- Biomedical Research (2009) Volume 20, Issue 3
Biochemical Changes in Alcoholic Hepatitis with Phyllanthus Amarus Therapy: A Study
Padmaja Nikam*, Shashikant Nikam, Ajit Sontakke1, Chitra Khanwelkar21Department of Biochemistry, Belgaum Institute of Medical Sciences, Belgaum, Karnataka, India,
2Departments of Pharmacology, KIMS, Karad, India
- *Corresponding Author:
- Padmaja Nikam
Department of Biochemistry
Belgaum Institute of Medical Sciences,
District Hospital Campus
Belgaum-590 001
Karnataka, India.
E-mail: nikam31@gmail.com
Accepted date: May 17 2009
Abstract
Alcoholic hepatitis is a leading cause of morbidity and mortality. This study focuses on Phyllanthus Amarus therapy and its effect on liver in alcoholic hepatitis. The therapy tries to protect the liver by investigating liver profile enzymes, antioxidant enzymes, antioxidant vitamins and lipid peroxidation. The study consists of 50 well diagnosed alcoholic hepatitis males aged between 33 to 55 years. The control group includes 50 age-matched normal healthy persons. Oxidative stress was assessed by estimating lipid peroxidation [LPO]. Parameters like serum bilirubin, total proteins and activity of liver profile enzymes were chosen. The activity of enzymatic antioxidants, superoxide dismutase [SOD], glutathione peroxidase [GPx], catalase, and levels of non- enzymatic antioxidant vitamin E and vitamin C were measured in plasma or erythrocytes. Methods used in the study are mainly enzyme kinetics by auto-analyzer and turbidimetry. Plasma LPO levels were significantly high but activity of SOD, GPx, catalase, and levels of vitamin E and vitamin C were significantly lowered in alcoholic hepatitis when compared with controls. After phyllanthus amarus therapy, for four and eight weeks, plasma LPO levels significantly decreased and activity of SOD, GPx, catalase and levels of vitamin E and C significantly increased in alcoholic hepatitis. This study concludes that the imbalance between oxidative stress and anti-oxidants may play an important role in alcoholic hepatitis. Elevated free radicals may cause hepatic cell loss and play a role in pathogenesis of alcoholic hepatitis. This study strongly suggests that the therapy of phyllanthus amarus increases activity antioxidants and reduces lipid peroxidation; protects liver from damage due to free radicals in alcoholic hepatitis.
Keywords
Alcoholic hepatitis, antioxidants, phyllanthus amarus, oxidative stress
Introduction
Hepatitis is a global public health problem, which is responsible for major chunk of morbidity and mortality [1]. Alcoholic hepatitis is a leading cause of morbidity and mortality throughout the world [2]. It is a major health care problem, accounting for 40% of deaths from cirrhosis and more than 30% cases of hepatocellular carcinoma [3]. Consistent heavy drinking or binge drinking is a primary risk factor for alcoholic hepatitis.
There is no specific treatment for alcoholic hepatitis. Complete abstinence from alcohol is the single most important treatment for alcoholic hepatitis. Patients with severe alcoholic hepatitis may benefit from treatment with corticosteroids, pentoxifylline, methionine, vitamin C, vitamin E and vitamin B- complex.
Alcohol-induced liver injury is linked to an oxidative stress resulting from raised free radical generation and lowered antioxidant defense and play a vital role in pathogenesis of alcoholic hepatitis [4]. The plant Phyllanthus amarus is bitter, astringent, stomachine, diuretic, febrifuge and antiseptic. Every part of the plant is used in dropsy, gonorrhoea, menorrhagia and other genital infections [5,6]. It is found effective in treatment of hepatitis without adverse effects. It has most promising application in hepatitis-B [7]. Considering these facts we planned to study the effect of Phyllanthus amarus therapy in protection of liver in alcoholic hepatitis with the help of investigating liver profile enzymes [serum glutamate oxaloacetate transminase (SGOT), serum pyruvate oxaloacetate transaminase (SGPT), alkaline phosphatase (ALP), γ glutamyl transferrase (GGT), 5’ nucleotide phosphatase (5’ NTP) and lactate dehydrogenase (LDH) ], antioxidant enzymes (SOD, Gpx, catalase), antioxidant vitamins (vitamin-E and vitamin-C) and lipid peroxidation.
