Research Article - Biomedical Research (2017) Volume 28, Issue 15
Association of serum Aβ1-42, cystatin C and UA with parkinson's disease
Jing Lei, Jianhua Ma, Ruobing Liang and Xiaoning Zhang*
Department of Neurology, the First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, P. R. China
- *Corresponding Author:
- Xiaoning Zhang
Department of Neurology
The First Affiliated Hospital of Xinjiang Medical University
Urumqi, Xinjiang, P. R. China
Accepted on July 10, 2017
Abstract
Objective: This study is to investigate the association of β-amyloid 1-42 (Aβ1-42), Cystatin C (Cys C), Uric Acid (UA) with Parkinson’s disease (PD).
Methods: A total of 108 PD patients and 108 healthy individuals were enrolled. The serum levels of Aβ1-42, Cys C and UA were measured. The Operating Characteristic Curve (ROC) was drawn, and then the Area Under the Curve (AUC), 95% Confidence Interval (CI), sensitivity and specificity were calculated.
Results: Serum Aβ1-42 and UA levels in PD patients were significantly lower than those in healthy controls. Serum Cys C level in PD patients was significantly higher. The AUC of Aβ1-42 was 0.644, 95% CI was 0.570~0.718, diagnostic sensitivity and specificity were 52.8% and 74.1% and the cut-off point was 0.912 μg/L. The AUC of UA was 0.633, 95% CI was 0.557~0.708, sensitivity and specificity were 65.7% and 64.8% and the cut-off point was 281 μmol/L. Serum UA in male PD patients was significantly higher than that in female PD patients. Serum Cys C in PD patients were significantly higher than that in healthy controls. Serum Aβ1-42 was negatively correlated with HY grade. Serum Cys C was positively correlated with HY grade. Serum UA was negatively correlated with HY classification, UPDRS score and the III scores.
Conclusion: Serum Aβ1-42 and UA may contribute to PD diagnosis. Cystatin C may be involved in the development of PD and be helpful in assessing the PD severity.
Keywords
Parkinson’s disease (PD), Serum Aβ1-42, Serum cystatin C (Cys C), Serum uric acid (UA).
Introduction
Parkinson’s disease (PD) is a degenerative disorder of the central nervous system with slow progression, which often occurs in the elderly and is incurable. Generally, when PD are diagnosed based on specific clinical symptoms, 50%~70% irreversible degeneration of the Substantia Nigra pars compacta (SNc) neuronal death have occurred already in the brain [1].
The causes of PD are not clear so far. Studies have shown that oxidative stress, mitochondrial dysfunction, immune inflammation, and neurotrophic factor reduction co-participate in the development and progression of PD [2-4]. The β- amyloid 1-42 (Aβ1-42) is a biomarker for PD, which induces the degeneration of the dopamine (DA) neurons and changes the morphology of DA neurons in a dose-dependent manner [5-7]. Moreover, Aβ deposition in the cerebral cortex causes the oxidative stress, the activation of glial cells, and the induction of inflammatory cascade [8]. Cystatin C (Cys C) has been investigated as a reliable indicator of renal function [9]. More recently, Cys C has been reported to be correlated to neurological diseases, such as the cerebrovascular disease and the Alzheimer’s disease [10,11]. Cys C is involved in the inflammatory response through regulating the cysteine protease activity, while the nerve inflammation plays an important role in the development of PD [12-14]. Therefore, Cys C may be associated with PD occurrence. The blood Uric Acid (UA) is an antioxidant and free radical scavenging factor [15]. Cells under oxidative stress are more susceptible to the injuries caused by free radicals and metal ions [16]. Based on the literature review, we hypothesize that serum content of Aβ1-42, Cys C and UA may be closely related to the pathogenesis of PD. To test this hypothesis, the relationship of Aβ1-42, Cys C and UA with PD was investigated in this study.
