Research Article - Biomedical Research (2017) Volume 28, Issue 22
Basal ganglia cerebral infarction patient fMRI imaging analysis before and after acupuncture-medicine therapy
Aihong Guo1#, Fengli Hao1#, Futai Li1*, Bingju Wang1, Lianfeng Liu2, Zhiru Zhao1 and Chunhong Yao1
1Department of Neurology, Xianyang Hospital of Yan’an University, Xianyang, 712000, PR China
2Department of Medical Imaging, Xianyang Hospital of Yan’an University, Xianyang, 712000, PR China
#These authors contributed equally
- *Corresponding Author:
- Futai Li
Department of Neurology
Xianyang Hospital of Yan’an University
PR China
Accepted date: October 09, 2017
Abstract
Basal ganglia cerebral infarction can cause cerebral vascular dementia, which seriously influence patient’s quality of life. Thus, early prevention and treatment are extremely important. At present, the common methods used for treatment include drug therapy, acupuncture therapy, and rehabilitation treatment, etc. Research found that acupuncture-medicine therapy can significantly improve patient’s prognosis and quality of life. This study applied acupuncture-medicine therapy to treat patients with basal ganglia cerebral infarction, and used functional magnetic resonance imaging (fMRI) technology to observe its clinical curative effect. A total of 80 basal ganglia cerebral infarction patients were randomly divided into four groups. All of the four groups received cerebral infarction drug therapy, including anti-atherosclerosis, lipid regulation, cranial nerve nutrition, and improve cerebral circulation. Group D, as the control group, received routine drug treatment for cerebral infarction. Group A, B, and C were given acupuncture therapy, rehabilitation therapy, and acupuncture plus rehabilitation therapy on the basis of control group. Degree of nervous functional defects (NIHSS), motor function, and activity of daily life were compared. fMRI was performed to observe cerebral cortex activation area under the same stimuli. Four groups showed statistical difference on NIHSS score, clinical efficacy score, Barthel Index score, and SM1 region activation area (P<0.05). Acupuncture-medicine therapy has prominence effect for the treatment of patients with basal ganglia cerebral infarction. fMRI can objectively indicate the cortex recovery, suggesting that it can track functional recovery after treatment and provide a new direction for clinical diagnosis, treatment, and prognosis.
Keywords
Basal ganglia cerebral infarction, Medical therapy, Acupuncture therapy, Rehabilitation therapy, MRI
Introduction
Cerebral infarction is one of the most common nervous system diseases in clinic that is harmful for human life. Recently, the incidence of cerebrovascular disease gradually increased. Epidemiological investigation shows that cerebral infarction is the leading cause of disability in our country, while its fatality rate is only after malignant tumor [1,2]. Cerebral infarction occurrence is due to brain blood vessels (atherosclerosis or vasospasm) stenosis or obstruction caused blood supply deficiency and hypoxidosis, eventually leading to brain tissue necrosis, accompanied by nerve dysfunction and daily life disability. Following ischemia deterioration and time extension, the degree of nerve dysfunction also gradually increases [3]. Studies have proved that there are some reversible dysfunctional brain tissues around the infarction brain tissue, which become the basis of ischemic cerebrovascular disease treatment and rehabilitation [4,5]. Identifying survival brain tissue has important significance for acute cerebral infarction treatment options and prognosis prediction, especially with limb function disorder.
Basal ganglia cerebral infarction is common in clinic. It refers to the infarction occurred in the basal ganglia region. Brain small artery occurs occlusion on the basis of vascular atherosclerosis, leading to cerebral ischemic infarction lesions [6,7]. The pathological changes scope is 2-20 mm, of which 2 to 4 mm is most common. Basal ganglia cerebral infarction usually has no obvious symptoms, whereas some patients may affect brain function, leading to intelligence progressive deterioration and cerebral vascular dementia at last. Thus, effective treatment is immediately needed once found. Common treatment method includes drug therapy, acupuncture therapy, and rehabilitation treatment, etc. Study found that acupuncture-medical therapy can significantly improve patient’s prognosis and quality of life [4].
Functional magnetic resonance imaging (fMRI) is a type of imaging technique based on magnetic resonance technology, which mainly focuses on brain region activity (brain function) investigation [8]. fMRI is characterized as high comparative, nondestructive, good repeatability, flexible and feasible method, and accurate positioning, thus can be used to observe human brain neural function activities directly [9,10]. As it not only can help ischemic cerebrovascular disease early discover and diagnosis, but also can track prognosis, fMRI received more and more attention in recent years to improve patient’s prognosis and life quality.
