Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2023) Volume 13, Issue 101
Bacterial etiology in acute exacerbations of COPD
Kamaldeep Kaur1*, Anusha P2, Ratna Kumari2, Lakshmi21Department of Medical Microbiology, Shankracharaya Institute of Medical Sciences, Bhilai, Chattisgarh
2Department of Microbiology, Andhra Medical College, Maharanipeta , Visakhapatnam, Andhra Pradesh
- *Corresponding Author:
- Kamaldeep Kaur
Department of Microbiology
Shri Shankaracharya Institute of Medical Sciences, India
E-mail: kamaldeepkaur_06@yahoo.in
Received: 30-Aug -2023, Manuscript No. AABPS-23-104913; Editor assigned: 01-Sep -2023, PreQC No. AABPS-23-104913(PQ); Reviewed: 14-Sep -2023, QC No. AABPS-23-104913; Revised: 20-Sep-2023, Manuscript No. AABPS-23-104913(R); Published: 29-Sep -2023, DOI: 10.35841/ aabps-13.101.191
Citation: Kaur K, Anusha P, Kumari R, et al. Bacterial etiology in acute exacerbations of COPD. Asian J Biomed Pharmaceut Sci. 2023;13(101):191
Abstract
Background: Chronic Obstructive Pulmonary Disorder (COPD) is a major cause of morbidity and one of the principal causes of death worldwide. The objective of this study is to identify the bacterial etiological agents in patients with acute exacerbations of COPDs by sputum culture and their antibiogram. Methods: This is a prospective study comprising 100 diagnosed patients of COPD taken as GOLD Grade 4 who were admitted in the Department of Pulmonary Medicine, TB and Chest Hospital which comes under King George Hospital, Visakhapatnam during a period from May 2015 to May 2016. Results: Out of 100 patients, 83 were males of which 87% were smokers. The most common organisms isolated were Gram negative bacilli in 51 patients and only 26 were Gram positive cocci. The most common Gram-negative isolate was Klebsiella pneumoniae 27 cases followed by Pseudomonas aeruginosa in 19 cases. The most common Gram-positive isolate was Streptococcus pneumoniae in 16 cases followed by Streptococcus pyogenes in 6 cases and Staphylococcus aureus in 2 cases.
Keywords
COPD, Bacterial aetiology, acute exacerbation, Smokers, Antibiogram.
Introduction
Chronic Obstructive Pulmonary Disorder (COPD), a common preventable and treatable disease which is characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to noxious particles or gases [1].
Cigarette smoking or inhalation of dust or fumes are important contributing factors. The disease is defined by the clinical symptoms in which excessive mucus production leads to coughing up sputum on most days during at least 3 consecutive months for more than two successive years. During episodes of acute exacerbations patient has increased expectorations and is usually associated with increased clinical symptoms, signs and functional disability [2].
Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD) is defined as an acute change in a patient’s baseline dyspnea, cough and/or sputum beyond dayto- day variability sufficient to warrant a change in therapy. Exacerbation may be triggered by an infection with bacteria or viruses or by environmental pollutants [3].
Exacerbations of COPD have considerable impact on health care system at both primary and tertiary care levels as they are the major reason for antibiotic use and admissions; additionally, exacerbations lead to indirect costs because of days lost from work [4].
A strong association exists between tobacco smoking and the occurrence of COPD. The reported smoker: nonsmoker prevalence ratio varied from 1.6 to 10.2. The difference in the prevalence of COPD among males and females could be due to the differences in their levels and type of smoking. Women, spending more time indoors for cooking than men who are exposed to biomass fuel combustion products and are prone to develop COPD [1].
Chronic clinical course of COPD is punctuated by periods of increased symptoms that are termed “acute exacerbations” [5].
COPD is classified according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria Potential pathways by which bacteria could contribute to the course and pathogenesis of AECOPD were identified. Bacteria cause a substantial proportion of acute exacerbations of chronic bronchitis which leads to considerable morbidity and mortality.