Materials and Methods
Participants
A total of 108 PD patients who were admitted during May 2014 and Dec 2015 to the Department of Neurology of the First Affiliated Hospital of Xinjiang Medical University were enrolled. At the same time period, 108 healthy participants with matched sex, age, and ethnicity were also enrolled and served as the control group. PD was diagnosed according to the UK Parkinson’s Disease Society Brain Bank criteria. Besides, the exclusion criteria of PD patients were: (1) Patients with other dementia (such as Alzheimer’s disease and vascular dementia) or with other conditions (such as hypertension, diabetes, gout, thyroid disease, liver cirrhosis, glomerulonephritis, renal failure, heart failure, blood diseases, systemic infectious disease); (2) Patients received surgery or radiotherapy or chemotherapy in recent months; (3) Patients with a history of trauma; (4) Patients took antibiotics or potassium-sparing diuretics.
The inclusion criteria for healthy participants were: No PD or family PD history; good health without other diseases or recent discomfort and the physical examination results were normal. Healthy participants who received surgery, radiotherapy and chemotherapy in recent months, or had a history of trauma, or took antibiotics or potassium-sparing diuretics, or mental abnormalities were excluded.
The prior written and informed consents were obtained from every patient. The study was approved by the ethics review board of the First Affiliated Hospital of Xinjiang Medical University.
Grouping criteria
According to the clinical Hoehn-Yahr (HY) classification, also known as the Unified Parkinson’s Disease Assessment Rating Scale (UPDRS) Part V, PD patients were grouped into earlier PD (HY grade<3; n=64) and advanced PD (HY grade ≥ 3; n=44). Besides, all the participants were divided into different groups based on their different gender and PD (Table 1). There was no significant difference between different groups in age and gender composition (p>0.05).
| PD patients | Healthy participants | p value | |
|---|---|---|---|
| Age (y, mean ± SD) | 64.79 ± 9.51 | 64.1 ± 8.45 | 0.58 |
| Gender | |||
| Male | 62 (57.4) | 59 (54.6) | 0.68 |
| Female | 46 (42.6) | 49 (45.4) |
Table 1. The basic information of PD patients and healthy participants.
General data collection
Patients who were preliminary diagnosed as PD were required to fill a form to collect their demographic information, medical history and family history. PD patients in the “open period” (symptoms relieved) received UPDRS grading. UPDRS includes five parts: Part I reflects the mental, behavioural and emotional condition; Part II evaluates the daily activities; Part III assesses the movement of PD patients. Each item in the above three parts contains five levels, grading from 0 to 4 points. The higher score indicates the more severe PD symptoms. Part IV of UPDRS reveals the complications in order to evaluate the dyskinesia’s, clinical fluctuations, and other complications. For part IV, the total score is 23, and higher score indicates the symptom aggravation. Part V (H-Y grading) involves 8 grades, which is used to evaluate the PD progression. The higher score indicates the more serious degree of PD progression.
Blood sample collection and measurement of Aβ1-42, UA and Cys C
The fasting peripheral venous blood samples of all participants were collected. After centrifugation at 3000 r/min for 10 min, the serum was isolated. Aβ1-42 level in serum was tested using human serum Aβ1-42 ELISA assay kit (China Huamei Biological Engineering Co., Ltd. Wuhan) according to the instruction. Serum UA was determined using the peroxidase method on the Beckman CX4 automatic biochemical analyzer (Beckman Coulter, Inc.). Cys C was measured using latex-enhanced immunoassay turbidimetric method on the Beckman CX4 automatic biochemical analyzer (Beckman Coulter, Inc.).
Statistical analysis
SPSS 17.0 statistical software was used for data analysis. Data of age, serum Aβ1-42, Cys C and UA level, and UPDRS score are shown as mean ± SD. Independent t-test was used to conduct comparisons between two groups. Comparisons among multiple groups were analyzed using one-way ANOVA. If the difference was statistically significant, LSD method was further performed to study the difference between two groups. In addition, data of gender and ethnicity are shown as a relative composition ratio (%). Chi-square test was used to analyze these data. Spearman rank correlation analysis was performed. ROC was plotted. The area under the curve (AUC), 95% CI, cut-off point, sensitivity and specificity were calculated. P<0.05 indicated a statistically significant difference.
