Research Article - Current Pediatric Research (2022) Volume 26, Issue 10
Association of CAT rs1001179 and SOD2 rs4880 Gene polymorphisms with Cerebral Palsy in Hypoxic-Ischemic Encephalopathy
Osama A Elfiki1*, Neveen T Abed1, Akram E Elsadek1, Eman G Behiry2, Tarek M Ghareeb3
1Department of Pediatric, College of Medicine, Benha University, Egypt
2Deptartment of Clinical Pathology, College of Medicine, Benha University, Egypt
3Department of Pediatric, Ministry of Health, Benha University, Egypt
- *Corresponding Author:
- Osama A
Pediatric Department
Faculty of Medicine
Benha University
Egypt
E-mail: abujamaljameel@gmail.com
Received: 02 October, 2022, Manuscript No. AAJCP-22-76478; Editor assigned: 05 October, 2022, PreQC No. AAJCP-22-76478(PQ); Reviewed: 12 October, 2022, QC No. AAJCP-22-76478; Revised: 19 October, 2022, Manuscript No. AAJCP-22-76478(R); Published: 27 October, 2022, DOI:10.35841/0971-9032.26.10.1643-1649.
Abstract
Background: Perinatal hypoxic-ischemic brain injury remains a serious condition that causes significant mortality and long-term morbidity. It leads to a cascade of neurotoxic events involving energy failure and the accumulation of Reactive Oxygen Species (ROS). In the immature brain with its reduced capacity for defence against ROS, the resultant tissue damage may lead to severe neurological sequelae such as epilepsy and/or Cerebral Palsy (CP). Aim: was to investigate the association between Catalase (CAT) and Superoxide Dismutase (SOD2) genes polymorphisms and development of cerebral palsy in infants with Hypoxic-Ischemic Encephalopathy (HIE). Methods: This case control study included 100 children assigned to two equal groups: Group (I): Fifty patients with cerebral palsy due to (HIE) and Group (II): fifty healthy children as controls. Genomic DNA was extracted from venous blood and genotyped for SOD2 rs4880 and CAT rs1001179 using the process of Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP). Results: The polymorphic CAT rs1001179 allele demonstrated a statistically significant association with the presence of CP (OR=2.249, 95%CI=1.311–3.859, P<0.05). Patients with at least one polymorphic CAT rs1001179 T allele were more prone to develop CP (P<0.05) compared to noncarriers. The investigated SOD2 rs4880 polymorphism was not significantly associated with the risk of CP (P=0.663). Conclusion: CAT rs1001179 polymorphism could be used to identify children who are more likely to develop CP after perinatal HIE.
Keywords
Catalase, Superoxide dismutase, Cerebral palsy, Hypoxic-ischemic encephalopathy.
Introduction
Cerebral Palsy (CP) is a non-progressive disorder of the developing brain principally affecting the motor system [1]. It is the most common physical disability in childhood, with a prevalence of 2 to 3 per 1000 newborns [2]. CP is not a specific disease but a group of movement and posture disorders that are accompanied by disturbance of sensation, cognition or other brain functions [3].
Hypoxic-ischemic encephalopathy (HIE; also referred to as birth asphyxia) is defined as disturbed cerebral function due to lack of oxygen to the brain following antenatal/perinatal adverse events [4]. It remains the leading cause of neonatal mortality and morbidity and affects 1 to 8 per 1000 live births worldwide [5]. Of the individuals affected by HIE, 15%–20% will die in the postnatal period, and an additional 25% will develop severe and permanent neurological sequelae, such as cerebral palsy, epilepsy, global developmental delay, intellectual disability, behavioral disorders and autism spectrum disorders [6]. Hypo perfusion, in conjunction with hypoxia, leads to a cascade of events including acidosis, release of inflammatory mediators and free radical formation including reactive oxygen species (ROS) [7]. These biochemical substances result in loss of normal cerebral autoregulation and diffuse brain injury (neuronal cell death).
Antioxidant enzymes such as manganese Superoxide Dismutase (SOD2) and Catalase (CAT) detoxify superoxide anion and hydrogen peroxide and constitute the primary defense against ROS. The activity of these antioxidant enzymes protects cells from ROS and is influenced by functional genetic polymorphisms in antioxidant genes [8].