Material and Methods
The research design included three study groups:
Group I: 50 healthy control male subjects.
Group II: 25 patients suffering from alcoholic hepatitis for past six months.
Group III: 25 patients suffering from alcoholic hepatitis for over six months.
All the 50 male patients of well diagnosed alcoholic hepatitis, aged between 33 to 55 years, were selected from the Out Patient Department (OPD) of Corporation hospital Sangli, Govt. Medical College hospital Miraj and Civil hospital Sangli. All the patients in this study group were diagnosed with the help of complete medical history, personal history including drinking habits, physical examination, specific laboratory tests, liver function tests, cellular blood counts, bleeding time, electrolyte tests and ultrasonographic examination.
There were symptoms and signs like abdominal tenderness, spider like blood vessels in the skin, ascites, poor appetite, jaundice, low grade fever, fatigue, portal hypertension, mental confusion etc. All these patients were kept totally free from alcohol in the time of therapy. Age-matched 50 normal healthy males as per International Federation of Clinical Chemistry (IFCC) were included as control group in this study. Patients and controls gave consent to participate in this study when informed of the details, purpose of study. For this study, the institution’s ethical committee approval was taken. Patients with associated renal diseases, non-alcoholic liver diseases, lung diseases, thyroid diseases, gastro-intestinal diseases, tobacco chewers and smokers that could alter the required parameters were excluded from the study.
Sample Collection
About 10 ml of fasting venous blood samples were collected under sterile condition from alcoholic hepatitis patients before starting any therapy and from normal healthy subjects. After four weeks and eight weeks therapy 10 ml of fasting venous blood samples were collected from alcoholic hepatitis patients under sterile condition. Five ml blood was taken in sterile dry and acid washed ethylenediaminotetra acetic acid (EDTA) bulbs and five ml blood in plain bulbs. Plasma was separated by centrifuging the blood at 3000rpm for 20 minutes at 4°c [8,9]. This plasma was used for estimation of malondialdehyde (MDA) and vitamin E. The packed cells were used for the analysis of vitamin C, SOD, catalase and GPx.
The sera separated were used for the investigation of parameters like total bilirubin, total proteins and activity of enzymes SGPT [10], SGOT [11], ALP [12], GGT [13], 5’ NTP [14] and LDH [14]. All these parameters were estimated in healthy subjects and all cases before and after the therapy with phyllanthus amarus. At the end of four, eight weeks, changes in these parameters from base line values were taken. MDA was determined as the measure of thiobarbituric acid reactive substances (TBARS) [15].
Erythrocytes ascorbic acid levels were estimated by the method of Tietz [16]. Plasma separated was used for estimation of vitamin E by the method of Baker H et al. [17] SOD was determined in the hemolysate by the method of Mishra and Fridovich based on the inhibition of autoxidation of epinephrine to adenochrome at pH 10.2 [18]. Catalase activity was measured by the method of Beer and Seazer [19] and GPx activity by paglia and valentine in erythrocytes [20].
Statistical Analysis
All values are presented as mean plus/minus SD. Statistical significance was analyzed by Student’s ‘t’ test.