Results
The serum Aβ1-42, Cys C and UA levels in healthy and PD participants
As shown in Table 2, the serum Aβ1-42 and UA levels in PD patients were 1.02 ± 0.47 μg/L and 261.51 ± 81.68 μmol/L, respectively, significantly lower than that in the healthy controls, which were 1.18 ± 0.45 μg/L and 293.95 ± 76.45 μmol/L (p<0.05). While, the serum Cys C content in PD patients was 0.98 ± 0.22 mg/L, significantly higher than the 0.88 ± 0.16 mg/L in the control group. This result indicates that the serum Aβ1-42 and UA level are significantly lower in PD patients than healthy controls, however, the serum content of Cys C is higher in PD patients than that in the healthy controls.
| Indicator (Mean ± SD) |
PD patients | Healthy participants | p value |
|---|---|---|---|
| Aβ1-42 (µg/L) | 1.02 ± 0.47 | 1.18 ± 0.45 | 0.01 |
| Cys C (mg/L) | 0.98 ± 0.22 | 0.88 ± 0.16 | <0.001 |
| UA (µmol/L) | 261.51 ± 81.68 | 293.95 ± 76.45 | 0.003 |
Table 2. The serum Aβ1-42, Cys C and UA levels in healthy and PD participants.
The diagnostic value of serum Aβ1-42, Cys C and UA for PD
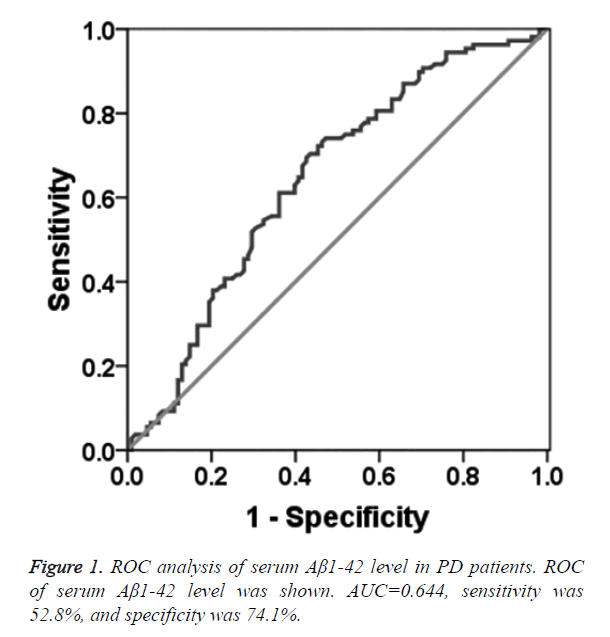
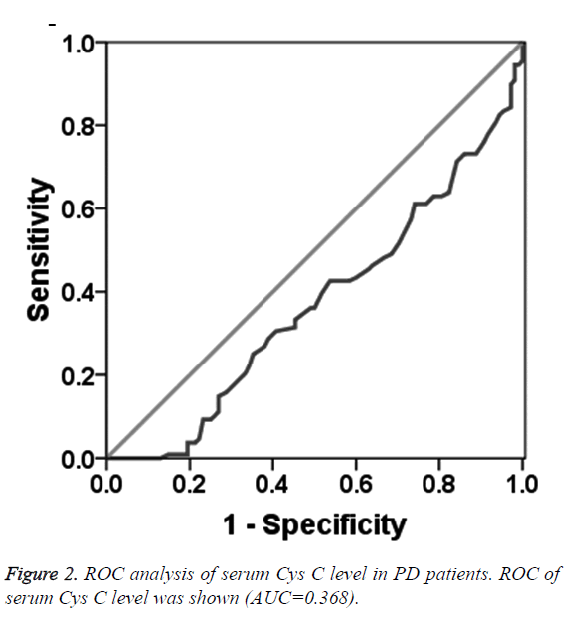
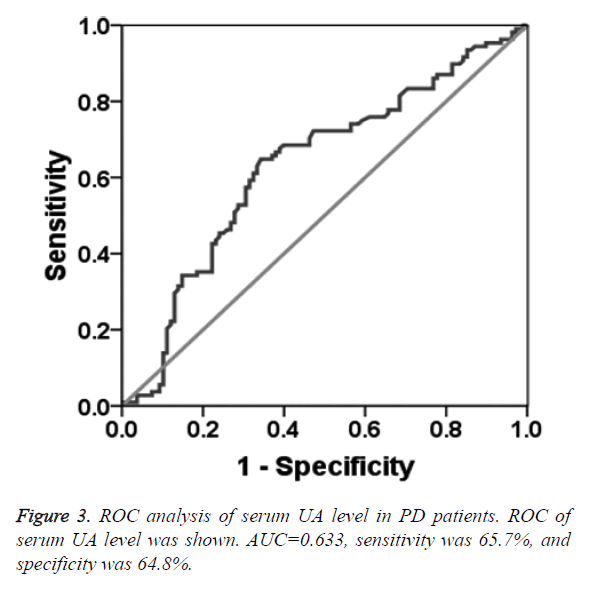
To determine the diagnostic value of Aβ1-42, Cys C and UA for PD, the ROC curve was plotted. The AUC of serum Aβ1-42 was 0.644 (95% CI: 0.570~0.718, p=0.000, Figure 1). The diagnostic sensitivity and specificity of serum Aβ1-42 were 52.8% and 74.1%, respectively, and its corresponding cut-off was 