The SOD2 rs4880 polymorphism changes the amino acid sequence in the mitochondrial leading sequence (p.Ala16Val), resulting in lower MnSOD activity [9]. The CAT rs1001179 polymorphism alters the transcription factor binding site in the promoter region, with the polymorphic T allele leading to enhanced gene transcription [10]. The aim of our study was to assess whether rs1001179 of CAT gene and rs4880 of SOD2 gene polymorphisms are associated with cerebral palsy in infants with hypoxic-ischemic encephalopathy.
Patients and Methods
This case control study was conducted on 100 subjects; cases were selected from pediatric neurology clinic, Benha University Hospital.
They were divided into two groups:
- Group (I): included 50 patients who were diagnosed by pediatric neurologists according to history of perinatal asphyxia, neurological examination and imaging studies as Cerebral Palsy (CP).
- Group (II): included 50 apparently healthy children age and sex matched as a control group.
Inclusion criteria
- Age: 6 months - 4 years
- All patients with neonatal HIE grades II to III according to the sarnat and sarnat classification.
Exclusion criteria
- Patients with chromosomal anomalies, metabolic disorders or genetic syndromes.
- Patients with HIE grade I or any other medical conditions lead to neonatal brain disorders or CP.
Ethical considerations
All participants’ parents gave written informed consent. The ethics committee at Benha Faculty of medicine, Benha University approved the study.
All studied children were subjected to:
Detailed medical history: Age, gender, gestational age, special interest to information on the risk factors associated with CP, which can be summarized into maternal and neonatal factors.
- Complete physical and neurological examination.
- Neonatal HIE severity was graded clinically according to Sarnat and Sarnat classification [11].
- Brain imaging: CT and MRI.
Genotyping: The test was done in two main steps:
- Extraction of genomic DNA: The blood samples were used for DNA extraction using the gene JET whole blood genomic DNA purification mini kit (thermo fisher scientific, Germany) according to the manufacturer’s protocol.
- Single Nucleotide Polymorphism (SNP): SNP detection; Genotyping of CAT gene SNP (rs1001179) and SOD2 gene SNP (rs4880) were performed using the TaqMan SNP Genotyping assays (Applied Biosystems, USA).The PCR amplification was done using stepone plus real time PCR instrument (applied biosystems, USA).
Statistical Analysis
Categorical variables were summarized as numbers and percentages while continuous variables were summarized as means ± standard deviations. Differences between cases and controls were compared by either Student’s t-test for continuous variables or Chi-square test for categorical variables.
Unconditional logistic regression was used to calculate the Odd Ratios (ORs) and 95% Confidence Intervals (CIs) for the CP risk associated with the SOD2 and CAT polymorphisms. Statistical analysis was performed using IBM SPSS Statistics, version 22.0 (IBM Corporation, Armonk, NY, USA). A p value of <0.05 was considered statistically significant. A genotype distribution follows Hardy-Weinberg equilibrium in the studied groups. To assess deviation from Hardy–Weinberg equilibrium (HWE), a standard chi-square test was used.
Results
This study included 100 children; divided into two groups. Group (I): included 50 patients (28 males and 22 females) with CP, their mean age was 18 ± 11.6 months. Group (II): 50 apparently healthy age and gender matched individuals (24 males and 26 females) as a control group and their mean age was 17.28 ± 12 months. Characteristics data of all children are presented in Table 1. There were no significant differences between both groups as regard to sex, age, gestational age, birth weight and mode of delivery, while Apgar score at 5 minutes showed significant decrease in patients than controls (p=0.001).
| Data | Patients | Control | test | p. value | |
|---|---|---|---|---|---|
| Sex | Male (%) | 28 (56%) | 24 (48%) | X2: 0.641 | 0.423 |
| Female (%) | 22 (44%) | 26 (52%) | |||
| Age (months) | Range | 6–48 | 6–48 | T: 0.341 | 0.734 |
| Mean ± SD | 18.080 ± 11.622 | 17.280 ± 12.081 | |||
| Gestation (weeks) | Range | 37–42 | 37–42 | T: 1.067 | 0.288 |
| Mean ± SD | 38.54 ± 1.46 | 38.24 ± 1.35 | |||
| Birth weight (kg) | Range | 2.7–4.5 | 2.8–4.4 | T: 0.864 | 0.446 |
| Mean ± SD | 3.47 ± 0.50 | 3.55 ± 0.56 | |||
| Mode of delivery | CS (%) | 27 (54%) | 24 (48%) | X2:0.363 | 0.548 |
| NVD (%) | 23 (46%) | 26 (52%) | |||
| Apgar score at 5 minutes | Range | 1–4 | 7–10 | T: 26.563 | 0.001 |
| Mean ± SD | 2.62 ± 1.03 | 8.14 ± 1.05 | |||
| HIE Sarnat staging | Stage II (%) | 28 (56%) | |||
| Stage III (%) | 22 (44%) | ||||
Table 1. Demographic data of the studied cases.
Regarding our clinical data ,thirty nine patients (78%) with cerebral palsy have spastic clinical features. 16 patient of them have diplegia (32%), 13 patients have hemiplegia (26%) and 10 patients have quadriplegia (20%). The athetoid or dyskinetic type of cerebral palsy, affecting 7 patients (14%). The rarest form, ataxic cerebral palsy, affects 1 patient (2%), three patients (6%) have mixed type of cerebral palsy (Table 2).
| CP type | CP distribution | No | % |
|---|---|---|---|
| Spastic: | 39 | 78 | |
| Diplegia | 16 | 32 | |
| Hemiplegia | 13 | 26 | |
| Quadriplegia | Spastic | 10 | 20 |
| Dyskinetic | 7 | 14 | |
| Ataxic | 1 | 2 | |
| Mixed | 3 | 6 |
Table 2. Clinical data of the studied cases (n=50).
Intellectual impairment occurs in 90% of patients with cerebral palsy. About one half of patients have seizures. Communication problems are common (60%), as well as growth problems (54%), impaired vision affect 20% of patients while impaired hearing affect 18% of patients (Table 3).
| Variables | No | % |
|---|---|---|
| Intellectual disability | 45 | 90 |
| Spasticity and contractures | 36 | 72 |
| Communication problems | 30 | 60 |
| Growth problems | 27 | 54 |
| Epilepsy | 25 | 50 |
| Impaired vision | 10 | 20 |
| Impaired hearing | 9 | 18 |
Table 3. Associated disorders among CP patients (n=50).
All children with cerebral palsy had a brain MRI scan assessed for the study. The MRI scans showed that perirolandic area involvement was the most common finding (32%), followed by Watershed zone involvement (24%), cortical/subcortical lesions (20%), basal ganglia lesions (16%) and mixed pattern (8%) (Table 4).
| MRI pattern | No | % |
|---|---|---|
| Peri-rolandic involvement | 16 | 32 |
| Watershed zone involvement | 12 | 24 |
| Cortical/subcortical damage | 10 | 20 |
| Basal ganglia involvement | 8 | 16 |
| Mixed pattern | 4 | 8 |
Table 4. Magnetic Resonance Imaging (MRI) pattern types of CP patients (n=50).
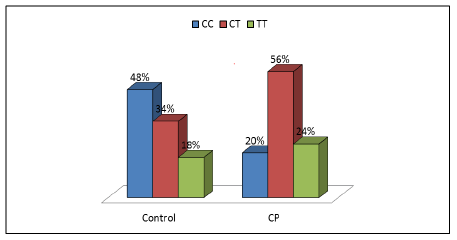
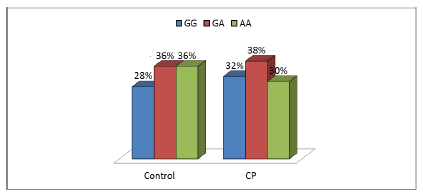
Genotype frequencies of the investigated polymorphisms SOD rs4880 and CAT rs1001179 in genes coding for antioxidant enzymes were compared in patients with CP and control group. SOD rs4880 polymorphism was not significantly associated with the risk of CP (P=0.663), while the polymorphic CAT rs1001179 allele demonstrated a statistically significant association with the presence of CP (OR=2.249, 95%CI=1.311–3.859, P<0.05) as shown in Table 5.
| Control N=50 | CP N=50 | p | OR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| rs1001179 CAT |
CC | 24 | 48 | 10 | 20 | Reference | ||
| CT | 17 | 34 | 28 | 56 | 0.004 | 2.346 | (1.313-4.190) | |
| TT | 9 | 18 | 12 | 24 | 0.043 | 2.057 | (1.023-4.138) | |
| CT+TT | 26 | 52 | 40 | 80 | 0.003 | 2.249 | (1.311-3.859) | |
| C | 65 | 65 | 48 | 48 | Reference | |||
| T | 35 | 35 | 52 | 52 | 0.015 | 1.548 | (1.087-2.205) | |
| rs4880 SOD |
GG | 14 | 28 | 16 | 32 | Reference | ||
| GA | 18 | 36 | 19 | 38 | 0.872 | 0.951 | (0.52-1.741) | |
| AA | 18 | 36 | 15 | 30 | 0.532 | 0.821 | (0.441-1.526) | |
| GA+AA | 36 | 72 | 34 | 68 | 0.663 | 0.887 | (0.519-1.518) | |
| G | 46 | 46 | 51 | 51 | Reference | |||
| A | 54 | 54 | 49 | 49 | 0.479 | 0.882 | (0.623-1.249) | |
Table 5. Association of rs1001179 and rs4880 genotypes and alleles with risk of CP susceptibility. OR, odds ratio; CI, confidence interval. Logistic regression analysis was used.
Patients with at least one polymorphic CAT rs1001179 T allele were more prone to develop CP (P<0.05) compared to non-carriers as shown in Figures 1 and 2.
Discussion
HIE remains a serious condition that causes significant mortality and long-term morbidity. It leads to the formation of Reactive Oxygen Species (ROS) and the resultant cell and tissue damage may cause neurological sequelae such as cerebral palsy and/or epilepsy [12].
The antioxidant enzymes SOD2 and CAT represent the first line of defense against ROS by detoxifying the superoxide anion and hydrogen peroxide. Their activity and ability to protect cells and tissues from ROS and their damaging products are influenced by functional genetic polymorphisms in antioxidant genes [7].
Our study regarding sex, revealed no significant difference between CP group and controls, this agrees with Esih et al., [8] Abdel Rasol et al., [13] and Torres-Merino et al., [14]. But several studies reported a significant association between male sex and CP. This has been explained by several factors such as a greater biological vulnerability in males in terms of cerebral structure, genetic predisposition and the influence of female hormones on a possible reduction of the consequences of brain damage [15-18]. Regarding Apgar score at 5 minutes, our results showed significant decrease in CP patients than controls (p=0.001).
This finding is supported by several studies; they reported that low Apgar score at 5 minutes was strongly associated with later diagnosis of cerebral palsy. Given that Apgar score is a measure of vitality shortly after birth, this finding suggests that the causes of cerebral palsy are closely linked to factors that reduce infant vitality. Low Apgar score is also linked to perinatal asphyxia, immaturity, malformations, and impairments of the central nervous system [14,19,20].
Regarding our clinical data, the most common type was spastic CP 78% and the least one was ataxic CP 2%. This finding is supported by study done by Yang et al., [17] a total of 43 studies were included in this systematic review, for the prevalence of CP by different classifications, they found that, spastic CP was the highest and ataxic CP the lowest.
Regarding the associated disorders among our CP patients, intellectual disability was the most common (90%), and the least one was impaired hearing (18%). This was agreed by study done by Gincota et al., [21], they include 207 children with CP, among all children, intellectual disability was the most common (59%) and hearing impairment was the least one (5%). In our study, MRI scans for CP group showed that perirolandic area involvement was the most common finding (32%), followed by Watershed zone involvement (24%), cortical/subcortical lesions (20%), basal ganglia lesions (16%) and mixed pattern (8%).
Our findings are consistent with Rana et al. [22], they found that involvement of perirolandic area was the most common imaging ?nding followed by watershed zone involvement, cystic encephalomalacia, basal ganglia involvement and mixed pattern. This was explained on the basis of that, areas of high metabolic demand containing a high concentration of excitatory amino acid receptors, such as the deep gray matter and perirolandic cortex are commonly damaged in acute severe ischemia. In comparison to this, less severe but more prolonged ischemic insults affect the cortical and sub-cortical watershed regions.
In our study we investigated the association between rs1001179 of CAT gene and rs4880 of SOD2 gene polymorphisms with cerebral palsy in infants with hypoxic-ischemic encephalopathy. We found that, genotype frequency of SOD rs4880 polymorphism was not significantly associated with the risk of CP (P=0.663), while the polymorphic CAT rs1001179 allele demonstrated a statistically significant association with the presence of CP (P<0.05). Patients with at least one polymorphic CAT rs1001179 T allele were more prone to develop CP (P<0.05, OR>1) compared to non-carriers.
In agreement with us, Kulak et al., [23], in their study on antioxidant enzymes in children with cerebral palsy, they compared the activities of SOD, glutathione peroxidase1 (GPX1) and Glutathione Reductase (GR) in plasma and erythrocytes in 34 children with CP and 61 normal controls. They found no significant difference in the activities of SOD, GPX and GR in plasma between children with CP and the control group.
Similar to our findings, Esih et al., [24], investigated the impact of common functional polymorphisms in the antioxidant genes SOD2, GPX1 and CAT in 80 patients with neonatal HIE grades II and III, 51 of them had CP and 29 without CP. They found no significant difference in the activities of SOD2 and GPX1 between children with CP and children without CP but they found that CAT polymorphism was significantly more frequent in children who developed CP compared to patients without CP after HIE.
Contrary to our findings, another study done by Esih et al. [8] to evaluate the association between the polymorphisms in genes of antioxidant and inflammatory pathways in newborns treated with therapeutic hypothermia and the development of epilepsy or CP at two years follow-up in 55 patients with neonatal HIE treated with TH. 16 patients developed epilepsy and 15 children developed CP. They found that polymorphisms in antioxidant genes CAT rs1001179, SOD2 rs4880 and GPX1 rs1050450 were not associated with neurological outcome.
CAT converts hydrogen peroxide to water and oxygen, thereby mitigating its toxic effects [25]. Its activity and ability to protect cells and tissues from ROS and their damaging products are influenced by functional genetic polymorphisms in antioxidant genes [8]. CAT rs1001179 polymorphism alters the transcription factor binding site in the promoter region, with the polymorphic T allele leading to enhanced gene transcription [9].It is thus possible that even though the CAT T allele has been associated with higher levels of CAT transcription resulting in increased blood CAT protein levels this allele could also be associated with lower enzyme activity in the immature brain [26].
Lower CAT activity may lead to decreased protection against ROS, which may result in irreversible neurotoxic damage to the immature brain [27]. The association between enhanced oxidative damage and the activity of CAT has been as well reported in some chronic inflammatory diseases such as asthma in children. Inflammation is also involved in the neurotoxic cascade in acute perinatal hypoxic-ischemic brain injury [28]. Our finding may point to a possible association of CAT rs1001179 polymorphism with development of CP after perinatal asphyxia.
To the best of our knowledge, our study was one of the first studies to investigate the associations of antioxidant polymorphisms with cerebral palsy after perinatal asphyxia as there are very limited data on antioxidant enzyme polymorphism in relation to the risk of cerebral palsy after perinatal asphyxia. Furthermore, the strength of our study is that our study group was homogenous as we only included term newborns after HIE.
Our study had some limitations: due to the retrospective nature of our study it was not possible to collect relevant data of maternal factors, such as medication taken during pregnancy, as they are possible confounders which could be addressed if our study had been performed prospectively. Furthermore, it is well known that many intrinsic pathophysiological mechanisms are involved during hypoxic-ischemic events leading to hypoxic-ischemic neonatal brain injury and its sequelae. As it would be impossible to retrospectively evaluate each of them, we decided to use the Sarnat and Sarnat classification as a well-recognized tool for the clinical assessment of HIE.
Conclusion
Our study revealed that SOD rs4880 polymorphism was not significantly associated with the risk of CP, while the polymorphic CAT rs1001179 allele demonstrated a statistically significant association with the presence of CP.
Patients with at least one polymorphic CAT rs1001179 T allele were more prone to develop CP compared to non-carriers, so CAT rs1001179 polymorphism could be used to identify children who are more likely to develop CP after perinatal HIE.
Recommendations
Future large-scale, controlled trials are needed to further understand the association between the antioxidant gene polymorphisms and development of cerebral palsy in patients with hypoxic-ischemic encephalopathy.
Better understanding of the role of polymorphisms in antioxidant genes may in future provide improved neuro-protective strategies to reduce brain injury in asphyxiated newborns.
Acknowledgements
Authors would like to thank all children and their parents who participated in this study.
Conflicts of Interest
The authors declare that they have no conflict of interest regarding the publication of the current study.
Source of Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Derrick M, Alexander D, Xinhai J, et al. A Model of CP from fetal hypoxia-ischemia. Stroke 2007; 38 (II): 731-735.
- Patel DR, Neelakantan M, Pandher K, et al. Cerebral palsy in children: A clinical overview. Transl Pediatr 2020; 9(Suppl1): S125–135.
- Downs J, Blackmore AM, Epstein A, et al. The prevalence of mental health disorders and symptoms in children and adolescents with cerebral palsy: A systematic review and meta-analysis. Dev Med Child Neurol 2018; 60(I): 30-38.
- Glass HC. Hypoxic-ischemic encephalopathy and other neonatal encephalopathies. Continuum (Minneap Minn) 2018; 24: 57-71.
- Lee BL, Glass HC. Cognitive outcomes in late childhood and adolescence of neonatal hypoxic-ischemic encephalopathy. Clin Exp Pediatr 2021; 64(12): 608-618.