Results
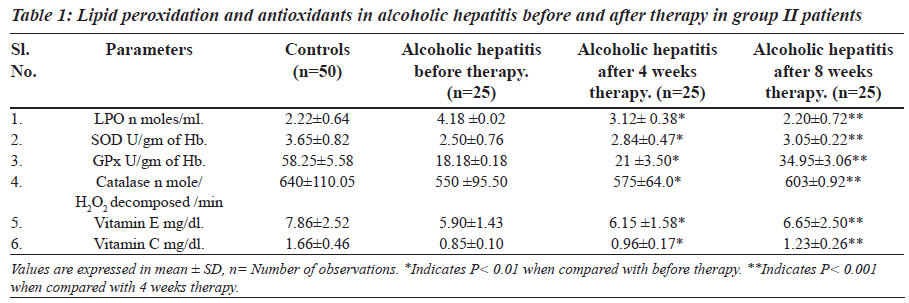
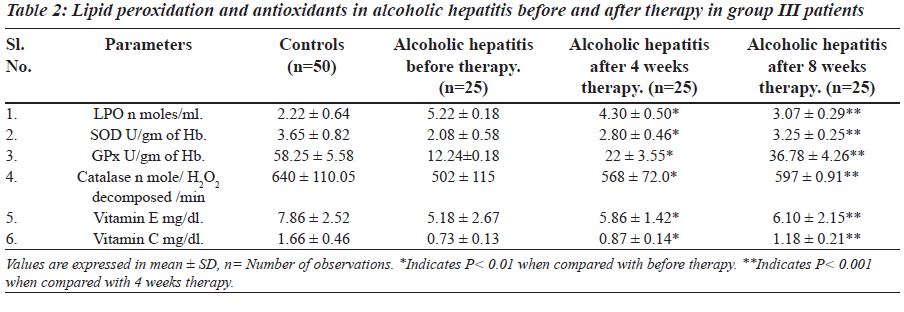
The levels of lipid peroxidation, vitamin E, vitamin C and activity of SOD, catalase and GPx are presented in Tables 1 and 2. The levels of lipid peroxidation were significantly higher in group II and group III in comparison with controls.
The levels of vitamin E, vitamin C and activity of antioxidant enzymes SOD, GPx and catalase were significantly lower in group II and group III patients.
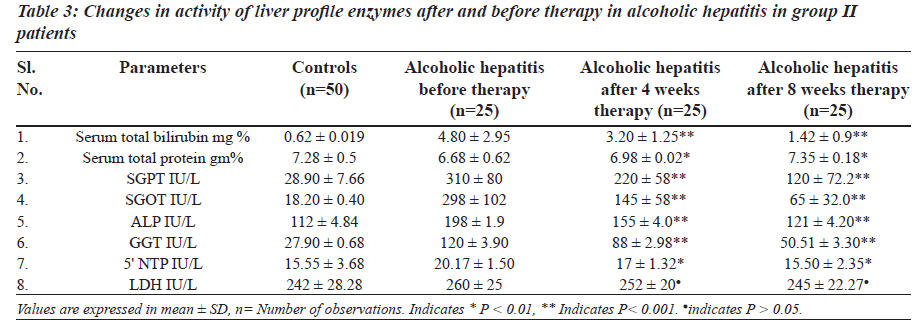
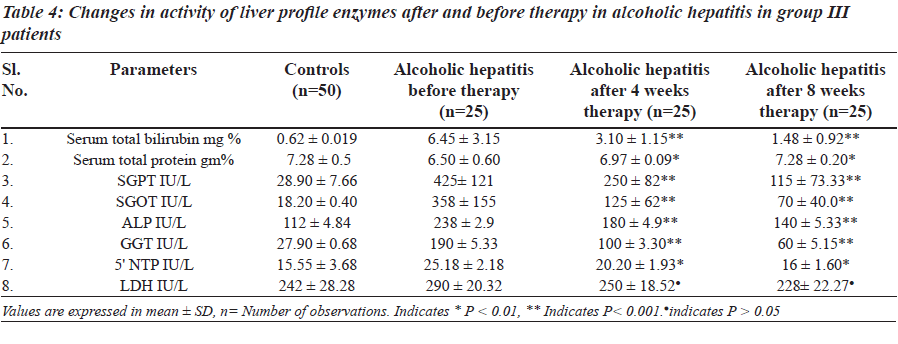
The levels of total brilirubin (Tables 3 and 4) and the activity of liver profile enzymes SGPT, SGOT, ALP, GGT and 5’ NTP were significantly increased in group II and group III in comparison with control group. However LDH did not show any statisticaly significant changes in both group II and group III.
Discussion
MDA estimation is one of the most commonly used methods to monitor lipid peroxidation in biochemical samples. Lipid peroxidation is used as an indicator of oxidative stress in cell and tissues. Thiobarbituric acid reactive substances, the indicator of lipid peroxidation were significantly elevated in alcoholic hepatitis. There is association between increased levels of MDA and progression of alcoholic hepatitis. Alcohol may be reducing the activity of acetaldehyde dehydrogenase thus there is accumulation of acetaldehyde [21,22]. This may result in the formation of free radicals; such radicals may be attacking unsaturated fatty acids in membrane and organelles to produce lipid peroxides. This may cause decrease in membrane permeability. Thus, change in membrane fluidity causes cellular damage and necrosis.
