Research Article - Biomedical Research (2017) Volume 28, Issue 1
Association between the clinical classification of hypothyroidism and maternal and foetal outcomes, and the effects of levothyroxine supplemental replacement treatment for pregnancy
Min Zhang*, Hui-Xia Lu, Wei-Guo ZhangDepartment of Clinical Laboratory, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, PR China
- *Corresponding Author:
- Min Zhang
Department of Clinical Laboratory Zhongda
Hospital School of Medicine Southeast University PR China
Accepted on June 4, 2016
Abstract
Maternal hypothyroidism is closely associated with adverse maternal and foetal outcomes. This study aims to analyse the associations between the clinical classification of hypothyroidism and maternal and foetal outcomes, and evaluate the effects of levothyroxine (LT4) supplemental replacement treatment on maternal and foetal outcomes in pregnant women with subclinical hypothyroidism (SCH) or overt hypothyroidism (OH). Totally, 264 pregnant women were enrolled in this study. They were categorized into three groups: euthyroid, SCH and OH group. Our results showed that pregnant women with SCH had a higher free T4 (FT4) and thyroid-stimulating hormone (TSH) levels, while pregnant women with OH had a lower FT4 levels and a higher TSH levels. The rate of premature delivery in euthyroid women was 9.95% (24/241), and in SCH women was 33.3% (3/9), and in OH women was 21.4% (3/14), respectively. Among SCH women, one woman received LT4 treatment during the pregnancy, and had no premature delivery; while in the non-treated SCH women, 3 out of 8 women had premature delivery. More importantly, among OH women, 11 women received LT4 treatment during the pregnancy, and none of these treated-women had premature delivery; while 3 non-treated pregnant women with OH all had premature delivery. Our data suggested that the therapeutic effect of substitutive treatment with LT4 was significantly associated with different clinical classifications of hypothyroidism in pregnancy and the treatment should begin as soon as possible after diagnosis.
Keywords
Hypothyroidism, Free T4, Thyroid-stimulating hormone, Levothyroxine, Premature delivery.
Introduction
Hypothyroidism accounts for approximately 3.5% of pregnancies, and it can be classified into subclinical hypothyroidism (SCH: a thyroid-stimulating hormone (TSH) concentration exceeding the trimester-specific reference value in combination with a normal free T4 (FT4) concentration) and overt hypothyroidism (OH: a TSH concentration exceeding the trimester-specific reference value in combination with a decreased FT4 concentration) [1]. Hashimoto’s thyroiditis and iodine deficiency are the main causes of hypothyroidism, which reduces thyroid hormone levels [2,3]. Both hypothyroidism and thyroid autoimmunity have been found to be correlated with infertility [4].
However, in the case of SCH, adverse effects either in pregnancy or the offspring have not been found and the impact of treatment is controversial [5-7]. On the other hand, OH in pregnancy has been suggested to have adverse impacts on placental abruption, miscarriage, prematurity, gestational hypertension, skin developmental damage, and intellectual impairment in the offspring [8-11].
According to the guideline for SCH and OH put forward by the American Thyroid Association (ATA), SCH and OH have been proposed to be associated with adverse maternal and foetal outcomes [12], but no consensus has been reached about the need for universal thyroid function screening and particularly for the treatment of SCH during pregnancy. However, several studies have shown that levothyroxine (LT4) therapy in women with SCH or OH has been shown to improve the outcome of pregnancies [13,14], and LT4 has been suggested an optional treatment for OH, and this treatment has been shown to prevent obstetrical complications effectively [12,15]. Therefore, when SCH or OH is diagnosed during pregnancy, LT4 treatment has been recommended because of its potential benefits [12].
However, the difference with regard to the classification of SCH and OH with LT4 supplemental treatment is largely unknown, and in China, the recognition and treatment of thyroid disorder in pregnant women is also insufficient and few studies have examined the possible effects of SCH and OH on maternal outcomes as well as the beneficial effects of LT4 treatment. Therefore, the present study aimed to analyse the association between several clinical factors, especially the clinical classification of hypothyroidism, and the effects of LT4 treatment for pregnancy.
Materials and Methods
Ethics statements
This retrospective study was conducted at the Zhongda Hospital, and the study protocol was approved by the hospital's review board. The privacy of all subjects was guaranteed.
