- Biomedical Research (2014) Volume 25, Issue 2
Biochemical cellular damage indicators and in situ cell death in chronic alcohol consumption: Pseudomonas aeruginosa induced pneumonia rat model.
Melek Demir1*, Kazim Kartkaya2,A. Çevik Tufan3, Okan Can Arslan2, Güngör Kanbak21Pamukkale University, Faculty of Medicine, Department of Medical Microbiology, Denizli, Turkey
2Osmangazi University, Faculty of Medicine, Department of Biochemistry, Eskişehir, Turkey
3Pamukkale University, Faculty of Medicine, Department of Histology and Embryology, Denizli Turkey
- *Corresponding Author:
- Melek Demir
Pamukkale University
The School of Medicine
Department of Medical Microbiology
20070, Denizli, Turkey
Accepted January 11 2014
Abstract
In this study, our aim was to investigate whether cellular alterations occurred in liver and lung tissue in presence of chronic alcohol ingestion and Pseudomonas aeruginosa pneumonia related to oxidative stress and in situ cell death. Male wistar rats were divided into five groups: the sham group fed by normal solid diet, two control groups; one fed by normal liquid diet, and the other fed by liquid diet plus ethanol, two pneumonia groups induced by Pseudomonas aeruginosa; one fed by normal liquid diet, and the other fed by liquid diet plus ethanol. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nickend labelling analysis was performed to confirm in situ cell death. Serum alanine amino transferase and lactate dehydrogenase activities, and tissue and serum malondialdehyde levels, paraoxonase, arylesterase activities, and tissue caspase-3 activities were determined. Serum alanine amino transferase activities of both ethanol given groups were higher than the other groups (p<0.05). Liver malondialdehyde level was increased in ethanol with pneumonia group (p<0.05). Lung malondialdehyde levels were not different among groups. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labelling positive cell ratio in the liver was higher in both ethanol given groups and normal liquid diet with pneumonia group than sham and control group fed by normal liquid diet. Liver and lung caspase-3 activities were not different among groups. Although serum paraoxonase activities were lower in both pneumonia groups, it was not statistically significant. It may be interpreted as growing infection during chronic ethanol ingestion that causes increased liver damage through oxidative stress.
Keywords
Alcohol, P.aeruginosa, MDA, Paraoxonase, TUNEL
Introduction
Long-term and high dose alcohol consumption affects many organs and systems particularly liver, pancreas and gastrointestinal system [1]. Toxic effects of alcohol are associated with serum alcohol levels. Alcohol is metabolized in liver after ingestion. It has been shown cellular alteration related to chronic alcohol consumption in the various cells such as hepatocytes, lung, neuron and intestinal epithelial cells [2,3,4]. Chronic alcohol consumption cause deterioration of liver functions depending on accumulation of acetaldehyde which is alcohol metabolite in hepatocytes and generate cell injury with decreased levels of reduced glutathione [3,5]. Oxidative stress has an important role on the pathogenesis of alcoholic liver disease due to chronic alcohol consumption [2,6,7].
Paraoxonase (PON1) activities, malondialdehyde and other oxidative stress parameters are used as markers of liver injury besides classical liver enzymes such as alanin aminotransferase (ALT) [8,9,10].
Because of the effect on immune system, chronic alcohol consumption generate predisposition on infectious diseases in organism, especially lung infections [11-14]. Both gram positive and gram negative microbial agents cause pneumonia. Pneumonia incidence increases in people who drank alcohol [15]. Alcohol consumption history come into prominence in persons with community-acquired pneumonia related to especially Pseudomonas aeruginosa (P.aeruginosa) and Acinetobacter species [15,16]. Alcohol consumption independently increases risk and severity of acute respiratory distress syndrome (ARDS) [3,17]. Pneumonia caused P. aeruginosa was observed to increase in patients with chronic alcohol consumption, chronic obstructive lung infection, long term hospitalization, and patients who are connected to artificial respiratory apparatus [16,18,19].
This study was performed to investigate cellular alterations occurred in liver and lung tissues in P. aureuginosa induced pneumonia and chronic alcohol consumption and to reveal whether or not there is a relationship among these alterations and oxidative stress and in situ cell death.
Materials and Methods
This study was approved by Experiment Animals Ethical Council of Pamukkale University.
