Case Report - Current Pediatric Research (2017) Volume 21, Issue 2
Atypical Kawasaki disease presenting as refractory pneumonia.
Grazia Bossi1, Savina Mannarino2, Valentina Muratore1, Giampiero Beluffi3
1Department of Pediatrics, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
2Pediatric Cardiology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
3Pediatric Radiology Section, Department of Radiodiagnosis, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
- *Corresponding Author:
- Grazia Bossi
Department of Pediatrics
Fondazione IRCCS Policlinico San Matteo
V.le Golgi 19, 27100 Pavia, Italy.
Tel: +39.0382.502804
Fax +39.0382.527549
E-mail: g.bossi@smatteo.pv.it
Accepted date: March 24, 2017
Abstract
We report about a case of atypical Kawasaki disease presenting with persistent fever and pneumonia unresponsive to antibiotic therapy. No other signs or symptoms suggestive for Kawasaki disease were evident at illness onset or thereafter. Despite the puzzling clinical presentation, diagnosis has been made timely. Intravenous immunoglobulin was administered within the first two weeks of fever, along with aspirin, but the child recovered only after steroid therapy. Although a transient coronary dilation was evident at diagnosis, no long term cardiac sequelae were observed. Atypical Kawasaki disease usually affects infants and toddlers and, due to delayed diagnosis and treatment, is strongly associated with an increased risk of permanent heart damage. The lung consolidation as clinical presentation of atypical Kawasaki disease has been described in a very few cases. A high index of suspicion is required in children with refractory pneumonia, fever and persistently high inflammatory markers. In these cases, pediatricians should consider atypical Kawasaki disease as a possible alternative diagnosis, because early clinical suspicion and prompt treatment with intravenous immunoglobulin and aspirin dramatically abate the high risk of permanent coronary damage.
Keywords
Kawasaki disease, Immunoglobulin, Pediatricians.
Introduction
Kawasaki Disease (KD) is an acute, systemic, self-limiting febrile vasculitis of unknown etiology that usually affects infants and young children [1]. The diagnosis of the classic form of KD relies on the presence of high spiking fever lasting at least 5 days, with four of the following clinical signs: unilateral cervical lymphadenopathy, polymorphous exanthema, erythema and swelling of hands/feet, oral mucosal changes, bilateral non-purulent bulbar conjunctivitis [2].
In the so-called atypical KD, that usually affects infants, a part from fever, the clinical signs can be fewer or different from those of the classic disease, so that its diagnosis is more difficult and often delayed [3,4]. If the disease is left untreated, 10-15% of patients develop coronary artery lesions that could be permanent and KD is currently the leading cause of acquired childhood heart disease in western countries [5]. Fortunately, timely treatment with Intravenous Immunoglobulin (IVIG) during the first 10 days of illness, by day 7 if possible, dramatically abates the risk of developing coronary artery aneurysms from 25% to 4% [2,6].
Despite its importance, a timely diagnosis of KD is often a challenge because it still relies on non-specific clinical signs, and no definite laboratory tests have yet been identified. Diagnosis of KD can be even more difficult in patients who do not fulfill the diagnostic criteria. A clinical presentation with less than the required criteria for KD and/or unusual clinical features (“incomplete” or “atypical” KD) is more common in infants younger than 6 months or children older than 5 years of age and very often causes a delay in diagnosis and treatment, that results in an exceeding incidence of coronary aneurysms [7-13].
Due to its systemic nature, multiorgan involvement is possible in both the classic and in the incomplete/atypical forms of the disease, but, in contrast to other vasculitides, lung involvement is very rare, usually asymptomatic and characterized by a wide range of clinical and radiological findings [14-27]. Among the possible clinical presentations of atypical KD, isolated lung involvement is considered a very uncommon feature and almost all the cases reported in literature involve children in the first year of life. [17,23,24]. The largest Italian epidemiology study on KD did not report any case of pneumonia even in the atypical form of the disease [28]. We describe a child with unresolving pneumonia as the main presenting symptom of atypical KD.