This study used fMRI to observe the curative effect of acupuncture-medicine therapy on basal ganglia cerebral infarction, explored the relationship between limb disabilities recovery and primary sensory cortex activation, and investigate the significance on cerebral infarction treatment selection and prognosis.
Materials and Methods
Clinical information
Inclusion criteria: 1) In accordance with cerebral infarction diagnosis criteria established by Chinese medical association. 2) Basal ganglia cerebral infarction patients detected by CT or MRI within five years in our hospital. 3) First time cerebral infarction without cognition impairment, ipsilateral unilateral limb dyskinesia, strength less than level 3. 4) Without severe heart, lung, liver, and kidney diseases or complications. 5) The patient had signed informed consent.
Exclusion criteria: 1) does not meet the inclusion criteria. 2) Ipsilateral limb muscle strength achieves level 3 and obvious cognitive dysfunction. 3) Transient ischemic attack. 4) Large cerebral infarction area, accompanied by cerebral hernia, severe coma, intracranial vascular malformation, or bleeding patient. 5) Patients with poor compliance, including irregular therapy, discontinued, or plus other treatment without authorization.
A total of 80 basal ganglia cerebral infarction patients were randomly equally divided into four groups, of which group A, B, C were treatment group and group D was control. There were 12 males and 8 females in group A with mean age at 65.13 ± 6.78 (46-75) years old, 9 males and 11 females in group B with mean age at 63.78 ± 7.19 (47-76) years old, 13 males and 7 females in group C with average age at 64.54 ± 8.27 (45-73) years old, and 9 males and 11 females in group D with average age at 64.34 ± 5.78 (47-74) years old. No statistical differences were observed on age, gender, concomitant disease (including hypertension and hyperlipidemia), and infarct severity among groups (P>0.1).
Treatment method
All of the four groups received cerebral infarction drug therapy, including anti-atherosclerosis, lipid regulation, cranial nerve nutrition, and improve cerebral circulation. Group D, as the control group, received routine drug treatment for cerebral infarction. Group A, B, and C were given acupuncture therapy, rehabilitation therapy, and acupuncture plus rehabilitation therapy on the basis of control group.
Therapeutic evaluation
Degree of nervous functional defects (NFD) (evaluated by NIHSS scale published by NIH), activity of daily life (Barthel index, BI), and clinical curative effect before and after treatment were evaluated according to The Chinese medical association the fourth national cerebrovascular disease conference in 1995 [11].
Disability degree was divided into four levels: (1) level 0, no dependence, and do not need other care; (2) level 1, mild dependence, need few other care; (3) level 2, moderate dependence, most need other care; (4) level 3: heavy dependence, all need other care.
Clinical efficacy was graded according to the function defect degree and disability degree: (1) basic cure, function defect score decreased by 91~100%, and the disability degree is level 0; (2) remarkable progress: function defect score decreased by 46~90%, and the disability degree is level 1~3; (3) progress: function defect score decreased by 18~45%; (4) invalid, function defect score decreased by 17%; (5) deterioration, function defect score decreased by less than 17%.
fMRI investigation
Ten patients were randomly selected from each group before and after treatment for fMRI investigation. Primary sensory region (SM1) was tested when the patient received stimulus to observe its changes before and after treatment. fMRI was performed on 3.0 T Siemens magnetic resonance system to scan all brain results. Cerebral infarction patients active clench movement was designed as the fMRI block in fMRI upper limb movement pattern. All the subjects received stimulus training at 1 day before scan. Subjects were familiar with the general rules, procedures, and environment of the test operation to alleviate psychological pressure. Patient was fixed in head, closed eyes, and keep quiet during the scanning process. Operator observed patient to complete the task during scan.
Statistical analysis
fMRI data was extracted and analyzed on SPM8 software on Matlab platform. SMI active region volume was calculated. Data processing was performed on SPSS19.0. Measurement data was presented as mean ± standard deviation (͞x ± s) and tested by t test. Enumeration data was presented asх2. P<0.05 was considered as statistical significance.