0.912 μg/L. While, the AUC of serum Cys C was 0.368 (95% CI: 0.295~0.442, p=0.001, Figure 2), suggesting that serum Cys C was not able to diagnose PD. Additionally, ROC of blood UA showed that the AUC was about 0.633 (95% CI: 0.557~0.708, p=0.001, Figure 3). The diagnostic sensitivity and specificity were 65.7% and 64.8%, respectively, and its corresponding cut-off was 281 μmol/L. This result indicates that the serum Aβ1-42 and UA level are valuable for PD diagnosis.
The serum content of Aβ1-42, Cys C and UA in male and female PD patients
PD patients were grouped according to the gender. Then, the differences in serum levels of Aβ1-42, Cys C and UA between male and female PD patients were compared. The serum UA levels in male PD patients was 288.05 ± 75.59 μmol/L, significantly higher than that in female PD patients (225.73 ± 76.42 μmol/L) (p<0.001). However, the comparison of serum Aβ1-42 and Cys C level in male and female PD patients indicated no statistical difference (p>0.05, Table 3). This result indicates that the serum UA level rather than the level of serum Aβ1-42 and Cys C may be correlated to the gender of PD patients.
| Indicators (Mean ± SD) |
Male PD patients | Female PD patients | p value |
|---|---|---|---|
| Aβ1-42 (µg/L) | 1.05 ± 0.44 | 0.98 ± 0.51 | 0.45 |
| Cys C (mg/L) | 1.01 ± 0.21 | 0.94 ± 0.22 | 0.06 |
| UA (µmol/L) | 288.05 ± 75.59 | 225.73 ± 76.42 | <0.001 |
Table 3. The serum content of Aβ1-42, Cys C and UA in male and female PD patients.