Case Report
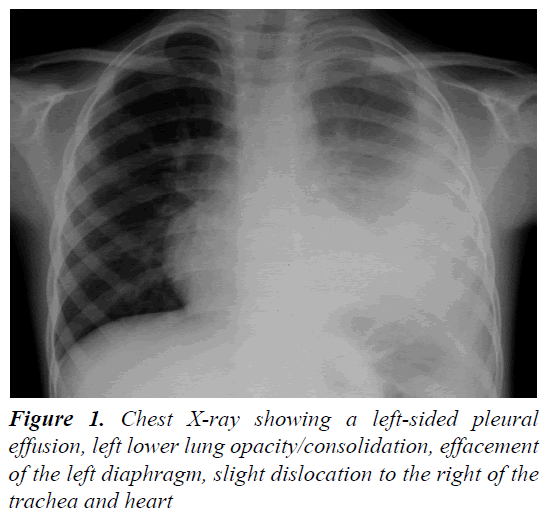
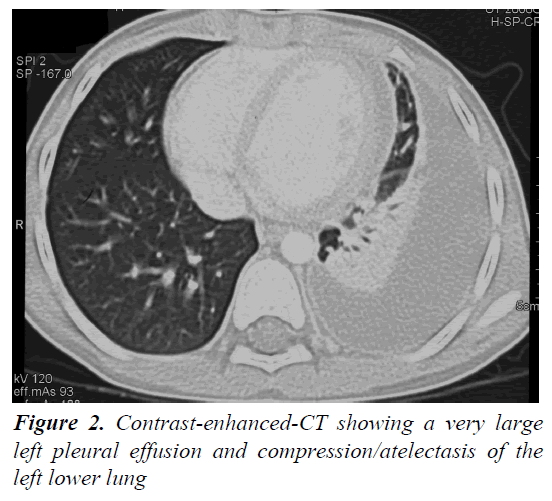
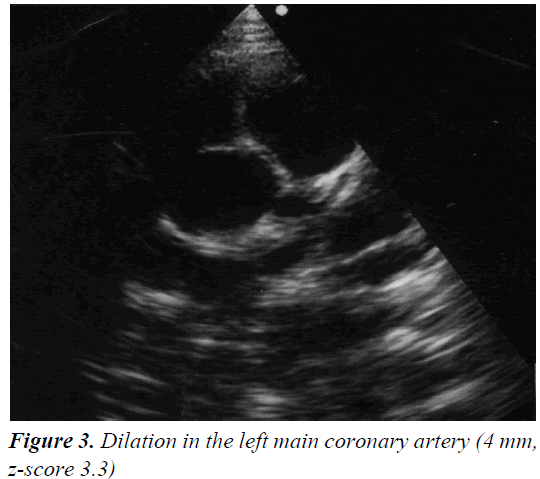
A 4 year and 8 month old, previously healthy, Caucasian boy was admitted due to fever, chest and abdominal pain persisting for three days. On examination he appeared irritable and presented hepatomegaly, dullness on chest percussion and absent breath sounds over the left pulmonary side. Chest radiograph showed the presence of a large left-sided pulmonary consolidation with pleural effusion. (Figure 1) On admission laboratory results were as follows: WBC count 25 × 109/l (PMN 85%), platelets 251 ×109/l, haemoglobin 10.9 g/dl, erythrocyte sedimentation rate (ESR) 93 mm/h, C-reactive protein (CRP) 22 mg/dl, albumin 3.6 g/l, sodium 133 mEq/l. Investigations for malignant, autoimmune and infectious diseases (culture, serology for mycoplasma, Epstein-Barr virus, cytomegalovirus, brucella, rickettsia, Mantoux test) were negative. In the following days, despite large spectrum antibiotic therapy, fever, irritability and chest pain persisted and the child’s conditions worsened. A contrast-enhanced chest Computed Tomography (CT) scan confirmed the presence of the pleural effusion with partial lung collapse, but ruled out lung parenchymal consolidation (Figure 2). The diagnosis of atypical KD was considered on day 9 of illness, based on persistent fever, pleural effusion, markedly elevated inflammatory laboratory markers (ESR 95 mm/h, CRP 20 mg/dl, WBC 28 × 109/l, PMN 88%), slightly increasing platelets count (474 × 109/l), sterile pyuria (26 WBC/μL) in urine sediment. Echocardiography showed a dilated left main coronary artery (LMCA; 4 mm; z-score 3.3 according to Parameter (z) Boston Circ 2007); right coronary artery was measuring 2.8 mm (RCA;z-score 1.97) and the left anterior descending artery was measuring 2.4 mm (z-score 1.04) (Figure 3). Two doses of IVIG were administered, together with aspirin (20 mg/kg per day), without any appreciable result. On day 12 of illness the child was still febrile, platelets had increased to 1.010 × 109/l, anemia persisted and mild hyper transaminasemia became evident (aspartate aminotranferase 65 mU/ml, alanine aminotransferase 119 mU/ml). Steroids were administered intravenously (30 mg/kg per day, for three consecutive days) resulting in immediate defervescence, clinical and radiological improvement and progressive normalization of laboratory findings. The echocardiografic findings at two weeks were unchanged, while one month after the diagnosis LMCA appeared reduced (3 mm, z-score 0.93) with LAD and RCA invariated (2.3 and 2.6 mm, z-score 0.71 and 1.42). Repeat echocardiogram performed two months after diagnosis showed no evidence of dilation in LMCA (2.6 mm, z-score -0.02) with normal LAD and RCA (both 2.2 mm, z-score 0.38 and 0.32). Nine years later the boy is in good conditions and without any cardiac sequela.