Results
NIHSS comparison before and after treatment
Four basal ganglia cerebral infarction groups NIHSS results before and after treatment showed that compared with control, NIHSS score significantly decreased in group A, B, and C. The treatment group presented better neurological deficit improvement than control (P<0.05). NIHSS score markedly declined in group C compared with group A and B, suggesting that group C had better effect on neurological deficit improvement than group A and B (P<0.05) (Table 1).
| Group | Cases | Before treatment | After treatment |
|---|---|---|---|
| Control | 20 | 27.45 ± 5.89 | 22.25 ± 5.65Δ |
| Group A | 20 | 26.78 ± 6.23 | 17.78 ± 5.34Δ* |
| Group B | 20 | 27.34 ± 5.17 | 18.67 ± 4.48Δ* |
| Group C | 20 | 26.79 ± 5.57 | 12.25 ± 5.23Δ* |
| ΔP<0.05, group A, B, and C compared with control; *P<0.05, group A and B compared with group C. | |||
Table 1: NIHSS comparison before and after treatment.
BI comparison before and after treatment
BI score obviously elevated in group A, B, and C after treatment compared with control (P<0.05). Group C presented significantly higher BI score after treatment compared with group A and B, indicating that group C showed better effect on daily living activity improvement than group A and B (P<0.05) (Table 2).
| Group | Cases | Before treatment | After treatment |
|---|---|---|---|
| Control | 20 | 23.45 ± 5.39 | 32.45 ± 7.85Δ |
| Group A | 20 | 22.38 ± 6.73 | 42.78 ± 8.14Δ* |
| Group B | 20 | 24.36 ± 7.37 | 43.67 ± 9.47Δ* |
| Group C | 20 | 23.29 ± 6.58 | 53.25 ± 9.43Δ* |
| ΔP<0.05, group A, B, and C compared with control; *P<0.05, group A and B compared with group C. | |||
Table 2: BI score comparison before and after treatment.
Clinical efficacy index comparison before and after treatment
Clinical efficacy index before and after treatment results demonstrated it markedly increased in group A, B, and C after treatment compared with control (P<0.05). Compared with group A and B, clinical efficacy index obviously elevated in group C after treatment, revealing that group C improved clinical efficacy better than group A and B (P<0.05) (Table 3).
| Group | Cases | Basic cure | Remarkable progress | Progress | Invalid | Deterioration | Overall response rate |
|---|---|---|---|---|---|---|---|
| Control | 20 | 2 | 4 | 4 | 8 | 2 | 50%Δ |
| Group A | 20 | 3 | 4 | 6 | 6 | 1 | 65%Δ* |
| Group B | 20 | 2 | 6 | 5 | 7 | 0 | 65%Δ* |
| Group C | 20 | 5 | 10 | 2 | 3 | 0 | 85%Δ* |
| ΔP<0.05, group A, B, and C compared with control; *P<0.05, group A and B compared with group C. | |||||||
Table 3: Clinical efficacy index comparison before and after treatment.
fMRI comparison before and after treatment
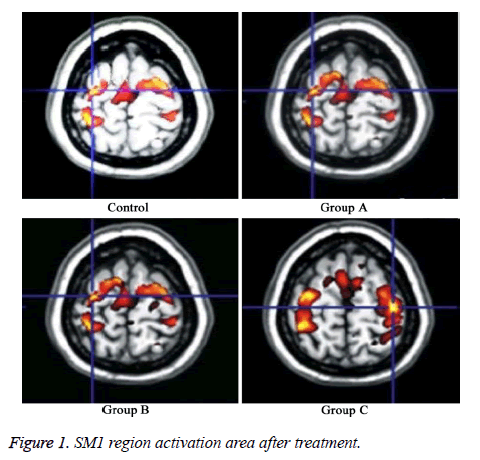
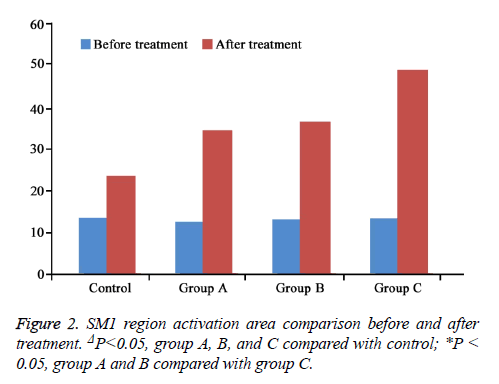
SM1 region activation area results showed that compared with control, the area significantly enlarged in group A, B, and C after treatment (P<0.05) (Figure 1). SM1 region activation area obviously elevated in group C compared with group A and B, suggesting that group C had larger activation volume compared with group A and B under the same Bandettini stimulus (P<0.05) (Figure 2).