Study groups
Male wistar rats were divided into five study groups as: sham group fed with normal solid diet (standard rat chow) (SC, n=6), control groups; fed with normal liquid diet (NLC, n=8) or liquid diet with ethanol (ELC, n=8), and Pneumonia groups; fed with normal liquid diet (NLP, n=8) or liquid diet with ethanol (ELP, n=8). Diet was prepared based on isocaloric liquid diet that was defined before [20,21], alcohol groups were fed with a liquid diet formulated so that 36 percent of daily calorie intake will be supplemented by ethanol [20,21]. All groups were fed with these diets for 6 weeks. Then, pneumonia was induced in two groups, mentioned above. One rat from each ELC and ELP groups died immediately after the intratracheal application. Two rats from ELP group died at 20. hours after intratracheal application and one rat from NLP group died at 21. hours after intratracheal application and immediately after this, all surviving rats were anesthetized, sacrificed and blood, liver and lung tissue samples were taken for microbiological, biochemical and histological examinations.
Pneumonia model and evaluation of bacterial growth
P. aeruginosa (PAO1) strain was used for bacterial pneumonia. Bacterial suspension was given intratracheally [22,23]. A single dose of 0.5ml/kg bacterial suspension (2x109 CFU/ml) was instilled in the trachea as previously described [22,23]. The rats in the control groups were given sterile saline instead of bacterial suspension.
All tissue samples were weighted under sterile conditions and homogenized in phosphate buffered saline (PBS). Aliquots were taken from homogenates and after a series of dilutions they were seeded to blood and EMB agar mediums. After overnight incubation at 37 °C, P. aeruginosa growth in the cultures were observed, counted and identified by routine microbiological methods. Bacterial growth were calculated as log10 CFU/g tissue
Histopathological evaluation of tissues and analysis of In Situ Cell Death
Ten micrometer sections were taken from paraffin embedded tissues stained with haematoxylin-eosin (H&E) and the alterations in the examined tissues were evaluated using a light microscope.
In situ cell death development was investigated by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining method using “In situ cell death detection kit” (Roche Diagnostics–Germany). Slides containing TUNEL marked tissue sections were analyzed with a light microscope (Olympus). For liver tissue, apoptotic cells observed in total investigated area were evaluated and results were expressed as increase of apoptotic cell ratio compared to sham group. For lung tissue, alveoli with similar size were evaluated and apoptotic cells per alveoli were analyzed. Evaluations were performed on a blind basis by histologists.
Measurement of tissue caspase-3 activity
Caspase-3 activity in liver and lung tissues was measured with commercial micro plate kit according to the manufacturer’s instructions (Chemicon International Temecula Co. USA) .
Measurement of tissue and serum malondialdehyde (MDA) levels
Tissue and serum malondialdehyde levels were measured according to method of Ohkawa et al. [24] which is based on measurement of absorbance of the product of reaction the between MDA and thiobarbituric acid at acidic environment, at 532 nm.
Measurement of serum and tissue paraoxonase (PON) and arylesterase (AE) activities
Paraoxonase and arylesterase activities were measured by using paraoxon and phenylacetate as the substrates, respectively as previously described [25]. Serum Paraoxonase activity was taken at 412 nm. The activity was calculated by using the molar extinction coefficient of pnitrophenol (€412: 18290 M-1/cm-1) [25]. Results of serum paraoxonase activity were expressed as U/L. Arylesterase activity of PON1 enzyme was taken at 270 nm. Enzyme activity was calculated using the molar extinction coefficient of phenol (€270: 1310 M-1/cm-1]) [25]. Results of serum AE was expressed as kilo units per litter (kU/L). For tissue enzyme activity; tissues samples were homogenized in ten volumes of 50 mM Tris-HCl (pH 8.0) containing 2 mM CaCl2 and the homogenate centrifuged at 10.000 x g for 15 min [25].
Enzyme activities were measured in tissue supernatants. Tissue results were expressed as mU/mg protein. Tissue protein concentrations were determined using standard protocols for Lowry method [26].
Other biochemical parameters
Alanine aminotransferase (ALT), lactate dehydrogenase (LDH) and gamma glutamyl transferase (GGT) activities in the serum were measured on Roche-Hitachi modular analyzer using a commercial kit (Roche Diagnostics Modular-P).
Statistical analysis
Results were given as Mean ±SD. SPSS 10.05 program was used for statistical comparisons. One-way ANOVA followed by Bonferroni’s post-hoc comparisons tests and Kruskal Wallis followed by Mann-Whitney U tests were performed for comparisons of biochemical parameters of serum and tissues. Mann-Whitney U test was used for comparison of bacterial grown and Odds ratio was used for comparison of liver TUNEL positive cell ratio in groups. P<0.05 was considered to be statistically significant.