The correlation analysis between UPDRS score and serum Aβ1-42, Cys C, UA levels in PD patients
To determine the correlation of UPDRS score with serum Aβ1-42, Cys C, UA levels in PD patients, the Spearman rank correlation analysis was performed. As shown in Table 4, the serum Aβ1-42 level was negatively correlated with HY stage (p<0.05), suggesting that the more severe HY classification is associated with the lower serum Aβ1-42 levels. There was no correlation between UPDRS score (total and Part I~IV score) and serum Aβ1-42 level in PD patients (p>0.05). Additionally, the serum Cys C level was positively correlated with HY stage (p<0.05), indicating that a more severe HY grade showed a higher Cys C level. Similarly, no correlation between serum Cys level and UPDRS score (total and Part I~IV score) (p>0.05) was found either. Additionally, the serum UA level was negatively correlated with HY grade (p<0.05). Thus, a more severe HY classification indicated a lower serum UA level. Besides, the serum UA level was negatively correlated to the UPDRS total score and the Part III score, suggesting that a higher score was related with a lower serum UA level (p<0.05). However, there was no significant correlation between serum UA level with other UPDRS scores (I, II and IV) (p>0.05).
| Indicators | Serum Aβ1-42 (μg/L) | Serum Cys C (mg/L) | Serum UA (μmol/L) | |||
|---|---|---|---|---|---|---|
| Rs value | P value | Rs value | P value | Rs value | P value | |
| UPDRS total score | 0.001 | 0.99 | 0.02 | 0.88 | -0.19 | 0.04 |
| UPDRS Part I score | -0.11 | 0.27 | 0.09 | 0.36 | -0.08 | 0.43 |
| UPDRS Part II score | -0.09 | 0.34 | 0.13 | 0.18 | -0.11 | 0.28 |
| UPDRS Part III score | 0.1 | 0.31 | -0.04 | 0.68 | -0.22 | 0.03 |
| UPDRS Part IV score | -0.16 | 0.1 | -0.11 | 0.26 | -0.17 | 0.08 |
| UPDRS Part V score (H-Y grade) | -0.25 | <0.001 | 0.25 | <0.001 | -0.23 | 0.001 |
Table 4. The correlation analysis of serum Aβ1-42, Cys C and UA levels in PD patients and the UPDRS score.
Discussion
The present research investigated the serum Aβ1-42, Cys C and UA levels in PD patients, and further demonstrated the role of these indicators for PD diagnosis and progression. Amyloid Precursor Protein (APP) was prone to produce Aβ after digestion by β- and γ-secretase enzyme. Cheng et al. demonstrated that the excessive accumulation of α-Synuclein (α-Syn) contributed to the synaptic damage and dysfunction. Soluble Aβ was more likely to induce synaptic damage and dysfunction than fibrillar Aβ [16]. The hyper-phosphorylation and aggregation of α-Syn were closely related to Aβ1-42 [16]. Clinton et al. also confirmed the interaction of Aβ with α-Syn, and this interaction could increase the synaptic damage, leading to PD aggravation [17]. In the present study, serum Aβ1-42 in PD patients was significantly lower than that in the healthy control group, suggesting the diagnostic value of serum Aβ1-42 for PD diagnosis. Besides, Siderowf showed that the Aβ1-42 levels in the Cerebrospinal Fluid (CSF) of PD patients reduced by 15%~20% with comparison to the healthy controls [18]. Aasly et al. demonstrated that the reduction of Aβ1-42 in PD patients was related to the dysfunction of striatal dopamine [19].
Previous studies mainly reported the level of Aβ1-42 in CSF. Maetzler et al. reported that the CSF Aβ1-42 levels in advanced PD patients were much lower than that in the healthy controls [20]. Consistently, our study showed that the serum Aβ1-42 levels in PD patients were much lower than that in healthy participants. Abnormal aggregation of Aβ and α-Syn occurs in the cortex and hippocampus as PD progresses. The Aβ oligomerization often result in blood-brain barrier damage and enhances the inward transport, which leads to the reduction of soluble Aβ in CSF [21]. This may be one of the reasons that Aβ1-42 level was reduced in patient serum. In addition, there was no correlation between serum Aβ1-42 levels and UPDRS scores in this study. Presumably, this may be because that brain lesions of PD patients occur in different brain regions. While, Shi et al. reported the Aβ1-42 level in the CSF of PD patients was positively correlated with its severity [22]. It is inconsistent with our findings, which may be a result of the small sample size of advanced PD patients in our study. Therefore, further follow-up study will perform to confirm our conclusion. Animal experiments show that when brain Cys C level declines, and less microglia can be activated by impaired Dopaminergic (DA) neurons and inflammatory responses is inhibited, leading to cell damage [23]. Dutta et al. found that the release of Cys C from damaged DA neurons involved in the induction of activated microglia, which further aggravated the neuronal loss of DA neurons [24].