Chronic colonization of the lower respiratory tract by bacterial pathogens amplifies the chronic inflammatory response present in COPD and leads to progressive airway obstruction (vicious circle hypothesis) [4] .Bacterial antigens in the lower airway induce hypersensitivity that enhances airway hyper reactivity and induces eosinophilic inflammation [4]. Studies have been performed and it has been shown that the growth of pathogenic bacteria during an episode of acute exacerbations has been more in terms of colony forming units (CFU) than during other times. Furthermore, the local inflammatory response of the host increases with increasing airway bacterial load [6]. Antibiotics are commonly prescribed empirically to patients presenting with COPD to treat presumed bacterial infection. The rise in bacterial resistance to antibiotics has focused our attention on the benefit of this practice, and more fundamentally the importance of bacterial infection in COPD, and its role in stimulating bronchial inflammation, which is the hallmark of this condition [7].
Still, many questions remain, and future studies will be needed to better define the mechanisms of bacterial invasion in the COPD patients and to develop effective vaccines to prevent exacerbation. In the meantime, we must rely on antibiotic therapy, and we will need prospective studies to corroborate preliminary findings showing that different patients may require different therapies; Thus, patient sub setting may be vital in the selection of antibiotic therapy for exacerbations of COPD.
Materials and Methods
All procedures performed in the study were by the ethical standards of the institutional and or national research committee and with the 1964 Helsinki declaration and its later amendments.
In this cross-sectional study, a total of 100 patients diagnosed as COPD Grade 4 as per GOLD guidelines were included in the study. The study was carried out at the Department of Microbiology with the help of Department of Pulmonary Medicine under the TB and Chest hospital, King George Hospital, Visakhapatnam, during the period from May 2015 to May 2016. The study comprises of all gender with their age group ranging from 45 to 80 years. For all the 100 patients admitted, detailed history was taken, those patients with increased cough, increased severity of dyspnoea, excessive sputum production and sputum sample was collected prior to the administration of antibiotics were included in the study.
Patients diagnosed with tuberculosis, bronchial asthma, lung abscess, lung carcinoma, ischemic heart disease and who were already on antibiotic treatment were excluded from the study.
Classification of severity of airflow limitation in COPD
In pulmonary function testing, a postbronchodilator FEV1/ FVC ratio of <0.70 is commonly considered diagnostic of COPD. In patients with this ratio.
• GOLD 1 – mild: FEV1 ≥ 80% predicted
• GOLD 2 – moderate: 50% ≤ FEV1 < 80% predicted
• GOLD 3 – severe: 30% ≤ FEV1 < 50% predicted
The GOLD guideline uses a combined COPD assessment approach based on patient’s symptoms and previous history of exacerbations into 4 groups
• Group A: low risk (0-1 exacerbation / year, not requiring hospitalization) and fewer symptoms
• Group B: low risk (0-1 exacerbation / year, not requiring hospitalization) and more symptoms
• Group C: high risk (≥ 2 exacerbations / year or one or more requiring hospitalization) and fewer symptoms
• Group D: high risk (≥ 2 exacerbations / year or one or more requiring hospitalization) and more symptoms
Bacterial infections are one of the most common causes of acute exacerbation of COPD. They account for 25% exacerbations alone [6].