Results
Histological changes and apoptotic alterations in tissues
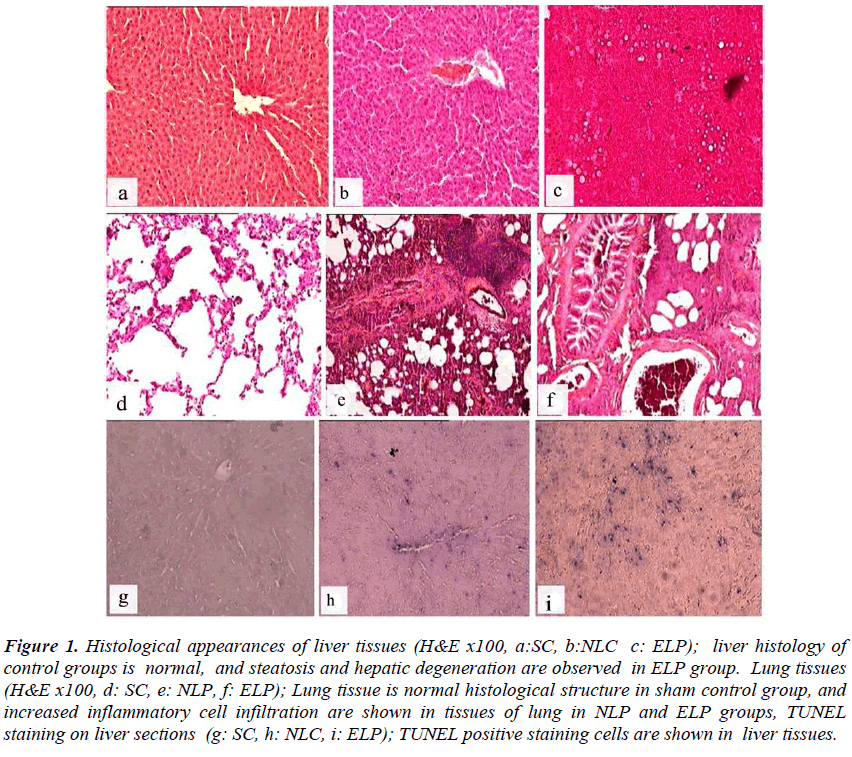
Liver tissue samples were histologically evaluated for cellular integrity, cellular degeneration, presence of necrosis and liver steatosis. Liver samples of sham group and rats receiving normal liquid diet were evaluated as normal in terms of histological tissue structure. In livers of both ELC and ELP rats, there were histopathological changes ranging from mild steatosis and minimal hepatic degeneration to intense steatosis and severe hepatic degeneration (Figure 1). Normal morphological structure was observed in lung tissue samples of sham and NLC groups while mononuclear cell infiltration was observed in tissues of lung samples in ELC group. In both pneumonia groups, increasing of neutrophil and mononuclear cell infiltration were observed in lung tissue
Figure 1: Histological appearances of liver tissues (H&E x100, a:SC, b:NLC c: ELP); liver histology of control groups is normal, and steatosis and hepatic degeneration are observed in ELP group. Lung tissues (H&E x100, d: SC, e: NLP, f: ELP); Lung tissue is normal histological structure in sham control group, and increased inflammatory cell infiltration are shown in tissues of lung in NLP and ELP groups, TUNEL staining on liver sections (g: SC, h: NLC, i: ELP); TUNEL positive staining cells are shown in liver tissues.
(Figure 1). Increased TUNEL positive cell were observed in liver tissue in experiment groups compared to sham group (Figure 1). TUNEL positive liver cell ratio was higher in both ethanol given (control and pneumonia) groups and normal liquid diet plus pneumonia group than sham and control group fed by normal liquid diet. Odds ratio (95% confidence intervals) for TUNEL positive liver cell was found to be 1.83 (1.47-2.27) in the NLC, 4.65 (3.86-5.59) in the ELC, 6.24 (5.17-7.54) in the NLP groups and 7,75 (6.43-9.35) in the ELP group. The ratio of TUNEL positive alveolar epithelial cells of study groups was similar with sham group.