Study population
Between January 2014 and May 2014, data of 264 pregnant women were used in this retrospective study. All subjects were screened and gave birth at the hospital, and had resided in the local area for at least 4 years. Women with the following conditions were excluded: previous use of thyroxin or antithyroid drugs, other autoimmune disease, congenital heart disease, and elevated serum transaminase or creatinine level. Thyroid function was tested in the second trimester. Information about the following demographic and clinical characteristics was collected through questionnaires administered during examination: demographic characteristics (e.g., age, address, occupation, educational level, income), medical history (menstrual history, childbearing history, other diseases, medication use), health behaviour (smoking and exposure to husbands’ smoking, alcohol consumption), general physical parameters (body weight, blood pressure, cardiopulmonary function, oedema), obstetric parameters (fundal height, abdominal girth, foetal heart sound, pelvic examination when necessary), and laboratory assessments (screening for gestational diabetes mellitus, human immunodeficiency virus, syphilis, routine blood and urinary tests, hepatic and renal functions, blood type, electrocardiography, electronic foetal monitoring). All data were kept in a computerized database.
Laboratory assays and diagnosis of SCH and OH
TSH and FT4 concentrations were measured by electrochemiluminescence immunoassay (Cobas; Roche) and associated diagnostic kits. Inter- and intra-assay coefficients of variation for each hormone were 10%. The assessment of thyroid function was based on the following local trimesterspecific reference values (2.5th-97.5th percentiles): second trimester, TSH 0.27-4.2 mIU/L and FT4 0.93-1.70 ng/dL. Maternal thyroperoxidase antibody (TPOAbs) was measured using immunoassay (Cobas; Roche).
SCH was defined as a TSH concentration exceeding the trimester-specific reference value in combination with a normal FT4 concentration. OH was defined as a TSH concentration exceeding the trimester-specific reference value in combination with a decreased FT4 concentration. Pregnant women with normal TSH and FT4 levels were considered to be euthyroid and served as control subjects. Some women with SCH or OH were treated with LT4. For LT4 treatment, the initial dose of LT4 was 50 μg and the LT4 dose was considered to be adequate when serum TSH was 3.0 mIU/L during the remaining time of pregnancy. The subsequent testing results were absent in the patients achieving serum 3.0 mIU/L for the censor of these patients in the follow-up.
Definition of maternal and foetal outcomes
All participants underwent monthly antenatal examinations during gestation and delivery until they were discharged from the hospital. Maternal and perinatal outcomes based on specific guidelines were recorded during this period.
Premature delivery was defined as the delivery before the end of 37th week of gestational age. Apgar score was determined by evaluating the new-born on five simple criteria on a scale from zero to two and summing up the five values.
The resulting Apgar score ranges from zero to ten. The five criteria are appearance, pulse, grimace, activity, and respiration.
Statistical analysis
All data are expressed as means ± standard deviations or numbers and percentages. Statistical analysis was performed using the SPSS 16.0 software. Student’s t-test or One-way ANOVA was used to compare continuous variables (body weight, TSH and FT4 concentrations) and the chi-squared test was used to compare categorical measures (maternal and perinatal outcomes). Univariate and multivariate logistic regression models were built to model the relationship between outcomes and one or more independent variables, respectively. P<0.05 was considered to be statistically significant.
Results
Descriptive statistics of study population
The study population consisted of 264 women of whom 12.5% (33/264) had a premature delivery (<37 weeks of gestation). The mean FT4 and TSH levels were 0.92 ± 0.12 ng/dL and 2.50 ± 1.43 mIU/L, respectively, and all the other descriptive statistics were shown in Table 1. The prevalence of TPOAb positivity was 6.0% (16/264), among the TPOAb pregnant women, 2 women had premature delivery.
| Characteristic | Value |
| Before Pregnancy BW (kg) | 55.9 ± 8.3 |
| After Pregnancy BW (kg) | 71.7 ± 9.5 |
| FT4 (ng/dL) | 0.92 ± 0.12 |
| TSH (mIU/L) | 2.50 ± 1.43 |
| Preterm rate | 35/260 |
| Infant BW (kg) | 3.46 ± 0.49 |
| Apgar core | 9.9 ± 0.5 |
Descriptive statistics are for the study population of women without twin pregnancies, pre-existing thyroid disease, or fertility treatment. BW, body weight.