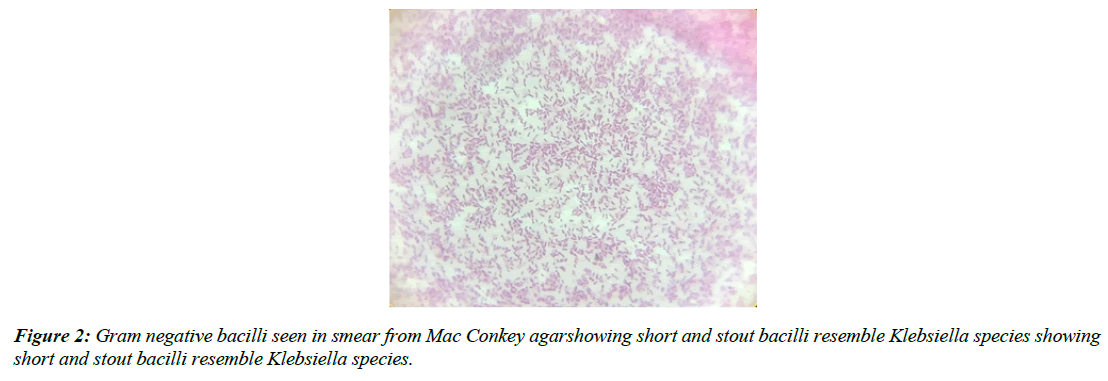
Patients were instructed and a deep coughed sputum sample was collected into a sterile wide mouth container with a screw cap. Smears were prepared and were stained by Gram staining technique (Figure 1-4) The smears were then examined and the quality of the sputum was assessed by Bartlett’s grading (Table 1) and having a score of 1 or more were processed further [8]. A final score of 0 or less indicates either lack of inflammatory response or presence of significant salivary contamination, thus invalidating the specimen. Selected sputa were processed microbiologically by using a calibrated loop, 0.01 ml onto the following culture media: blood agar, MacConkey agar and chocolate agar.
| No. of neutrophils per 10x low power field | Grade |
|---|---|
| <10 | 0 |
| 25-Oct | 1 |
| >25 | 2 |
| Presence of mucus | 1 |
| No. of epithelial cells per 10x low power field | Grade |
| 25-Oct | -1 |
Table 1: Bartletts grading of sputum
Incubated at 35 ± 2°C under aerobic conditions. Chocolate agar was incubated in a candle jar. A first reading was taken after 24 hrs, and a second final one was taken after 48 hrs of culture.
Results
A Total of 100 patients clinically diagnosed and admitted with AECOPD were studied. Bacterial infections of AECOPD were analysed. Organisms grown were identified based on their morphology, cultural characteristics and biochemical reactions their culture & sensitivity patterns to various antibiotics were also recorded.
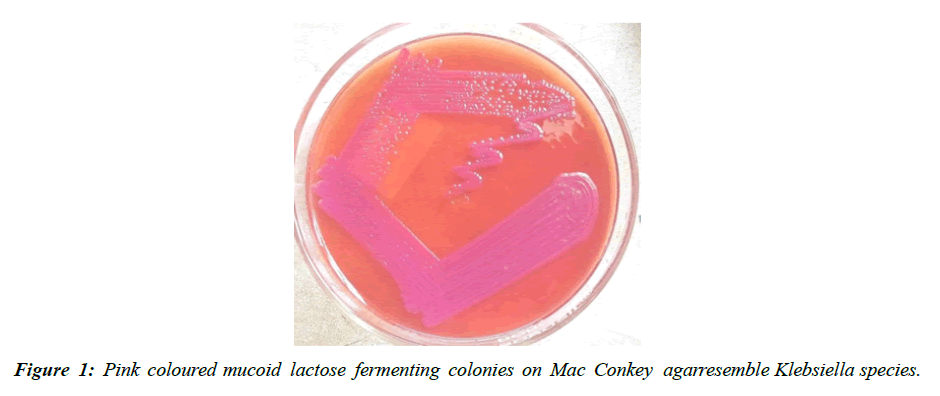
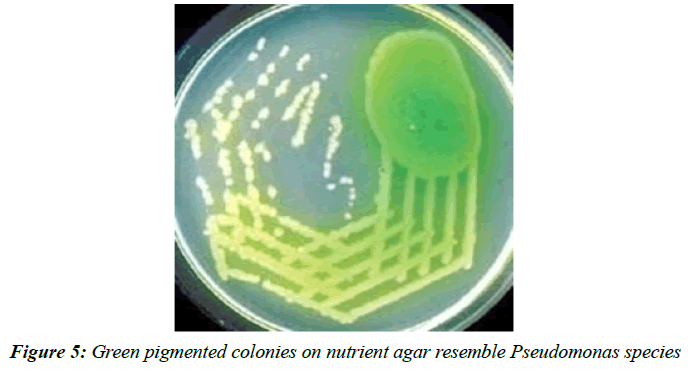
In 100 patients 83(83%) were males and 17(17%) were females. Male: female ratio was 4.8:1. As shown in (Table 2), among the 83 males 73 [88 %] were smokers, out of 17 females 9 [53 %], were smokers. 12 % were nons mokers. Majority of the patients in the study population were smokers. Out of the 100 specimens processed, 14 samples showed polymicrobial growth, 77 samples showed monomicrobial growth and the remaining ones were sterile. 77 samples that showed monomicrobial growth had Klebsiella pneumonia (Figure 1) the most common isolate, followed by Pseudomonas aeruginosa (Figure 5) and Streptococcus pneumonia (Figure 6).