The a c t ivi ty of l ive r prof i l e enzyme s we r e significantly increased in group II and group III. These enzymes are localized in the cell cytoplasm and cell mitochondria and found in bile as well. Elevated serum enzymes like SGPT, SGOT, ALP, 5’ NTP, GGT and LDH are indicative to cellular damage and loss of functional integrity of cell membrane in liver. Damage of liver cells causes leakage of cellular enzymes into serum [23]. In group II and group III after four- and eight-week therapy of Phyllanthus amarus there was significantly decreased activity of liver profile enzymes including the reduction in liver cell damage.
The increased concentration of bilirubin and significant rise activity of liver profile enzymes could be taken as an index of liver damage. After four-week therapy of Phyllanthus amarus there was significant decrease in LPO and it resumes to normal after eight weeks in group II and group III. Effect of alcoholic hepatitis showed increased oxidative stress by decreasing non enzymatic antioxidants vitamin E and vitamin C and increasing LPO. An alcoholic hepatitis increased oxidative stress may increase consumption of vitamin E and C. Vitamin E traps free radicals and interrupts the chain reaction that damage the cell [25]. Thus in group II and group III there is increased utilization of vitamin E and vitamin C due to oxidative stress.
SOD is an important antioxidant enzyme having scavenging effect against superoxide anion and catalase is responsible for detoxification of H2O2 produced by action of superoxide dismutase and inhibits formation of superoxide radicals [24]. Due to increased oxidative stress there may be increased utilization of enzymes in group II and group III to balance the decreased activity of antioxidant enzymes by oxidation through reactive free radicals. Thus, the activity of SOD, GPx, and catalase may decrease in group II and group III of alcoholic hepatitis. Decreased antioxidant enzymes and vitamin C and vitamin E might be causing oxiradical mediated injury and thus may contribute to liver damage. After eight weeks of therapy with Phyllanthus amarus in group II and in group III decreased levels of vitamin E and vitamin C has been comes to near normal. This indicates that the utilization of vitamin E and C is decreased and this may be responsible for raised levels of Vitamin E and vitamin C. Increased activity of antioxidant enzymes and vitamins in group II and group III of alcoholic hepatitis indicate that there might be regeneration of liver cells after therapy, which helps in curing hepatitis.
Phyllanthus amarus can detoxify the alcohol by scavenging free radicals formed from acetaldehyde and has an antioxidant activity [26]. It has been shown to increase protein biosynthesis [27] and increase the rate of regeneration of necrosed cells [28].
This study concludes that oxidative stress and antioxidants may be playing an important role in alcoholic hepatitis. Elevated free radicals may cause hepatic cell damage and play a role in pathogenesis of alcoholic hepatitis. The Phyllanthus amarus therapy is found to be equally effective in group II and group III patients of alcoholic hepatitis. The present study strongly suggests that the therapy with Phyllanthus amarus increases various antioxidants and reduces lipid peroxidation of hepatic cellular and intracellular membranes. Hence it ultimately protects liver damage due to free radicals in alcoholic hepatitis.
Acknowledgment
The authors thank Dr. Kalpana R. Sulhyan Dean Government Medical College Miraj, and Dr. M. R. Chandrshekar Director, Belgaum Institute of Medical Sciences, Belgaum for their help and support. We are also thankful to Medical Social Worker (MSW) of Corporation hospital Sangli,Civil hospital Sangli and Government Medical College hospital Miraj for constant counseling of alcoholic hepatitis patients to make this study possible.
References
- Reid A. Non alcoholic steatohepatitis. Gastroenterology 2001;121:710-4.
- Schiff N, Schiff ER. Diseases of the liver. 6th ed. J.B. Lippincott Company; 1987. p. 669-70.
- Libber CS. Alcoholic liver injury. Pathogenesis and therapy in 2001, Pathos Biol (Paris) 2001;49:738-42.
- Checha F, Kaplowitz N. Oxidative stress and alcoholic liver disease. Alcohol Health and Res World 1997;21:321-4.