Discussion
One male patient with prolonged fever, clinical signs of refractory pneumonia, eventually diagnosed as having atypical KD, is described in this report. No other symptoms or signs suggestive for KD were evident, neither at the onset of disease, nor when the diagnosis was made or during the follow-up. The first line therapy with two doses of IVIG, although it was administered before the 10th days, failed to achieve remission of clinical symptoms and reduction of the inflammatory markers. After the second line treatment with steroids, complete remission of clinical and radiological findings, along with normalization of the laboratory parameters were obtained. A coronary dilation was observed in the acute phase of the disease but it proved to be transient with complete normalization of coronary diameter within two months of diagnosis.
The present case is noteworthy for at least two reasons.
The first noteworthy feature of the case is the age of the patient, who was about 5 year old. In literature atypical KD is reported to be more common in children younger than 6 months. Our experience demonstrates that atypical KD must be suspected regardless of age.
The second noteworthy feature of the case is the clinical presentation of atypical KD as an apparent unresolving lobar pneumonia and prolonged fever, without any other clinical symptom or sign of the disease.
In literature there are very few reports about lung involvement in KD and in almost all cases KD followed respiratory infection as its complication. In our case lung involvement was the first and the main clinical feature of atypical KD.
The diagnosis of KD was initially suspected because persistent fever and suggestive changes in laboratory markers and, ultimately, was confirmed when coronary artery changes were detected on echocardiogram. The child was promptly and successfully treated with IVIG and aspirin as the first line and then with steroids, and the coronary dilation resolved.
Our experience shows that, in front of any case of protracted fever unresponsive to antibiotic therapy and inflammatory markers persistently high, also when signs and symptoms of pulmonary involvement are the prominent features of the clinical picture, the possible diagnosis of atypical KD should be considered, preferably before the evidence of coronary damage.
Moreover, our case confirms that, by limiting the delay in IVIG treatment (early in the second week of fever), possibly with the addition of the steroid, persistent heart damage can be successfully avoided, also when coronary artery changes are already evident, because they can be transient and reversible with therapy.
In conclusion, our experience underscore the need to maintain a high index of suspicion for atypical KD in case of infants and older children presenting with continuous fever and pleural effusion mimicking refractory pneumonia, coupled with increased inflammatory parameters and high platelet count in the second or third week of illness. In these cases, it is mandatory to perform periodic cardiac echocardiogram to rule out possible transient or persistent cardiac damage. The potential life-threatening cardiovascular complication of KD not properly and timely treated, emphasizes the importance of early diagnosis and treatment, especially in case of atypical disease.
References
- Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 1967; 16: 178-222.
- Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol 2016 114: 1708-1733.
- Cimaz R, Sundel R. Atypical and incomplete Kawasaki disease. Best Pract Res Clin Rheumatol 2009; 23: 689-697.
- Yu JJ. Diagnosis of incomplete Kawasaki disease. Korean J Pediatr 2012; 55: 83-87.