| Smoking status | Male | Female | Total [%] |
|---|---|---|---|
| Smoker | 73 | 9 | 82% |
| Non-Smoker | 10 | 8 | 18% |
Table 2: Incidence of smoking
The antibiotic discs which were tested for antimicrobial susceptibility upon Mueller Hinton agar by Kirby Bauer disc diffusion method.
With the help of antibiotic scale from HIMEDIA, the following sensitivity patterns were noted. The antibiotic susceptibility patterns of isolates of Gram negative baccilli are depicted in (Table 3a &3b). A total of 26 number of Gram positive cocci were isolated. (Table 4-6).
| Different species of Gram negative bacilli isolated | Number of bacterial isolates |
|---|---|
| Klebsiella pneumoniae | 27 |
| Pseudomonas aeruginosa | 19 |
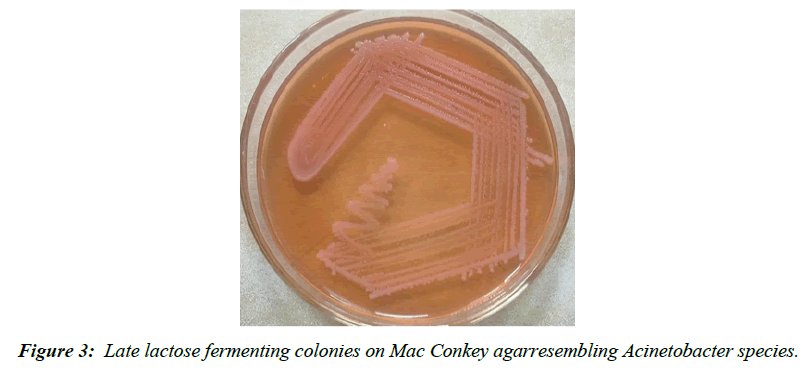
| Acinetobacter baumanii | 5 |
Table 3A: Isolates of gram negative bacilli growth
| Gram positive cocci isolated | Number of isolates |
|---|---|
| Streptococcus pneumoniae | 18 |
| Streptococcus pyogenes | 6 |
| Staphylococcus aureus | 2 |
Table 3B: Isolates of Gram positive cocci.