In addition, our study suggested that serum Cys C level was not sufficient to identify or diagnose PD. However, it was positively correlated with HY classification. Moreover, serum Cys C level in PD patients was significantly higher than that in healthy controls. Increased Cys C level was positively correlated with PD progression, suggesting that serum Cys C might contribute to the early screening for PD. Previous studies in rats demonstrated that injection of 6-Hydroxy-Dopamine (6- OHDA) as well as 1-Methyl-4-Phenyl-1, 2, 3, 6- Tetrahydropyridine (MPTP) into the nigrostriatal brain led to an effective dopaminergic neuronal degeneration [25,26]. Also, 6-OHDA could up-regulate the Cys C expression in the dopaminergic neurons. Meanwhile, our results showed gender had little effect on serum Cys C levels of PD patients
Besides, our results showed that the serum UA level in PD patients was significantly lower than that in the healthy control group, suggesting that serum UA might contribute to PD diagnosis. Also, we found that serum UA level was related to gender of PD patients. Additionally, our data showed serum UA level in PD patients was negatively correlated with H-Y grade in PD patients, and the serum UA level in the early PD patients was significantly lower than that in the control group. Meanwhile, serum UA levels in PD patients were negatively correlated to the UPDRS total and part III score. A possible explain is that excessive free radicals, together with low UA levels, weaken the antioxidant effect and damage the membranes, causing disorders in the mitochondrial electron transport and neuronal metabolism and eventually leading to brain damages [27]. Besides, low level of UA was not able to chelate iron, which resulted in more DA neurons exposed to iron environment, leading to cell death and the aggravation of the PD athletic performance [27].
In this study, the serum Aβ1-42 content in PD patients and healthy participants were tested by ELISA. The results showed that serum Aβ1-42 level in PD patients was lower than that in healthy controls, and the area under ROC curve of serum Aβ1-42 was 0.644 (95% CI: 0.570~0.718, P=0.000). The diagnostic sensitivity and specificity were 52.8% and 74.1%, respectively. According to the principle of maximum sensitivity and specificity, the corresponding threshold was 0.269 g/L, indicating that the serum Aβ1-42 as a potential marker for PD diagnosis. For patient with serum Aβ1-42 level of 0.912 μg/L, 52.8% PD patients were diagnosed and 74.1% non-PD patients were excluded. Similarly, the serum UA level was also significantly lower in PD patients than that in healthy participants. The area under the ROC curve of serum UA for PD diagnosis was 0.633 (95% CI: 0.557~0.708, P=0.001), and the diagnostic sensitivity and specificity were 65.7% and 64.8%, respectively. According to the principle of maximal sum of sensitivity and specificity, the threshold of serum UA level was 281 μmol/L, suggesting that the serum UA level may contribute to the PD diagnosis. Additionally, 65.7% PD patients were diagnosed and 64.8% non-PD patients were excluded, if the serum UA level was 281 μmol/L. The serum Cys C level in PD patients was significantly higher than that in the healthy participants. Further analysis of the ROC curve of the serum Cys C revealed that the area under the ROC curve was 0.368, indicating that serum Cys C was not able to diagnose PD. Considering the small size of patient sample, further study still needs to conduct. In clinical study, the serum indictors should be combined with the prevalence rate of PD, especially in the process of PD screening. The sensitive indicators should be used to find more possible PD patients, and then further clinical examination, including symptoms, physical examination and relevant scales should be applied to exclude the false positive PD patients.