- Edwards AB, Anderton RS, Knuckey NW, et al. Perinatal hypoxic-ischemic encephalopathy and neuroprotective peptide therapies: A Case for Cationic Arginine-Rich Peptides (CARPs). Brain Sci 2018; 8(8): 147.
- https://www.frontiersin.org/articles/10.3389/fnsyn.2021.709301/full
- Esih K, Goricar K, Soltirovska-Salamon A, et al. Genetic polymorphisms, gene-gene interactions and neurologic sequelae at two years follow-up in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Antioxidants (Basel) 2021; 10(9): 1495.
- Sutton A, Imbert A, Igoudjil A, et al. The manganese superoxide dismutase Ala16Val dimorphism modulates both mitochondrial import and mRNA stability. Pharmacogenet Genomics 2005; 15: 311e9.
- Forsberg L, Lyrenas L, de Faire, et al. A Common functional C-T substitution polymorphism in the promoter region of the human catalase gene influences transcription factor binding, reporter gene transcription and is correlated to blood catalase levels. Free Radic Biol Med 2001; 30: 500e5.
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976; 33: 696–705.
- Riljak V, Kraf J, Daryanani A, et al. Pathophysiology of perinatal hypoxic-ischemic encephalopathy - biomarkers, animal models and treatment perspectives. Physiol. Res. 65 (Suppl. 5): S533-S545.
- Abdel Rasol HA, El Ghaffara NK, El?Sayeda YS, et al. The effect of catalase enzyme gene polymorphism A-21T (rs7943316) on epilepsy and its drug resistance after hypoxic ischemic brain injury. Egypt J Lab Med 32; 1-7.
- Torres-Merino S, Moreno-Sandoval HN, Thompson-Bonilla MDR, et al. Association between rs3833912/rs16944 snps and risk for cerebral palsy in Mexican children. Mol Neurobiol 2019; 56(3): 1800-1811.
- Romeo DM, Venezia I, Pede E, et al. Cerebral palsy and sex differences in children: A narrative review of the literature. J Neurosci Res 2022; 00: 1–13.
- Abd Elmagid DS, Magdy H. Evaluation of risk factors for cerebral palsy. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2021; 57: 13.
- Yang S, Xia J, Gao J, et al. Increasing prevalence of cerebral palsy among children and adolescents in China 1988-2020: A systematic review and meta-analysis. J Rehabil Med 53(5).
- Yu T, Xia L, Bi D, et al. Association of NOS1 gene polymorphisms with cerebral palsy in a Han Chinese population: A case-control study. BMC Med Genomics 2018; 11(1): 56.
- Persson M, Razaz N, Tedroff K, et al. Five and 10 minute Apgar scores and risks of cerebral palsy and epilepsy: Population based cohort study in Sweden. BMJ 2018; 360: k207.
- Lie K, Groholt EK, Eskild A. Association of cerebral palsy with apgar score in low and normal birthweight infants: Population-based cohort study. BMJ 2010; 341: c4990.
- Gincota BE, Andersen GL, Torstein V, et al. Cerebral palsy in Moldova: Subtypes, severity and associated impairments. BMC Pediatr 2018; 18(1): 332.
- Rana L, Sood D, Chauhan R, et al. MR Imaging of hypoxic ischemic encephalopathy - Distribution Patterns and ADC value correlations. Eur J Radiol Open 2018; 5: 215-220.
- Kulak W, Sobaniec W, Solowej E, et al. Antioxidant enzymes and lipid peroxides in children with cerebral palsy. Life Sci 2005; 77: 3031e6.
- Esih K, Goricar K, Dolzan V, et al. The association between antioxidant enzyme polymorphisms and cerebral palsy after perinatal hypoxic-ischaemic encephalopathy. Eur J Paediatr Neurol 2016; 20(5): 704-8.
- Ighodaro OM, Akinloye OA. First line defence antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine 2018; 54: 287-293.
- Bastaki M, Huen K, Manzanillo P, et al. Genotype-activity relationship for Mn-superoxide dismutase, glutathione peroxidase 1 and catalase in humans. Pharmacogenet Genomics 2006; 16: 279e86.
- Vento M, Asensi M, Sastre J, et al. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J Pediatr 2003; 142: 240e6.
- Babusikova E, Jesenak M, Evinova A, et al. Frequency of polymorphism 262 c/t in catalase gene and oxidative damage in Slovak children with bronchial asthma. Arch Bronconeumol 2013; 49: 507e12.