| Antimicrobial susceptibility pattern | ||||||
|---|---|---|---|---|---|---|
| Klebsiella pneumoniae | Pseudomonas aeruginosa | A. baumanii | Drug total sensitivit y % | |||
| Sensitive | Resistant | Sensitive | Resistant | Sensitive | Resistant | |
| 27 | 0 | 19 | 0 | 5 | 0 | 100% |
| 17 | 10 | 15 | 4 | 3 | 2 | 68.60% |
| 26 | 1 | 17 | 2 | 4 | 1 | 92.15% |
| 25 | 2 | 16 | 3 | 2 | 3 | 84.31% |
| 20 | 7 | 15 | 4 | 3 | 2 | 74.50% |
| 26 | 1 | 15 | 4 | 3 | 2 | 86.27% |
| 5 | 22 | 2 | 17 | 2 | 3 | 17.60% |
Table 4: Antimicrobial susceptibility patterns in isolates of Gram negative bacilli
| Antimicrobial susceptibility pattern | ||||||
|---|---|---|---|---|---|---|
| Antibiotic discs | S. pneumonia | S. pyogenes | S. aureus | |||
| Sensitive | Resistant | Sensitive | Resistant | Sensitive | Resistant | |
| Meropenem | 16 | 0 | 8 | 0 | 2 | 0 |
| Ceftriaxone | 12 | 4 | 4 | 4 | 2 | 0 |
| Levofloxacin | 15 | 1 | 6 | 2 | 2 | 0 |
| Azithromycin | 14 | 2 | 6 | 2 | 1 | 1 |
| Tigecycline | 15 | 1 | 7 | 1 | 2 | 0 |
| Teicoplanin | 13 | 3 | 6 | 2 | 2 | 0 |
| Amoxyclav | 11 | 5 | 3 | 5 | 0 | 2 |
Table 5: Antimicrobial susceptibility patterns in isolates of in Gram positive cocci
| Total drug sensitivity percentage | |||
|---|---|---|---|
| Antibiotic | Gram negative bacilli sensitivity | Gram positive cocci sensitivity | Total sensitivity of the drug |
| Meropenem | 100% | 100% | 100% |
| Ceftriaxone | 68.60% | 69.20% | 68.90% |
| Levofloxacin | 92.15% | 88.46% | 90.48% |
| Piperacillin tazobactum | 84.31% | - | 84.31% |
| Azithromycin | 74.50% | 80.76% | 77.63% |
| Gentamycin | 86.27% | - | 86.27% |
| Amoxyclav | 17.60% | 53.80% | 35.70% |
| Tigecyclin | - | 92.30% | 92.30% |
| Teicoplanin | - | 80.70% | 80.70% |
Table 6: Shows total drug sensitivity percentage
Discussion
Inspite of the multifactorial etiopathogenic nature of AECOPD, infection plays a very important and unique role, impairing not only ventilator function of the lung but also restricting patient daily routine activities.
The present study confirms previous data reporting a role of bacterial infection in AECOPD during hospital admissions for acute exacerbations. Presence of bacterial pathogens was found in 91 % of all admitted patients.
Remarkably, the incidence of positive sputum cultures in 91% of our patient population was quite similar to previous studies [9-11].
The present study also showed a signifigant number of higher males 88% which were chain smokers had COPD infection as compared to females which was 53% were non-smokers. This finding was in conformity with other co-workers who reported similar findings [12]. The male predominance in the current study can be attributed to smoking and COPD being common among females can be due to indoor air pollution while domestic work. Aleemullah, et al. obtained pathogenic organisms from 109 (73.65%) sputum samples. Out of 148 samples processed Gram negative organisms were isolated predominantly 68(62.39%). While Gram positive organisms accounted for 41(37.61%) [13]. Saikat basu, et al. showed 21 positive sputum cultures. Among them, 76.20% were males and 23.80% were females. The prevalence of Gram negative bacteria was 71.42% and Gram positive bacteria was 28.58%. Klebsiella pneumoniae was the commonest isolated (33.33%) followed by P. aeruginosa (19.05%) and Staphylococcus aureus (14.30%) [14].
Chakraborty, et al. isolated pathogenic bacteria from 203 (60.1%) samples. Gram negative bacteria were isolated from 79.8 percent (162/203) while the rest were Gram positive. Klebsiella species was the commonest (49.2%; 100/203) Gram negative isolate from the sputum samples. Among the Gram negative organisms, carbapenem had the highest sensitivity (90.2%) followed by amikacin, ciprofloxacin and piperacillin tazobactam. Linezolid was found to be 100 percent sensitive amongst the Gram positive organisms while both amoxicillin clavulanate and azithromycin showed a rather low sensitivity profile overall [15].
Pradhan K.C. et al, conducted a study on the sputum samples of 100 cases, suffering from chronic obstructive pulmonary disease among them 16 cases had chronic bronchitis. The isolates obtained were Klebsiella spp - 40%, Staphylococcus aureus - 26%, Pseudomonas aeruginosa - 8%, Escherichia coli - 2%, Proteus species - 1% [16].