Table 1. Descriptive statistics of 264 women.
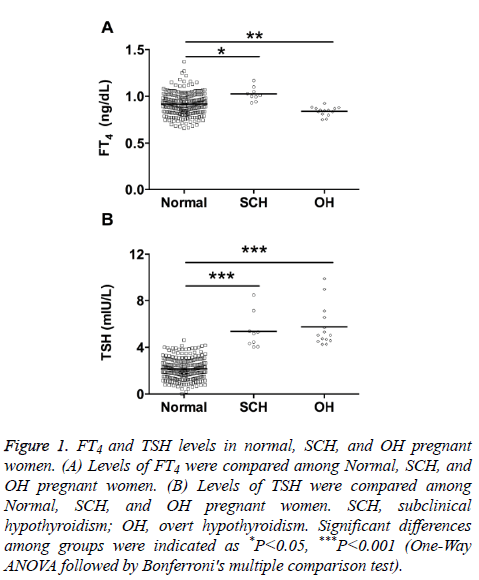
FT4 and TSH levels in normal, SCH, and OH pregnant women
The study population of 264 women was divided into three groups according to the Endocrine Society and American Thyroid Association guidelines.
Results from Figure 1 showed that the FT4 levels in SCH group were significantly higher than that in normal group, and the FT4 levels in OH group were significantly lower than that in normal group (Figure 1A); the TSH levels from SCH and OH group were both significantly higher than that in control group (Figure 1B).
Figure 1: FT4 and TSH levels in normal, SCH, and OH pregnant women. (A) Levels of FT4 were compared among Normal, SCH, and OH pregnant women. (B) Levels of TSH were compared among Normal, SCH, and OH pregnant women. SCH, subclinical hypothyroidism; OH, overt hypothyroidism. Significant differences among groups were indicated as *P<0.05, ***P<0.001 (One-Way ANOVA followed by Bonferroni's multiple comparison test).
SCH, OH, and the risk of premature delivery
As shown in Table 2, the rate of premature delivery in euthyroid women was 9.95% (24/241), and in SCH women was 33.3% (3/9), and in OH women was 21.4% (3/14), respectively (Table 2), and in OH women group, there are 11 women received LT4 treatment.
| Before Pregnancy BW (kg) | After Pregnancy BW (kg) | Rate of premature delivery | Infant BW (kg) | Apgar Score | |
|---|---|---|---|---|---|
| Euthyroid | 56.1 ± 8.6 | 71.7 ± 9.9 | 24/241 | 3.46 ± 0.44 | 9.9 ± 0.5 |
| SCH | 53.3 ± 8.0 | 69.4 ± 9.0 | 9-Mar | 3.47 ± 0.53 | 9.9 ± 0.5 |
| OH | 56.6 ± 7.5 | 73.0 ± 8.7 | 14-Mar | 3.42 ± 0.58 | 9.9 ± 0.3 |
Abbreviations: BW: Body Weight; SCH: Subclinical Hypothyroidism; OH: Overt Hypothyroidism.
Table 2. SCH, OH and the risk of premature delivery.
The other characteristics including body weight of women before and after pregnancy, body weight of new-borns, and the Apgar score of new-borns among euthyroid, SCH, and OH group were not significantly different among groups (Table 2).
The effect of LT4 treatment on the premature delivery in SCH women
Among SCH women, 1 woman who received LT4 treatment during the pregnancy did not have premature delivery; while in the non-treated SCH women, the rate of premature delivery was 37.5%, and was not significantly different from LT4- treated groups (Table 3).
| Before Pregnancy BW (kg) | After Pregnancy BW (kg) | Rate of premature delivery | Infant BW (kg) | Apgar Score | |
|---|---|---|---|---|---|
| Control | 55.6 ± 6.4 | 73.0 ± 8.5 | 8-Mar | 3.48 ± 0.54 | 9.8± 0.61 |
| LT4 | 53.0 ± 8.0 | 69.1 ± 8.9 | 0/1 | 3.67 ± 0.60 | 10.0 ± 0 |
Abbreviations: BW: Body Weight; SCH: Subclinical Hypothyroidism; OH: Overt Hypothyroidism.
Table 3. The effect of LT4 treatment on the premature delivery in SCH women.