- Paranjape P. Indian Medical plants. Forgotten healers. Delhi: Chaukhamba Sanskrit Prathisthan; 2001. p. 48-9.
- Bharatiya VB. Selected Medicinal plants of India. Bombay: Tata Press; 1992. p. 235-7.
- Thagarajan SP, Subramanian S. Thirunalasundar: Effect of Phyllanthus amarus on chronic carriers of hepatitis B- virus. Lancet 1998;2:764-6.
- Digde JF, Mitchell G, Hanahan DJ. The preparation and characterization of hemoglobin free ghosts for human red blood cells. Arch Biochem Biophysics 1968;110:119-30.
- Quist EH. Regulation of erythrocyte membrane shape by calcium. Biochem Biophys Res Common 1980;92:631-7.
- Moss DW, Henderson AK. Clinical enzymology. In: Burtis CA, Ashwood ER, editors. Tietz text book of clinical Chemistry. 3rd ed. Philadelphia: W.B. Saunders; 1994. p. 617-721.
- Henry JB. Clinical diagnosis and management by laboratory methods. Philadelphia: W.B. Sounders and company; 1979. p. 365-70.
- Tietz NW. Fundamentals of Clinical Chemistry. 2nd ed. Philadelphia: W.B. Saunders and company; 1976. p. 622-8.
- Wenger WC, Lott JA. Enzymes in Clinical Chemistry, Theory, analysis and correlation. In: Kaplan LA, Pesce AJ, editors. C. V. Mosby; Toronto: 1994. p. 1079-134.
- Kaplan LA, Pesce AJ, Kazmierczak SC. Clinical Chemistry, Theory, Analysis, Correlation. 4th ed. 2003. p. 503-9.
- Buege JA, Aust AD. Microsomal lipid peroxidation. In: Methods, Enzymol Estbrook RW, Pullman ME, editors. New York: Academic Press; 1987. p. 302-10.
- Tietz NW. In: Tietz NW, editor. Textbook of Clinical Chemistry. Philadelphia: London, Toronto: W.B. Saunders Company; 1986. p. 960-2.
- Baker H, Frank D, Winley NC. In: Clinical vitaminology methods and Interpretation. New York: Interscience, Publisher; 1968. p. 169-76.
- Mishra HP, Fridovech I. The role of superoxide anion in the auto oxidation of epinephrine and a simple assay for superoxide dismutase. J of Biol Chem 1972;247:3170-5.
- Beer RF, Sizer TW. A spectroscopic method for measuring the breakdown of hydrogen peroxide by catalase. Journal of Biol Chem 1952;195:133-40.
- Paglia De, Valentine WW. Studies on the quantitative qualitative characterization of erythrocyte glutathione peroxidase. Journal of Lab Clin Med 1967;70:158-9.
- Barry RC, Mc Gain JD. Acetaldehyde alone may initiate hepatocellular damage in acute alcoholic liver diseases. Gut 1985;26:1065-70.
- Bockus HL. Alcoholic hepatitis. Gastroenterology. Vol. 3. 2nd ed. Philadelphia and London: W.B. Saunders Company; 1996. P. 579-85.
- Subir Kumar Das, Hiran KR, Mukherjee S, Vasudevan DM. Oxidative stress is the primary event: effect of ethanol consumption in brain. Ind J of Clin Biochem 2007;22:99-104.
- Wendel A. Glutathione peroxidise. In: Jokby WB, editor. Enzymatic basis of detoxification. New York: 1980. p. 333-48.
- Nikam S, Nikam P, Ahaley SK. Role of free radical and antioxidant imbalance in pathogenesis of Parkinson’s disease. Biomedical Res 2009;20:55-8.
- Tagrajan SP, Jayaram S, Villicammai T. Phyllanthus amarus and hepatitis B. Lancet 1990;336: 949-50.
- Tagrajan SP, Thirunalasunder SS. Effect of phyllanthus amarus on chronic carrier of hepatitis B-virus. Lancet 1998;2:764-6.
- Oudhia P, Tripthi RS. Prospects of cultivation of medical plants in Chittisgarh India. Recent progress in medical plants. Crop improvement production technology, trade and commerce. Vol. 5. Sci Thch Publ USA: 2002. p. 211-36.