In the current study Gram negative bacilli 51 out of 77 isolates (66.23%) outnumbered the Gram positive cocci 26 out of 77 isolates (33.76%) in monomicrobial isolates.
In the study by Aleemullah, et al. Saikat basu, et al. Chakraborty, et al. the isolates of Gram negative bacilli (62.39%) were more than the Gram positive cocci (37.61%) which is comparable to the present study [17-19].
In the studies like Pradhan et al, Chawla, et al. Soler, et al. Hariom Sharan et al, Chakraborty, et al. Alemullah, et al. Narayangowda, et al. Jayasimha, et al and Rakhee, et al. had similar findings [20-22]. Another study done by Mahmoud Ahmed et al showed a difference in bacteriological profile showing that there was a predominance of Gram positive cocci than Gram negative bacilli.
In this study, 3 commonest organisms isolated were Klebsiella pneumoniae in 27 cases (35.06%), Pseudomonas aeruginosa in 19 cases (24.67%) and Streptococcus pneumoniae in 18cases (23.37%).
Studies by Chawla et al, Basu et al, Chakraborty, et al. showed that Klebsiella pneumonia (33.33%) was predominantly isolated followed by Pseudomonas aeruginosa (25.9%).
Similarly other studies done by Pradhan, et al. Hariom, et al. Saxena, et al. and Narayanagowda, et al. also suggested the higher isolation of K. pneumoniae as the predominant organism [23].
As the time has passed over, Enterobacteriaceae family organisms and Pseudomonas species have dominated the bacterial flora during acute exacerbations leading to increased severity of the disease. Multidrug resistance and biofilm formation by these organisms have further worsen the situation limiting the therapeutic option.
In the studies conducted by N. Arora, et al. (25.8%), Patel, et al. (32%), Rakesh, et al.(38.1%), Suseela, et al. (22%), Shashibhushan, et al. (42%) and Vishwamber, et al. (45%), the predominant pathogen isolated was Streptococcus pneumoniae [24-29].
Other pathogens isolated were A. baumanii 05 cases (6.49%), Streptococcus pyogenes 06 cases (7.79%) and S. aureus in 02 cases (2.59%).
In the study conducted by Hariom Sharan, et al. of (5.13%), Suseela, et al. 3% is the rate of isolation for A.baumanii comparable to the present study.
Investigation for diagnosis of AECOPD sputum culture is a very simple and useful technique. It offers easy detection, aetiology & complications due to bacteria causing the severe infection. If followed in a well-disciplined way, it can replace the costlier diagnostic methods like immune diffusion. Management of AECOPD can be achieved by the study of antibiogram which helps in creating a correct treatment protocol and hence antibiotics can be started empirically to treat the presumed bacterial infection. The mortality and morbidity can be reduced by finding out resistant pathogens and hence the treatment can be planned according to the organisms. Amino penicillins like ampicillin and amoxicillin were formerly the standard treatment in AECOPD. Due to emergence of resistance among respiratory pathogens, their utility had been limited. Beta lactams, amoxyclav has been used in the study to know about the resistance patterns. Aminopenicillins with beta lactamase inhibitor is a better choice.
Cephalosporin’s demonstrated clinical efficacy and tolerability that can surpass the standard aminopenicillins. A third generation cephalosporin has been used to test its efficacy against pathogens. Quinolones like levofloxacin exhibit a broad spectrum of activity that includes both Gram positive and Gram negative organisms causing AECOPD. It was proven to be better than the other quinolones in treating Pseudomonas infection.
Macrolides are the most potent drugs used for treating sputum related infections Azithromycin has been used to see its efficacy. The newer and powerful drugs available are carbapenems and the drug used is meropenem.
The antibiotic meropenem, had 100% sensitivity to all the isolates tested was this was comparable to the study done by Chakraborty, et al. were carbapenems show 90.2% sensitivity. In the study conducted by Rakhee, et al imipenem had 85% sensitivity [22] In our study 3rd generation cephalosporin used was ceftriaxone which showed a sensitivity of 68.9%. In his study, Shashibhushan, et al. stated that ceftriaxone is the most effective drug [28]. In his study, Vishwambhar, et al. isolated Streptococcus pneumonia which was 100% sensitive to cephalosporins, a little higher than the present study [29].