In summary, our study revealed that serum Aβ1-42 and UA levels were significantly lower while the serum Cys C level was significantly higher in PD patients than in the healthy controls. The serum Aβ1-42, Cys C and UA may be related to the pathogenesis of PD and be potential markers for PD diagnosis, particularly, serum Cys C level may be valuable for assessing PD severity.
Acknowledgement
This study was supported by High-tech Research and Development Project of Science and Technology Department of Xinjiang Uygur Autonomous Region (grant No. 201417101).
Disclosure
All authors have read the journal’s policy on disclosure of potential conflicts of interest and have none to declare.
All authors have read the journal’s authorship agreement that the manuscript has been reviewed and approved by all named authors.
References
- Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson's disease with (18F) dopa PET. J Neurol Neurosurg Psychiatry 1998; 64: 314-319.
- Lin WC, Chou KH, Lee PL, Huang YC, Tsai NW, Chen HL, Cheng KY, Wang HC, Lin TK, Li SH, Chen MH, Lu CH, Lin CP. Brain mediators of systemic oxidative stress on perceptual impairments in Parkinson's disease. J Transl Med 2015; 13: 386.
- Wang W, Wang X, Fujioka H, Hoppel C, Whone AL, Caldwell MA, Cullen PJ, Liu J, Zhu X. Parkinson's disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat Med 2016; 22: 54-63.
- Gustot A, Gallea JI, Sarroukh R, Celej MS, Ruysschaert JM, Raussens V. Amyloid fibrils are the molecular trigger of inflammation in Parkinson's disease. Biochem J 2015; 471: 323-333.
- Millard SP, Lutz F, Li G, Galasko DR, Farlow MR, Quinn JF, Kaye JA, Leverenz JB, Tsuang D, Yu CE, Peskind ER, Bekris LM. Association of cerebrospinal fluid Abeta42 with A2M gene in cognitively normal subjects. Neurobiol Aging 2014; 35: 357-364.
- Bian H, Van Swieten JC, Leight S, Massimo L, Wood E, Forman M, Moore P, de Koning I, Clark CM, Rosso S, Trojanowski J, Lee VM, Grossman M. CSF biomarkers in front temporal lobar degeneration with known pathology. Neurol 2008; 70: 1827-1835.
- Zhang W, Wang Y, Hong J-S. To explore the mechanism of Alzheimer's disease in the clinical features of Parkinson's disease. Chin J Neurol 2008; 15: 334-338.
- Alam Q, Alam MZ, Mushtaq G, Damanhouri GA, Rasool M, Kamal MA, Haque A. Inflammatory process in Alzheimer's and Parkinson's diseases: central role of cytokines. Curr Pharm Des 2016; 22: 541-548.
- Ayub S, Khan S, Ozair U, Zafar MN. Cystatin C levels in healthy kidney donors and its correlation with GFR by creatinine clearance. J Pak Med Assoc 2014; 64: 286-290.
- Zang L, Fu P, Liu F, Wu M, Huang YQ, Li L, Zang J. The correlation of serum cystatin C level with the severity of carotid atherosclerosis in patients with type 2 diabetes mellitus. Sichuan Da Xue Xue Bao Yi Xue Ban 2012; 43: 882-887.
- Ghosh D, Brewer GJ. Externalcys/cySS redox state modification controls the intracellular redox state and neurodegeneration via Akt in aging and Alzheimer's disease mouse model neurons. J Alzheimers Dis 2014; 42: 313-324.
- Eriksson P, Deguchi H, Samnegard A, Lundman P, Boquist S, Tornvall P, Ericsson CG, Bergstrand L, Hansson LO, Ye S, Hamsten A. Human evidence that the cystatin C gene is implicated in focal progression of coronary artery disease. Arterioscler Thromb Vasc Biol 2004; 24: 551-557.