Bacterial growth in pneumonia groups
Bacterial growth was found to be greater than 103 CFU/g tissues and infection signs were observed by histological evaluation in both pneumonia groups. The amount of grown bacteria in right lung tissue was found to be similar in the ELP and NLP groups. For left lung, however, significantly increased bacterial growth was observed in the ELP group compared to the NLP (5,41±0,57 log10 CFU/g tissue vs 4,43±0,39 log10 CFU/g tissue respectively; p<0.05 )
Results of biochemical parameters
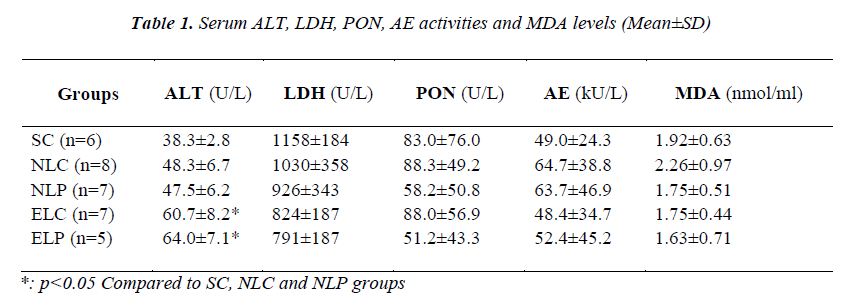
Serum ALT, LDH, PON and AE activities and MDA levels of study groups were given in Table 1. GGT activities were not evaluated due to their activities were under of limit of detection. In the ethanol treated study groups, serum ALT activities were significantly increased (p<0.05). No significant difference was found in LDH activities among study groups. Although serum paraoxonase activities were lower in the pneumonia groups, the differences were not statistically significant. Serum MDA levels were not different between the groups.
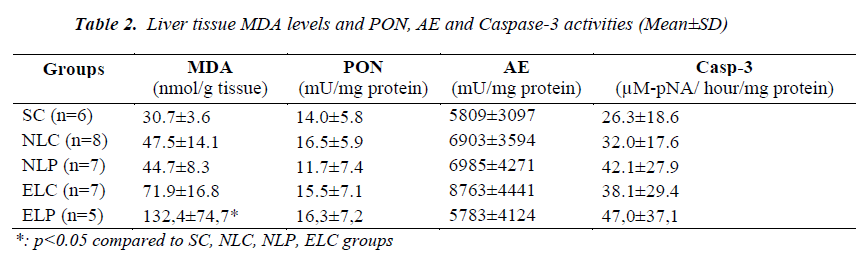
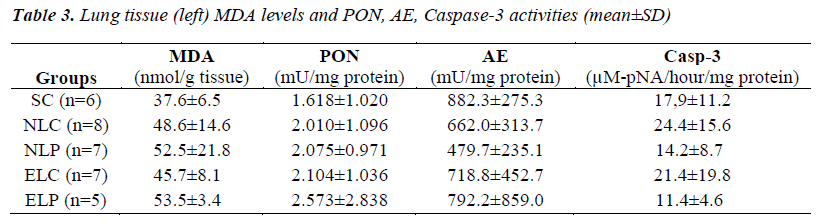
Liver tissue MDA levels, PON, AE and caspase-3 activities of study groups were given in Table 2. Liver MDA levels of ELP group were significantly increased (p<0.05) compared to other groups. ELC group had also increased MDA levels but this was not significant. No difference in liver PON, AE and Caspase-3 activities was found among study groups (p>0.05). No significant difference in both left and right lung tissues MDA levels, PONs, and Caspase-3 activities was found among study groups (p>0.05). Left lung tissue MDA level, PON, AE and Caspase-3 activities of the study groups were given in Table 3.
Discussion
Chronic alcohol consumption can cause several nutritional problems. Due to its multiple effects on metabolism, investigating the effects of alcohol on multiple systems has gained interest [5,27,28]. Chronic alcohol consumption is a major risk factor for mortality and morbidity due to its detrimental effects on multiple systems. Alcohol is mainly oxidised in liver, a number of potentially dangerous by-products are generated, such as acetaldehyde and highly reactive molecules called free radicals [2,5].
In this study, the effects of alcohol consumption on liver were investigated using histopathological and biochemical parameters. Almost normal liver histology and morphology was observed in the NLC and NLP groups while various degrees of hepatic degeneration and steatosis were observed in alcohol received the ELC and ELP groups.
Disruption of liver cellular integrity affects the serum activities of enzymes abundant in liver. ALT activities of alcohol groups were found to be significantly elevated compared to other groups in this study and this finding was consistent with histological findings. Clinical and experimental studies showed alcohol related increase in serum ALT activities in alcoholic liver damage [27,28,29]. We observed no significant differences in groups in terms of serum LDH activities. A study on cultured cells showed that low doses of ethanol reduced LDH release from human and rat hepatocytes; however, highest ethanol induced increase in cell necrosis [30]. Another study on cultured cells also revealed that ethanol has a dose dependent effect on liver cells. It has been shown that there was dose-dependent correlation between LDH leakage with the ethanol concentrations [31]. In our study, a mild but statistically non significant decrease in LDH activities of alcohol given groups was observed. This may be linked to the dual effect of alcohol mentioned above.
Free oxygen radicals play an important role in ethanol induced liver damage [5,7]. Microsomes and mitochondria and major cell organelles affected by alcohol consumption [32]. Several clinical and experimental studies investigated the relationship between alcohol and liver lipid peroxidation [27,28,29]. In our study no difference was observed in serum MDA levels among groups; however liver MDA levels were significantly elevated in the ethanol given pneumonia group. In the presence of an infection, activated Kupffer cells secrete several chemical mediators which cause tissue damage through oxygen radicals formed during the immune response against the infection agent. Oxidative stress mediated by CYP2E1 pathway is also known to be responsible for cellular damage in alcoholic liver disease [32]. In a study, it was reported that LPS or pyrazole did not significantly increase MDA levels, but the combination of LPS plus pyrazole caused a significant increase in MDA levels and serum ALT activities [33]. In another study, it was shown that enteral LPS administration potentiates alcoholic liver necrosis. Serum ALT activities were significantly increased in rats received alcohol plus LPS, compared to both controls and rats received LPS alone [34]. In our study no difference in liver MDA levels were also observed compared to sham and control groups in the alone presence of infection. However in the presence of alcohol plus infection, histological markers of liver damage and also serum ALT activities and liver MDA levels were observed to be increased. Increase in liver MDA levels of ELP group were greater than of ELC group. This may be due to that simultaneous presence of alcohol and infection may increase liver damage through mediation by free oxygen radicals. Two gram negative bacteria as P.aeruginosa and Acinetobacter species cause more lung infection in chronic alcohol consumers [15,35]. No significant difference in lung MDA levels was found among study groups in our study. A study performed by direct intratracheal application of LPS to lung showed MDA levels similar to control group [36].
PONs, an enzyme synthesized by liver, is associated with HDL and also known to have antioxidant properties [9,37]. In our study, serum PON activity was decreased in both ELP and NLP groups compared to sham, NLC and ELC groups. Although it was not statistically significant, we consider this decrease important. The PON activities, which were undiminished enough in the ethanol groups may be associated with duration of alcohol ingestion. We think that if duration of alcohol ingestion had continued a few more weeks, significant decrease in serum PON activities would be observed. No significant difference was found among groups in terms of liver PON activities.
P.aeruginosa secrets many virulence factors. In P.aeruginosa infections, quorum sensing is critical for biofilm formation and pathogenicity. Bacteria that use quorum sensing constitutively produce and secrete certain signaling molecules (called autoinducers). Acyl-homoserine lactone (AHL) molecule is an important auto inducer. AHL molecules secreted by P. aeruginosa strains have immunomodulatory activity and affects on various hemodynamic parameters of the host and also have an important role on the pathophysiology of lung infections [38,39,40]. AHL molecules contain lactone ring. PON enzymes were reported to have lactonase activity [38,39]. Serum-PON1 prevented P. aeruginosa quorum-sensing and biofilm formation in vitro by inactivating the quorum- sensing signal. AHL products secreted by P. aeruginosa are preferentially inhibited by PON2 [41,42]. In tracheal epithelial cells, it was reported that PON2 inhibits P.aeruginosa QS signals but it is not effective in intact epithelium cells [42]. In our study, no significant difference was found in lung PON activities among study groups.
In our study, increased liver apoptotic cell ratio was observed in both ethanol groups compared to NLC and sham group. These results are consistent with a previous study which shows increased liver apoptosis in chronic alcohol consumption [43]. In our study was shown that increased number of liver apoptotic cells were in ELP and NLP groups more than ELC group. It was revealed that presence of infection increases liver cell apoptosis ratio. In this study, it was also observed that more liver cells undergo apoptosis in simultaneous presence of ethanol consumption and pneumonia infection. In autoimmune hepatitis and infection induced liver apoptosis were mediated by increased mononuclear cell infiltration and production of cytokines [44,45]. In our study, increased TUNEL positive cell ratio in infection generated groups compared to control and sham groups may be related to this.