In his study Chakraborty, et al. had 37% of sensitivity rate for ceftriaxone which is much less compared to the present study [15].
In the present study quinolones used were levofloxacin which showed a sensitivity of 90.48%., comparable to study by Vishwambhar, et al. which showed 89% efficacy with levofloxacin and 80% sensitivity to ciprofloxacin. Studies by Chakraborty, et al and Kumar, et al have efficacy of 62% and 76.9% for ciprofloxacin which are too low [30,31].
Much lower rates of sensitivity to seen with Narayanagowda, et al. with 54.55% and Saxena, et al. with 25.53% for ciprofloxacin and 22.34% for levofloxacin.
Periodic isolation, identification and resistant status of pathogens responsible for AECOPD will help us formulate appropriate treatment protocol and this will be of immense use in reducing mortality and morbidity besides reducing the volume of antibiotics and development of resistance to antibiotics. The protective host immune responses that develop after exacerbation needs to be characterized to facilitate vaccine development. Further, the interaction among different etiologic factors such as environment, bacteria, viruses and atypical pathogens needs to be better understood to treat exacerbations and develop novel as well as cost effective preventive and therapeutic strategies.
Conclusion
There have been significant advances in our understanding of aetiology of acute exacerbations of COPD (AECOPD). Frequent exacerbations appear to be associated with worsening health outcomes and effort should focus on prompt and effective treatment of each episode. Bacterial pathogens, mostly Gram negative bacilli are found to be the chief etiological agents in AECOPD. Antibiotic therapy should be initiated early depending on the culture. Susceptibility patterns of that particular region should be determined to prevent antibiotic resistance and to decrease health costs. However treatment options are limited and further research is needed to clarify the mechanisms that commence and sustain exacerbations and to identify new therapeutic agents. Better and more specific approaches to boost immune competence are currently under study.
References
- Vijayan V.K et al. Chronic obstructive pulmonary disease” Indian J Med Res. 2013; 137(2):251-269
- Crofton, Douglas. Chronic Bronchitis and Emphysema Chapter 23 in Crofton and Douglas’s Respiratory Disease -Vol 1. 5th Edt. Chronic bronchitis and emphysema P.No.616-619.
- Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117(5):398S-401S.
- Jindal SK, Aggarwal AN, Gupta D. Disease and Its Association with Smoking. Indian J Chest Dis Allied Sci. 2001;43:139-471.
- Beasley V, Joshi PV, Singanayagam A, et al. Lung microbiology and exacerbations in COPD. Int J Chron Obstruct Pulmon Dis. 2012:555-69.
- David Ebner et al; History of COPD; lung institute
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. The lancet. 2009;374(9691):733-43.
- Koneman EW, Allen SD, Janda WM, et al. Diagnostic microbiology. The nonfermentative gram-negative bacilli. Philedelphia: Lippincott-Raven Publishers. 1997:253-320.
- Beasley V, Joshi PV, Singanayagam A, et al. Lung microbiology and exacerbations in COPD. Int J Chron Obstruct Pulmon Dis. 2012:555-69.
- Burge S, Wedzicha JA. COPD exacerbations: Definitions and classifications. Eur. Respir. J. 2003;21(41 suppl):46s-53s.
- Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur. Respir. J. 2007;29(6):1224-38.
- Khatun T, Das A, Banik GC, et al. Bacteriological Spectrum of Sputum in Acute Exacerbation of Chronic Obstructive Pulmonary Disease (COPD). J Med. 2022;23(1).
- Aleemullah MF, Krishnamurthy V, Harish M, et al. Bacteriological profile of patients with AECOPD-hospital based study. Int J Curr Microbiol App Sci. 2016;5(4):84-90.