- Levy E, Sastre M, Kumar A, Gallo G, Piccardo P, Ghetti B, Tagliavini F. Codeposition of cystatin C with amyloid-beta protein in the brain of Alzheimer disease patients. J Neuropathol Exp Neurol 2001; 60: 94-104.
- Annanmaki T, Pessala-Driver A, Hokkanen L, Murros K. Uric acid associates with cognition in Parkinson's disease. Parkinsonism Relat Disord 2008; 14: 576-578.
- Cutler RG, Camandola S, Malott KF, Edelhauser MA, Mattson MP. The role of uric acid and methyl derivatives in the prevention of age-related neurodegenerative disorders. Curr Top Med Chem 2015; 15: 2233-2238.
- Cheng F, Vivacqua G, Yu S. The role of α-Synuclein in neurotransmission and synaptic plasticity. J Chem Neuroanat 2011; 42: 242-248.
- Clinton LK, Blurton-JM, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: a acceleration of Neuropathology and cognitive decline. J Neurosci 2010; 30: 7281-7289.
- Siderowf A, Xie SX, Hurtig H, Weintraub D, Duda J, Chen-PA, Shaw LM, Van Deerlin V, Trojanowski JQ, Clark C. CSF amyloid Aβ1-42 predicts cognitive decline in Parkinson disease. Neurol 2010; 75: 1055-1061.
- Aasly JO, Shi M, Sossi V, Stewart T, Johansen KK, Wszolek ZK, Uitti RJ, Hasegawa K, Yokoyama T, Zabetian CP, Kim HM, Leverenz JB, Ginghina C, Armaly J, Edwards KL, Snapinn KW, Stoessl AJ, Zhang J. Cerebrospinal fluid amyloidβand tau in LRRK2 mutation carriers. Neurol 2012; 78: 55-61.
- Maetzler W, Liepelt I, Berg D. Progression of Parkinson's disease in the clinical phase: potential markers. Lancet Neurol 2009; 8: 1158-1171.
- Wan W, Cao L, Liu L, Zhang C, Kalionis B, Tai X, Li Y, Xia S. Aβ (1-42) oligomer-induced leakage in an in vitro blood-brain barrier model is associated with up-regulation of RAGE and metalloproteinase, and down-regulation of tight junction scaffold proteins. J Neurochem 2015; 134: 382-393.
- Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Kim HM, Leverenz JB, Montine TJ, Ginghina C, Kang UJ, Cain KC, Wang Y, Aasly J, Goldstein D, Zhang J. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol 2011; 69: 570-580.
- Tizon B, Sahoo S, Yu H, Gauthier S, Kumar AR, Mohan P, Figliola M, Pawlik M, Grubb A, Uchiyama Y, Bandyopadhyay U, Cuervo AM, Nixon RA, Levy E. Induction of autophagy by cystatin C: a mechanism that protects murine primary cortical neurons and neuronal cell lines. Plos One 2010; 5: e9819.
- Dutta G, Barber DS, Zhang P, Doperalski NJ, Liu B. Involvement of dopaminergic neuronal cystatin C in neuronal injury-induced microglial activation and neurotoxicity. J Neurochem 2012; 122: 752-763.
- Xu L, Sheng J, Tang Z, Wu X, Yu Y, Guo H, Shen Y, Zhou C, Paraoan L, Zhou J. Cystain C prevents degeneration of rat nigral dopaminergic neurons:in vitro and in vivo studies. Neurobiol Dis 2005; 18: 152-165.
- Dutta G, Barber DS, Zhang P, Doperalski NJ, Liu B. Involvement of dopaminergic neuronal cystatin C in neuronal injury-induced microglial activation and neurotoxicity. J Neurochem 2012; 122: 752-763.
- Shen C, Guo Y, Luo W, Lin C, Ding M. Serum Urate and the risk of Parkinson’s disease: results from a meta-analysis. Can J Neurosci Nurs 2013; 40: 73-79.