Discussion
fMRI is a new kind of neuroimaging method with the principle as applying MRI to measure neuron activities induced hemodynamic changes [12,13]. fMRI is widely used in the field of brain function location because of its non-invasive and no radiation exposure [14]. There are many kinds of fMRI research methods, including magnetic resonance spectral imaging (MRS), diffusion weighted imaging (DWI), perfusion weighted imaging (PWI), and cerebral cortex thickness and blood oxygen saturation level detection (BOLD). BOLD method is the fMRI on narrow sense [15,16]. fMRI can detect the brain neuronal activity area corresponding to cognitive, sensory, and motor functions, and gain function and morphology image at the same time [17]. As fMRI can reflect the pathological state of brain tissue damage and brain tissue perfusion information earlier, it makes the individualized treatment possible according to each patient’s pathological and physiological stage.
It was believed that central nervous system may appear plasticity changes after cerebral infarction, mainly as hibernant nerve tissue activation, involvement nerve tissue metastasis, and disabled nerve function activation [18,19]. Foreign scholars proposed that hibernant neural tissue can undertake the function lost by damaged nerve tissue, which became the pioneer of nervous system restructuring theory [20,21]. Central nervous system plasticity theory thought that the brain has plasticity. Dysfunction improvement after treatment is mainly because of central nervous system plasticity changes [22]. It was also found that the main areas of brain activation focused on SM1. It plays an indispensable role in controlling movement execution. Primary motor cortex activation changes could be observed under fMRI [23,24]. This study discovered that SM1 area activation volume increased after treatment. Group C presented significantly larger area than group A and B, suggesting that effective treatment can obviously improve patient’s prognosis. It was also found that nerve function defect degree, daily life ability, clinical efficacy and fMRI markedly elevated after acupuncture-medicine therapy. Furthermore, conventional drug therapy plus acupuncture and rehabilitation therapy is more effective. fMRI illustrated that acupuncture-medicine therapy can reduce the patient’s limbs dyskinesia, promote the hibenant and disabled nerve tissue recombination and domination, thus to improve functional recovery and prognosis.
To sum up, acupuncture-medicine therapy showed obvious clinical efficacy on basal ganglia cerebral infarction by improving limb dyskinesia and quality of life, and reducing complication and fatality rate. fMRI can directly detect central nerve system recovery, which provides new direction for cerebral infarction diagnosis and prognosis.
References
- Arora AJ, Bolla H, Arora R, Kaul S, Yarlagadda J. Basal ganglia infarction due to mild trauma in a case of mineralizing angiopathy: A rare entity. Neurol India 2015; 63: 771-772.
- Fritzsch D, Reiss-Zimmermann M, Lobsien D, Quaschling U, Hoffmann KT. Arteriovenous shunts and capillary blush as an early sign of basal ganglia infarction after successful mechanical intra-arterial thrombectomy in ischaemic stroke. Eur Radiol 2015; 25: 3060-3065.
- Shankaran S, McDonald SA, Laptook AR, Hintz SR, Barnes PD, Das A, Pappas A, Higgins RD, Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr 2015; 167: 987-993.
- He ZP, Lu H. Aquaporin-4 gene silencing protects injured neurons after early cerebral infarction. Neural Regen Res 2015; 10: 1082-1087.
- d'Esterre CD, Aviv RI, Morrison L, Fainardi E, Lee TY. Acute Multi-modal Neuroimaging in a Porcine Model of Endothelin-1-Induced Cerebral Ischemia: Defining the Acute Infarct Core. Transl Stroke Res 2015; 6: 234-241.
- Garge SS, Vyas PD, Modi PD, Ghatge S. Crohns disease with central nervous system vasculitis causing subarachnoid hemorrhage due to aneurysm and cerebral ischemic stroke. Ann Indian Acad Neurol 2014; 17: 444-447.
- Nardai S, Dobolyi A, Pal G, Skopal J, Pinter N, Lakatos K, Merkely B, Nagy Z. Selegiline promotes NOTCH-JAGGED signaling in astrocytes of the peri-infarct region and improves the functional integrity of the neurovascular unit in a rat model of focal ischemia. Restor Neurol Neurosci 2015; 33: 1-14.