- Basu et al, Epidemiological study of Bacterial Microbiology in AECOPD patients of Kolkatta India, Asian J Pharm. Clin. Res.,vol.6,Issue 1;2013;112-116.
- Chakraborty A, Choudhury A, Debnath J, et al. Bacteriological profile and antibiotic sensitivity pattern in acute exacerbation of advanced cases of chronic obstructive pulmonary disease (COPD). J Evid Based Med Healthc. 2016;3(1):20-3.
- Pradhan KC, Kar S, Nanda BK. Bacteriology of chronic respiratory disease of non-tubercular origin. Indian J Pathol Microbiol. 1979;22(2):133-8.
- Chawla K, Mukhopadhyay C, Majumdar M, et al. Bacteriological profile and their antibiogram from cases of acute exacerbations of chronic obstructive pulmonary disease: A hospital based study. J Clin Diagnostic Res. 2008;2(1):612-6.
- Lin SH, Kuo PH, Hsueh PR, et al. Sputum bacteriology in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease in Taiwan with an emphasis on Klebsiella pneumoniae and Pseudomonas aeruginosa. Respirology. 2007;12(1):81-7.
- Sharan H. Aerobic bacteriological study of acute exacerbations of chronic obstructive pulmonary disease. J Clin Diagnostic Res: JCDR. 2015;9(8):DC10.
- Narayanagowda DS, Golia S, Jaiswal J, et al. A bacteriological study of acute exacerbation of chronic obstructive pulmonary disease over a period of one year.
- Al Ghobain M, Al-Hajjaj MS, Wali SO. Prevalence of chronic obstructive pulmonary disease among smokers attending primary healthcare clinics in Saudi Arabia. Ann Saudi Med. 2011;31(2):129-33.
- Raakhee BT, Sreenivasa RU, Subbarayudu S. Surveillance of Pseudomonas in COPD patients in a tertiary care hospital.
- Saxena S, Ramnani VK, Nema S, et al. Bacteriological profile in acute exacerbation of Chronic Obstructive Lung Disease (AECOPD). Ann Int Med Dent. Res. 2016;2(5):1.
- Arora N, Daga MK, Mahajan R, et al. Chronic Obstructive Airway Disease in a Hospital Based Study. Indian J of Chest Dise and All Sci. 2001;43:157-62.
- Patel AK, Luhadia AS, Luhadia SK. Sputum bacteriology and antibiotic sensitivity pattern of patients having acute exacerbation of COPD in India: A preliminary study. J Pulm Respir Med. 2015;5(1):238.
- Rakesh G, Kasturi T, Yuvarajan S. Bacterial agents causing acute exacerbations in Chronic Obstructive Pulmonary Disease (COPD) patients, their antibiograms to Extended Spectrum Beta-Lactamases (ESBL) production in a tertiary care hospital, India. Int J Curr Microbiol App Sci. 2013;2(11):273-82.
- Suseela KV, Rennis D, Patil S, et al. Bacterial profile and antibiotic susceptibility in chronic obstructive pulmonary disease patients with acute exacerbation: A cross sectional study in a tertiary care hospital. Indian J Microbiol Res. 2016;3(3):317-21.
- Shashibhushan BL, Nagaraja C, Arun BJ, et al. Bacteriological profile and antibiotic sensitivity pattern in sputum culture of chronic obstructive pulmonary disease patients. Int J Adv Med. 2016;3(3):671-4.
- Vishwambhar et al, Gram Positive Bacterial Pathogens in Acute Exacerbation of Copd And Antibiotic Sensitivity Pattern of These Organisms, IJAR. 2016;6(3).
- Kumar et al, Bacteriological Profile of Sputum and their Antibiogram in Cases Of acute Exacerbations Of Chronic Obstructive Pulmonary Disease from A Rural Tertiary Care Hospital, JARBS. 2012; 4(2): 115-119.
- Lin SH, Kuo PH, Hsueh PR, et al. Sputum bacteriology in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease in Taiwan with an emphasis on Klebsiella pneumoniae and Pseudomonas aeruginosa. Respirology. 2007;12(1):81-7.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref