaction than ERRM and more detached particles after 7 and 30 days. However, it contradicts the results registered by Taha et al. [29]. They concluded that the MTA produced significantly less irritation than ESRRM at 1 and 3 weeks while confirmed more biocompatibility of ESRRM than after 6 weeks.
The difference in results may be attributed to different consistencies of putty and paste ESRRM. The putty used in this study had less setting time from the paste which did not actually set at 4 hours. Ma et al. [14] reported slightly higher cytotoxicity for the paste form of ESRRM compared to the putty form.
The event of coagulative necrosis observed in ESRRM putty group at 1 week revealed the ability of this material to induce calcification and mineralization [52].
The differences in the inflammatory reaction were observed in the present study in that the detached particles of MM-MTA were surrounded by severing inflammatory infiltrate, whereas the detached BD and ESRRM putty particles were surrounded by less infiltrate and encapsulated by a dense fibrous capsule. The differences in tissue response to MM-MTA, BD and ESRRM putty may be attributed to the difference in composition, consistency and setting reaction. MM-MTA contain heavy metals and toxic elements that were released to the surrounding tissue at the opening ends of the implanted silicone tubes [42], which would explain the increase in the mast cell number after 4 and 8 weeks in MM-MTA group that led to the persistence of inflammation.
Unfortunately, there is no in-vivo study comparing the biocompatibility and cytotoxicity between BD and ESRRM putty. This research considers the first study evaluates the tissue response to MM-MTA, BD and ESRRM putty together in the subcutaneous tissue of the rat model.
Conclusion
Under the limitation of this study, one can conclude that:
• The ESRRM putty was more biocompatible and produced less tissue irritation than MM-MTA and Biodentine during all time of experiments.
• MM-MTA and Biodentine showed an initial inflammatory response that diminished after 8 weeks.
• ESRRM putty, BD, and MM MTA can be biocompatible calcium silicate materials.
Conflict of Interest
Nil.
References
- Damas BA, Wheater MA, Bringas JS, Hoen MM. Cytotoxicity comparison of mineral trioxide aggregates and endosequence bioceramic root repair materials. J Endod 2011; 37: 372–375.
- Zhou HM, Shen Y, Wang ZJ, Li L, Zheng YF, Häkkinen L, Haapasalo M. In-vitro cytotoxicity evaluation of a novel root repair material. J Endod 2013; 39: 478-83.
- Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to Mineral Trioxide Aggregate. J Endod 1998; 24: 543-547.
- Mitchell PJ, Pitt Ford TR, Torabinejad M, McDonald F. Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials 1999; 20: 167-173.
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review—part III: clinical applications, drawbacks, and mechanism of action. J Endod 2010; 36: 400-413.
- Köseoğlu S, Pekbağr Yan K T, Kucukyilmaz E, Sağlam M, Enhos S, Akgün A. Biological response of commercially available different tricalcium silicate-based cements and pozzolan cement. Microsc Res Tech 2017; 80: 994-999.
- http://www.septodontusa.com/products/Biodentine
- Rajasekharan S, Martens LC, Cauwels RG, Verbeeck RM. Biodentine™ material characteristics and clinical applications: a review of the literature. Eur Arch Paediatr Dent 2014; 15: 147-158.
- Laurent P, Camps J, About I. Biodentine (TM) induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int Endod J 2012; 45: 439-448.
- Perard M, Le Clerc J, Meary F, Perez F, Tricot-Doleux S, Pellen-Mussi P. Spheroid model study comparing the biocompatibility of Biodentine and MTA. J Mater Sci Mater Med 2013; 24: 1527-1534.
- Silva EJ, Senna PM, De-Deus G, Zaia AA. Cytocompatibility of Biodentine using a three-dimensional cell culture model. Int Endod J 2016; 49: 574-580.
- Charland T, Hartwell GR, Hirschberg C, Patel R. An evaluation of setting time of mineral trioxide aggregate and EndoSequence root repair material in the presence of human blood and minimal essential media. J Endod 2013; 39: 1071-1072.
- Alanezi AZ, Jiang J, Safavi KE, Spangberg LS, Zhu Q. Cytotoxicity evaluation of endosequence root repair material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109: 122-125.
- Ma J, Shen Y, Stojicic S, Haapasalo M. Biocompatibility of two novel root repair materials. J Endod 2011; 37: 793–798.
- Martínez-Cortés M, Tinajero-Morales C, Rosales C, Uribe-Quero E. Cytotoxicity assessment of three endodontic sealing cements used in periapical surgery. In-vitro study. Rev Odont Mex 2017; 21: 40-48.
- Biological evaluation of medical devices. Part 6: Tests for local effects after implantation. Geneva, Switzerland: International Organization for Standardization 2016.
- Yaltirik M, Ozbas H, Bilgic B, Issever H. Reactions of connective tissue to mineral trioxide aggregate and amalgam. J Endod 2004; 30: 95–99.
- Parirokh M, Mirsoltani B, Raoof M, Tabrizchi H, Haghdoost AA. Comparative study of subcutaneous tissue responses to a novel root-end filling material and white and grey mineral trioxide aggregate. Int Endod J 2011; 44: 283–289.
- McNamara RP, Henry MA, Schindler WG, Hargreaves KM. Biocompatibility of accelerated mineral trioxide aggregate in a rat model. J Endod 2010; 36: 1851–1855.
- Song M, Kang M, Kim HC, Kim E. A randomized controlled study of the use of Pro-Root mineral trioxide aggregate and Endocem as direct pulp capping materials. J Endod 2015; 41: 11–15.
- Cintra LTA, Benetti F, de Azevedo Queiroz ÍO, Ferreira LL, Massunari L, Bueno CRE, de Oliveira SHP, Gomes-Filho JE. Evaluation of the Cytotoxicity and Biocompatibility of New Resin Epoxy-based Endodontic Sealer Containing Calcium Hydroxide. J Endod 2017; 43: 2088-2092.
- Lalis RM, Esaın ML, Kokubu GA, Willis J, Chaves C, Grana DR. Rat subcutaneous tissue response to modified Portland cement, a new mineral trioxide aggregate. Braz Dent J 2009; 20: 112–117.
- Zmener O. Tissue response to a new methacrylate-based root canal sealer: preliminary observations in the subcutaneous connective tissue of rats. J Endod 2004; 30: 348-351.
- Mussel RL, De Sa Silva E, Costa AM, Mandarim-De-Lacerda CA. Mast cells in tissue response to dentistry materials: an adhesive resin, a calcium hydroxide and a glass ionomer cement. J Cell Mol Med 2003; 7: 171–178.
- Kruger-Krasagakes S, Moller A, Kolde G, Lippert U, Weber M, Henz BM. Production of interleukin-6 by human mast cells and basophilic cells. J Invest Dermatol 1996; 106: 75–79.
- Catanzaro Guimaraes SA, Percinoto C. Effect of some endodontic materials on the influx of macrophages and multinucleated giant cell development in experimental granulomas. J Endod 1984; 10: 101–104.
- Khalil WA, Abunasef SK. Can Mineral Trioxide Aggregate and Nanoparticulate EndoSequence Root Repair Material Produce Injurious Effects to Rat Subcutaneous Tissues?. J Endod 2015; 41: 1151-1156.
- Pinheiro LS, Iglesias JE, Boijink D, Mestieri LB, Poli Kopper PM, Figueiredo JAP, Grecca FS. Cell viability and tissue reaction of NeoMTA Plus: An in-vitro and in-vivo study. J Endod 2018; 44: 1140-1145.
- Taha NA, Safadi RA, Alwedaie MS. Biocompatibility evaluation of EndoSequence Root Repair Paste in the connective tissue of rats. J Endod 2016; 42: 1523-1528.
- Bueno CRE, Vasques AMV, Cury MTS, Sivieri-Araújo G, Jacinto RC, Gomes-Filho JE, Cintra LTA, Dezan-Júnior E. Biocompatibility and biomineralization assessment of mineral trioxide aggregate flow. Clin Oral Investig 2018; 23: 1-9.
- Shahi S, Rahimi S, Lotfi M, Yavari H, Gaderian A. A comparative study of the biocompatibility of three root-end filling materials in rat connective tissue. J Endod 2006; 32: 776–780.
- Ramzi S. Cotran, Vinay Kumar, Tucker Collins, editors. Robbins Pathologic Basis of Disease. 6th ed. Singapore: Harcourt Asia Pte Ltd.; 2000; 50-112.
- Gomes-Filho JE, Bernabé PFE, Nery MJ, Otoboni-Filho JA, Dezan-Júnior E, de Moraes Costa MM, de Faria MD, Watanabe S, Gomes AC. Reaction of rat connective tissue to a new calcium hydroxide-based sealer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106: 71-76.
- Vita W, Reis MG, Santos TA, Carvalho AM, Mota C, Ramos EA. Comparison of the biocompatibility of grey mineral trioxide aggregate and sealapex plus zinc oxide in rat subcutaneous tissue. Clin Lab Res 2015; 21: 136-144.
- Collado-González M, Tomás-Catalá CJ, Oñate-Sánchez RE, Moraleda JM, Rodríguez-Lozano FJ. Cytotoxicity of GuttaFlow Bioseal, GuttaFlow2, MTA Fillapex, and AH Plus on Human Periodontal Ligament Stem Cells. J Endod 2017; 43: 816-822.
- Zhou HM, Du TF, Shen Y, Wang ZJ, Zheng YF, Haapasalo M. In-vitro cytotoxicity of calcium silicate-containing endodontic sealers. J Endod 2015; 41: 56–61.
- Simsek N, Alan H, Ahmetoglu F, Taslidere E, Bulut ET, Keles A. Assessment of the biocompatibility of mineral trioxide aggregate, bioaggregate, and biodentine in the subcutaneous tissue of rats. Niger J Clin Pract 2015; 18: 739-743.
- Chang SW, Lee SY, Kum KY, Kim EC. Effects of ProRoot MTA, Bioaggregate, and Micromega MTA on odontoblastic differentiation in human dental pulp cells. J Endod 2014; 40: 113-118.
- Opačić-Galić V, Petrović V, Jokanović V, Živković S. Histological Evaluation of Tissue Reactions to Newly Synthetized Calcium Silicate-and Hydroxyapatite-Based Bioactive Materials: in-vivo Study. Srp Arh Celok Lek 2017; 145: 370-377.
- Vosoughhosseini S, Lotfi M, Shabi S, Baloo H, Mesgariabbasi M, Saghiri MA, et al. Influence of White versus Gray Mineral Trioxide Aggregate on Inflammatory Cells. J Endod 2008; 34: 715-717.
- Camilleri J. A review of the methods used to study biocompatibility of Portland cement-derived materials used in dentistry. Malta Med J 2006; 18: 9-14.
- Kum KY, Kim EC, Yoo YJ, Zhu Q, Safavi K, Bae KS, Chang SW. Trace metal contents of three tricalcium silicate materials: MTA Angelus, Micro Mega MTA and Bioaggregate. Int Endod J 2014; 47: 704-710.
- Mori GG, Teixeira LM, de Oliveira DL, Jacomini LM, da Silva SR. Biocompatibility evaluation of biodentine in subcutaneous tissue of rats. J Endod 2014; 40: 1485-1488.
- Kang J, Lee B, Son H, et al. Biocompatibility of mineral trioxide aggregate mixed with hydration accelerators. J Endod 2013; 39: 497–500.
- Hill EE. Dental cements for definitive luting: a review and practical clinical considerations. Dent Clin North Am 2007; 51: 643–658.
- Setbon HM, Devaux J, Iserentant A, Leloup G, Leprince JG. Influence of composition on setting kinetics of new injectable and/or fast setting tricalcium silicate cements. Dent Mater 2014; 30: 1291-1303.
- Slompo C, Peres-Buzalaf C, Gasque KC, Damante CA, Ordinola-Zapata R, Duarte MA, de Oliveira RC. Experimental calcium silicate-based cement with and without zirconium oxide modulates fibroblasts viability. Braz Dent J 2015; 26: 587–591.
- Okiji T, Yoshiba K. Reparative dentinogenesis induced by mineral trioxide aggregate: A review from the biological and physicochemical points of view. Inter J Dent 2009; 1-12.
- Han L, Okiji T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int Endod J 2011; 44: 1081-1087.
- Silveira CM, Pinto SC, Zedebski Rde A, et al. Biocompatibility of four root canal sealers: a histopathological evaluation in rat subcutaneous connective tissue. Braz Dent J 2011; 22: 21-27.
- Hauman CH, Love RM. Biocompatibility of dental materials used in contemporary endodontic therapy: a review. Part 1. Intracanal drugs and substances. Int Endod J 2003; 36: 75–85.
- Moretton TR, Brown CE, Legan JJ, et al. Tissue reactions after subcutaneous and intraosseous implantation of mineral trioxide aggregate and ethoxybenzoic acid cement. J Biomed Mater Res 2000; 52: 528–533.