- Mitchell FM, Prasad SK, Greil GF, Drivas P, Vassiliou VS, Raphael CE. Cardiovascular magnetic resonance: Diagnostic utility and specific considerations in the pediatric population. World J Clin Pediatr 2016; 5: 1-15.
- Zubcevic S, Milos M, Catibusic F, Uzicanin S, Krdzalic B. Interictal Electroencephalography (EEG) Findings in Children with Epilepsy and Bilateral Brain Lesions on Magnetic Resonance Imaging (MRI). Acta Inform Med 2015; 23: 343-346.
- Tanaka N, Stufflebeam SM. Presurgical Mapping of the Language Network Using Resting-state Functional Connectivity. Top Magn Reson Imaging 2016; 25: 19-24.
- Nagaya N, Nishikimi T, Uematsu M, Kyotani S, Satoh T, Nakanishi N, Matsuo H, Kangawa K. Secretion patterns of brain natriuretic peptide and atrial natriuretic peptide in patients with or without pulmonary hypertension complicating atrial septal defect. Am Heart J 1998; 136: 297-301.
- Baker EH, Sloan JL, Hauser NS, Gropman AL, Adams DR, Toro C, Manoli I, Venditti CP. MRI characteristics of globus pallidus infarcts in isolated methylmalonic acidemia. AJNR Am J Neuroradiol 2015; 36: 194-201.
- Boehm-Sturm P, Farr TD, Adamczak J, Jikeli JF, Mengler L, Wiedermann D, Kallur T, Kiselev V, Hoehn M. Vascular changes after stroke in the rat: a longitudinal study using optimized magnetic resonance imaging. Contrast Media Mol Imaging 2013; 8: 383-392.
- Xu P, Lv L, Li S, Ge H, Rong Y, Hu C, Xu K. Use of high-resolution 3.0-T magnetic resonance imaging to characterize atherosclerotic plaques in patients with cerebral infarction. Exp Ther Med 2015; 10: 2424-2428.
- Dai Z, Chen F, Yao L, Dong C, Liu Y, Shi H, Zhang Z, Yang N, Zhang M, Dai Y. Application of diffusion tensor imaging in judging infarction time of acute ischemic cerebral infarction. Zhonghua Yi Xue Za Zhi 2015; 95: 2526-2531.
- Liu T, Li J, Zhao Z, Zhong Y, Zhang Z, Xu Q, Yang G, Lu G, Pan S, Chen F. Betel quid dependence is associated with functional connectivity changes of the anterior cingulate cortex: a resting-state fMRI study. J Transl Med 2016; 14: 33.
- Leithner C, Fuchtemeier M, Jorks D, Mueller S, Dirnagl U, Royl G. Infarct volume prediction by early magnetic resonance imaging in a murine stroke model depends on ischemia duration and time of imaging. Stroke 2015; 46: 3249-3259.
- Taylor AM, Harris AD, Varnava A, Phillips R, Hughes O, Wilkes AR, Hall JE, Wise RG. Neural responses to a modified Stroop paradigm in patients with complex chronic musculoskeletal pain compared to matched controls: an experimental functional magnetic resonance imaging study. BMC Psychol 2016; 4: 5.
- Nakayama S, Kamagata K, Sano T, Shimada Y, Tanaka Y, Fukui T, Urabe T, Aoki S, Hattori N, Motoi Y. Verbal dysflucency as a consequence of basal ganglia infarction with selective involvement of dorsolateral prefrontal fiber tract. J Neurol 2013; 260: 2427-2429.
- Wang J, Yu XD, Li GQ. Comparative study on short-term and long-term prognostic determinants in patients with acute cerebral infarction. Int J Clin Exp Med 2015; 8: 9855-9861.
- Kato N, Schilt S, Schneider H, Frey D, Kufeld M, Vajkoczy P, Picht T. Functional brain mapping of patients with arteriovenous malformations using navigated transcranial magnetic stimulation: first experience in ten patients. Acta Neurochir (Wien) 2014; 156: 885-895.
- Lu H, Hu H, He Z, Han X, Chen J, Tu R. Therapeutic imaging window of cerebral infarction revealed by multisequence magnetic resonance imaging: An animal and clinical study. Neural Regen Res 2012; 7: 2446-2455.
- Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, Kim YH. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke 2011; 42: 1357-1362.
- Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage 2012; 59: 2771-2782.