No difference was found among groups of our study in terms of liver caspase-3 activities. In a study, it was investigated liver procaspase-3 and caspase-3 activities after LPS application in rats fed with normal diet and ethanol treatment. A short term increase in caspase-3 activities was observed besides histological evidence of increased liver apoptosis after LPS application, but same situation was not observed after alcohol treatment, the authors suggested that ethanol exacerbates hepatotoxicity without enhancing caspase 3-dependent apoptosis [46]. In our study, no increase in liver caspase- 3 activities was observed but there was increased liver apoptotic cell ratio in the presence of alcohol plus infection. The small numbers of experiment animals may be a limiting factor in our study. Further studies should question the effects of simultaneous presence of alcohol consumption and infection on caspase-3 activity and also other pathways that are active in apoptosis.
We compared lung tissues of study groups in terms of TUNEL positivity. The ratio of TUNEL positive alveolar epithelial cells of study groups was similar with sham groups. Also, no difference was observed in lung caspase- 3 activity of infection groups compared to sham and control groups in our study. It was reported that airway epithelium is highly resistant to apoptosis [47]. In a study that P. aeruginosa induced lung infection model, it was reported apoptosis was rare in alveolar epithelial and capillary endothelial cells [48]. In an experimental infection model formed by both gram negative and positive bacteria, numerous apoptotic cells were observed in the inflammation zone of infection area but these cells was revealed to be lymphocytes and also apoptosis was not observed in respiratory epithelial or endothelial cell [49]. However some other studies have shown P. aeruginosa induced lung epithelial apoptosis [22,50]. In our study we observed that alcohol increased in situ cell death in liver cells and this was more severe in the simultaneous presence of infection. But, these findings were not observed in lung tissue. In infection generated groups, caspase-3 activities were found to be similar to controls in both liver and lung tissues. Apoptosis studies in alcohol and infection have used different clinical and experimental models. Besides the differences between methodologies the studies, the variation in the immune response of the hosts cause different individual responses to alcohol and infection.
In conclusion, this study showed that simultaneous presence of alcohol and infection increased cellular damage in liver. We also observed that liver MDA levels increased due to damage caused by free oxygen radicals but no sign change in PONs. Further studies investigating the relationship between alcohol, infection, cellular damage caused by free oxygen radicals and in situ cell death may reveal new approaches in this field.
Acknowledgement
This study was supported by Osmangazi University Research Foundation (No:200711028). We thank Dr. Onur Zorbozan and Barbaros Şahin for technical assistance and we thank Dr. Abdul N Hamood and Dr. Jane Colmer Hamood for bacteria strain.
References
- Caballería J. Current concepts in alcohol metabolism. Ann Hepatol. 2003;2: 60-68.
- Lieber CS. Alcohol and liver. The Mount Sinai J Med 2000; 67: 84-94.
- Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol 2004; 33: 191-197.
- McVicker BL, Tuma DJ, Casey CA. Effect of ethanol on pro-apoptotic mechanisms in polarized hepatic cells. World J Gastroenterol 2007; 13: 4960-4966.
- Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health 2003; 27:220-231.
- Navasumrit P, Ward TH, Dodd NJF, Connor PJO. Ethanol-induced free radicals and hepatic DNA strand breaks are prevented invivo by antioxidants:effects of acute and chronic ethanol exposure. Carcinogenesis 2000; 21: 93-99.
- Ramaiah S, Rivera C, Arteel G. Early-phase alcoholic liver disease: an update on animal models, pathology, and pathogenesis. Int J Toxicol 2004; 23: 217-231.
- Başkol, M, Başkol G, Deniz K, Özbakır Ö, Yücesoy M. A new marker for lipid peroxidation: Serum paraoxonase activity in non-alcholic steatohepatitis, Turk J Gastroenterol 2005; 16: 119-123.
- Ferré N, Camps J, Cabré M, Paul A, Joven J. Hepatic paraoxonase activity alterations and free radical production in rats with experimental cirrhosis. Metabolism 2001; 50: 997-1000.
- Rao MN, Marmillot P, Gong M, Palmer DA, Seeff LB, Strader DB, Lakshman MR. Light, but not heavy alcohol drinking, stimulates paraoxonase by upregulating liver mRNA in rats and humans. Metabolism 2003; 52: 1287-1294.
- Brown LA, Cook RT, Jerrells TR, Kolls JK, Nagy LE, Szabo G, Wands JR, Kovacs EJ. Acute and chronic alcohol abuse modulate immunity. Alcohol Clin Exp Res 2006; 30: 1624-1631.
- Friedman H, Newton C, Klein TW. Microbial ınfections, immunomodulation, and drugs of abuse. Clin Microbiol Rev 2003; 16: 209-219.
- Gamble L, Mason CM, Nelson S. The effects of alcohol on immunity and bacterial infection in the lung. Med Mal Infect 2006; 36: 72-77.
- Szabo G. Consequences of alchol consumption on host defence. Alchol and Alcholism 1999; 34: 830-841.
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc 2005; 2: 428-432
- Marik PE. The clinical features of severe communityacquired pneumonia presenting as septic shock. Norasept II Study Investigators. J Crit Care 2000; 15: 85-90.
- Guidot DM, Roman J. Chronic ethanol ingestion increases susceptibility to acute lung injury: role of oxidative stress and tissue remodeling. Chest 2002; 122: 309S-314S.
- Chastre J, Fagon JY, Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002; 165: 867-903.
- Fagon JY, Chastre J, Domart Y, Trouillet JL, Gibert C. Mortality due to ventilator-associated pneumonia or colonization with Pseudomonas or Acinetobacter species: assessment by quantitative culture of samples obtained by a protected specimen brush. Clin Infect Dis 1996; 23: 538-542.
- Lieber CS, DeCarli LM, Sorrell MF. Experimental methods of ethanol administration. Hepatology 1989; 10: 501-510.
- Yamada S, Wilson JS, Lieber CS. The effects of ethanol and diet on hepatic and serum gammaglutamyltranspeptidase activities in rats. J Nutr 1985; 115: 1285-1290.
- Le Bere, R, Faure K, Fauvel H, Viget NB, Ader F, Prangère T, Thomas AM, Leroy X, Pittet JF, Marchetti P, Guery BP. Apoptosis inhibition in P.aeruginosainduced lung injury influences lung fluid balance. Intensive Care Med 2004; 30: 1204-1211.
- Rezaiguia S, Garat C, Delclaux C, Meignan M, Fleury J, Legrand P, Matthay MA, Jayr C. Acute bacterial pneumonia in rats increases alveolar epithelial fluid clearance by a tumor necrosis factor-alpha-dependent mechanism. J Clin Invest 1997; 99: 325-335.
- . Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-358.
- Beltowski J, Jamroz-Wiśniewska A, Borkowska E, Wójcicka G. Differential effect of antioxidant treatment on plasma and tissue paraoxonase activity in hyperleptinemic rats. Pharmacol Res 2005; 51: 523-532.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265-275.
- Kanbak G, Inal M, Bayçu C. Ethanol-induced hepatotoxicity and protective effect of betaine. Cell Biochem Funct 2001; 19: 281-285.
- Uzun H, Simsek G, Aydin S, Unal E, Karter Y,Yelmen NK, Vehid S, Curgunlu A, Kaya S. Potential effects of L-NAME on alcohol-induced oxidative stress. World J Gastroenterol 2005; 11: 600-604.
- Gupta S, Pandey R, Katyal R, Aggarwal HK, Aggarwal RP, Aggarwal SK. Lipid peroxide levels and antioxidant status in alcoholic liver disease. Indian J Clin Biochem 2005; 20: 67-71.
- Castilla R, González R, Fouad D, Fraga E, Muntané, J. Dual effect of ethanol on cell death in primary culture of human and rat hepatocytes. Alcohol and Alcoholism 2004; 39: 290-296.
- Yang SS, Huang CC, Chen JR, Chiu CL, Shieh MJ, Lin SJ, Yang SC. Effects of ethanol on antioxidant capacity in isolated rat hepatocytes. World J Gastroenterol 2005; 11: 7272-7276.
- Ponnappa BC, Rubin E. Modeling alcohol's effects on organs in animal models. Alcohol Res Health 2000; 24: 93-104.
- Lu Y, Wang X, Cederbaum AI. Lipopolysaccharideinduced liver injury in rats treated with the CYP2E1 inducer pyrazole. Am J Physiol Gastrointest Liver Physiol 2005; 27: G308-G319.
- Mathurin P, Deng, QG, Keshavarzian A, Choudhary S,Holmes EW, Tsukamoto H. Exacerbation of alcholic liver injury by enteral endotoxin in rats. Hepatology 2000; 32: 1008-1017.
- Zhang P, Bagby GJ, Happel KI, Raasch CE, Steve Nelson S. Alcohol Abuse, Immunosuppression, and Pulmonary Infection. Current Drug Abuse Reviews 2008; 1: 56-67.
- Kinniry P, Amrani Y, Vachani A, Solomides CC, Arguiri E, Workman A, Carter J, Christofidou-Solomidou M. Dietary flaxseed supplementationameliorates inflammation and tissue damage in experimental models of acute lung injury in mice. J Nutr 2006; 136: 1545-1551.
- Furlong CE. Paraoxonases: An historical perspective. In: Mackness B, Mackness M, Aviram M, Paragh G, eds. The paraoxonases: Their role in diseasedevelopment and xenobiotic metabolism. The Netherland: Springer 2008; pp3-31.
- Draganov DI, Teiber JF. PONs’ natural substrates –The key for their physiological roles. In: Mackness B,Mackness M, Aviram M, Paragh G, eds. The paraoxonases:Their role in disease development and xenobiotic metabolism. The Netherland: Springer 2008; pp297-307.
- Stoltz DA, Ozer EA, Zabner J. Paraoxonases, quorum sensing, and Pseudomonas aeruginosa In: Mackness B, Mackness M, Aviram M, Paragh G, eds. The paraoxonases: Their role in disease development and xenobiotic metabolism. The Netherland: Springer 2008; pp307-321.
- Telford G, Wheeler D, Williams P, Tomkins PT, Appleby P, Sewell H, Stewart G, Bycroft, BW, Pritchard DI. The Pseudomonas aeruginosa quorumsensing signal molecule N-[3-oxododecanoyl]-LHomoserine lactone has immunomodulatory activity. Infect Immun 1998; 66: 36-42.
- Ozer EA, Pezzulo A, Shih DM, Chun C, Furlong C, Lusis AJ, Greenberg EP, Zabner J. Human and murine paraoxonase 1 are host modulators of Pseudomonas aeruginosa quorum-sensing. FEMS Microbiol Lett 2005;253: 29-37.
- Stoltz DA, Ozer EA, Ng CJ, Yu JM, Reddy ST, Lusis AJ, Bourquard N, Parsek MR, Zabner J, Shih DM. Paraoxonase-2 deficiency enhances Pseudomonas aeruginosa quorum sensing in murine tracheal epithelia. Am J Physiol Lung Cell Mol Physiol 2007; 292: L852-860.
- Zhao M, Laissue JA, Zimmermann A. TUNELpositive hepatocytes in alcoholic liver disease. A retrospective biopsy study using DNA nick endlabelling. Virchows Arch 1997; 431: 337-344.
- Ghavami S, Hashemi M, Kadkhoda K, Alavian SM, Bay GH, Los M. Apoptosis in liver diseases-detection and therapeutic applications. Med Sci Monit 2005; 11: RA337-345.
- Liu D, Li C, Chen Y, Burnett C, Liu XY, Downs S, Collins RD, Hawiger J. Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide.J Biol Chem 2004; 279: 48434-48442.
- Koteish A, Yang S, Lin H, Huang X, Diehl AM. Chronic ethanol exposure potentiates lipopolysaccharide liver injury despite inhibiting junN-terminal kinase and caspase 3 activation. J Biol Chem 2002: 277:13037-13044.
- Rajan S, Cacalano G, Bryan R, Ratner AJ, Sontich CU, van Heerckeren A, Davis P, Prince A. Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells: analysis of the effects of cystic fibrosis transmembrane conductance regulator dysfunction and bacterial virulence factors. Am J Respir Cell Mol Biol 2000; 23: 304-312.
- Hotchkiss RS, Dunne WM, Swanson PE, Davis CG, Tinsley KW, Chang KC. Buchman YG, Karl IE. Role of apoptosis in Pseudomonas aeruginosa pneumonia. Science 2001; 294: 1783.
- Schreiber T, Swanson PE, Chang KC, Davis CC, Dunne WM, Reinhart K, Hotchkiss RS. Both gramnegative and gram-positive experimental pneumonia induce profound lymphocyte but not respiratory epithelial cell apoptosis. Shock 2006; 26: 271-276.
- Grassme H, Kirschnek S, Riethmueller J, Riehle A, Kurthy G von, Lang F, Weller M, Gulbins E.CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science 2000; 290: 527-530.