- Taubert A, Rowley AH, Shulman ST. Seven-year national survey of Kawasaki disease and acute rheumatic fever. Pediatr Infect Dis J 1994; 13: 704-708.
- Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous G globulin. N Engl J Med 1986; 315: 341-347.
- Anderson MS, Todd JK, Glode MP. Delayed diagnosis of Kawasaki syndrome: An analysis of the problem. Pediatrics 2005; 115: e428-433.
- Chang FY, Hwang B, Chen SJ, et al. Characteristics of Kawasaki disease in infants younger than six months of age. Pediatr Infect Dis J 2006; 25: 241-244.
- Juan CC, Hwang B, Lee PC, et al. The clinical manifestations and risk factors of a delayed diagnosis of Kawasaki disease. J Chin Med Assoc 2007; 70: 374-379.
- Minich LL, Sleeper LA, Atz AM, et al. For the pediatric heart network investigators. Delayed diagnosis of Kawasaki disease: What are the risk factors. Pediatrics 2007; 120: 1434-1440.
- Pannaraj PS, Turner CL, Bastian JF, et al. Failure to diagnose Kawasaki disease at the extremes of the pediatric age range. Pediatr Infect Dis 2004; 23:789-791.
- Burns JC, Wiggins JW, Toews WH, et al. Clinical spectrum of Kawasaki disease in infants younger than 6 months of age. J Pediatr 1986; 109: 759-763.
- Burns JC, Glode MP. Kawasaki syndrome. Lancet 2004; 364:533-544.
- De Maddi F, Cinelli R, Rigante D, et al. Lung parenchymal consolidation as an uncommon presentation and cause of delayed diagnosis in atypical Kawasaki syndrome. Rheumatol Intern 2009; 29: 1373-1376.
- Do YS, Kim KW, Chun JK, et al. Risk factor for failure of initial intravenous immunoglobulin treatment in Kawasaki disease. Korean Circ J 2010; 4085: 239-242.
- Diana MC, Villa G, Gattorno M, et al. Sudden death in an infant revealing atypical Kawasaki disease. Pediatr Emerg Care 2006; 22:35-37.
- D?Souza S, Khubchandani RP, Shetty AK. Kawasaki disease presenting with hemorrhagic pleural effusion. J Trop Pediatr 2005; 52: 299-301.
- Falcini F, Vitale A, La Torre F, et al. Refractory pneumonia and high fever. Lancet 2009; 373: 1818.
- Itani MH, Zakhour RG, Haddad M, et al. Prolonged fever with pulmonary nodules in a 4 month old baby. Pediatric Infect Dis J 2010; 29: 784-788.
- Managoli S, Chaturvedi P. Kawasaki disease with coronary artery aneurysm and symptomatic pneumonia. Indian Pediatrics 2003; 40: 1106-1107.
- Manganelli P, Fietta P, Carotti M, et al. Respiratory system involvement in systemic vasculitides. Clin Exp Rheumatol 2006; 24: S48-59.
- Picazo M, Montes JGF, Fabrega MR, et al. Radiologic findings in the lungs of patients with Kawasaki disease. Radiologia 2006; 48: 14-18.
- Palmer Al, Walker T, Smith JC. Acute respiratory distress syndrome in a child with Kawasaki disease. South Med J 2005; 98: 1031-1033.
- Sengler C, Gaedicke G, Wahn U, et al. Pulmonary symptoms in Kawasaki disease. Ped Infect Dis J 2004; 23: 782-784.
- Uziel Y, Hashkes PJ, Kassem E, et al. Unresolving pneumonia as the main manifestation of atypical Kawasaki disease. Arc Dis Child 2003; 88: 940-942.
- Vojonow JA, Schanberg L, Sporn T, et al. Pulmonary complications associated with Kawasaki disease. J Pediatr 2002; 140: 786-787.
- Yavuz T, Nisli K, Yilmaz C, et al. Large pleural effusion necessitates chest tube drainage in a patient with Kawasaki disease. J Pediatr Child Health 2007; 43:191-192.
- Falcini F, Cimaz R, Calbri GB, et al. Kawasaki?s disease in northern Italy: A multi-center retrospective study of 250 patients. Clin Exp Rheumatol 2002; 20: 421-426.