There were also no significant differences in body weight of women before and after pregnancy, body weight of new-born, and the Apgar score of new-born’s among groups (Table 3).
The effect of LT4 treatment on the premature delivery in OH women
Among OH women, 11 women received LT4 treatment during the pregnancy, and none of these-treated women had premature delivery; while in the non-treated OH women, the rate of premature delivery was 100% (3/3), and LT4 treatment significantly reduced the premature delivery in OH women (Table 4).
| Before Pregnancy BW (kg) | After Pregnancy BW (kg) | Rate of premature delivery | Infant BW (kg) | Apgar core | |
|---|---|---|---|---|---|
| Control | 55.6 ± 6.4 | 73.0 ± 8.5 | 3-Mar | 3.44 ± 0.61 | 9.91 ± 0.35 |
| LT4 | 59.7 ± 10.3 | 72.1 ± 10.2 | 0/11* | 3.41 ± 0.46 | 10.0 ± 0 |
Abbreviations: BW: Body Weight; SCH: Subclinical Hypothyroidism; OH: Overt Hypothyroidism.
Table 4. The effect of LT4 treatment on the premature delivery in OH women.
There were no significant differences in body weight of women before and after pregnancy, body weight of new-borns, and the Apgar score of new-borns among groups (Table 4).
In order to evaluate the confounding factors in our findings, we further performed univariate and multivariate logistic regression analysis, and the results in Table 5 showed that only LT4 treatment retained its significance (p value less than 0.05) in both univariate and multivariate models.
| Univariate models | Multivariate models | |||||
|---|---|---|---|---|---|---|
| Parameters | OR | 95% CI | p value | OR | 95% CI | p value |
| Before Pregnancy BW | 1.8 | 1.1-9.6 | 0.171 | - | - | - |
| After Pregnancy BW | 0.1 | 0.21-0.9 | 0.121 | - | - | - |
| Age | 1.3 | 1.2-14.1 | 0.921 | - | - | - |
| Infant BW | 4.3 | 3.2-16.2 | 0.92 | - | - | - |
| Apgar Score | 0.4 | 0.01-0.72 | 0.12 | - | - | - |
| LT4 treatment | 9.2 | 4.0-11.5 | 0.022 | 9.2 | 4.0 - 11.8 | 0.018 |
Abbreviations: Bw: Body Weight; CI: Confidence Interval; OH: Overt Hypothyroidism; OR: Odds Ratio.
Table 5. Logistic regression models of premature delivery in pregnant women treated for OH.
Discussion
In the present study, we performed a hospital-based observational study and focus our analysis on the association between hypothyroidism and maternal and foetal outcomes in 264 pregnant women, and our results showed that compared to pregnant women with euthyroid, pregnant women with SCH had a higher FT4 and TSH levels, while pregnant women with OH had a lower FT4 levels with a higher TSH levels. In our study, pregnant women with OH had a higher rate of premature delivery than women with euthyroid; while pregnant women with SCH were not associated with adverse maternal and foetal outcomes. More importantly, the LT4 treatment in pregnant women with OH reduced the rate of premature delivery.
Pregnancy has pronounced effects on thyroid physiology [16,17]. During pregnancy, thyroxin-binding globulin was elevated, which caused an increase in total concentrations of triiodothyronine and thyroxine, major hormones secreted by the thyroid. Human chorionic gonadotropin (HCG) was found to be elevated during the first trimester, and it is a weak thyroid activator, which causes a slight decrease in the serum TSH concentration [18,19]. Therefore, the serum TSH concentration is low in the first trimester, and then increases significantly in the second and third trimesters; on the other hand, the FT4 level typically increases during the period of peak HCG level in the first trimester, and decreases after the first trimester [20].
It is important for us to use of gestational-age-specific threshold values in order to have accurate diagnosis of thyroid disorders; and trimester-specific reference values for thyroid function have been established for the population using women with normal singleton pregnancies [21]. In our study, we classified the pregnant women into euthyroid, SCH and OH based on the FT4 levels and TSH levels recorded from the second trimester. In our study, the incidence of SCH among pregnant women in our study was 33.3%, which is higher than previously reported values (2-5%) [22], and this inconsistency may be explained by the small population of pregnant women enrolled in our study. SCH has been suggested to be associated with an increased risk of adverse pregnancy complications.
However, in comparison to OH, data regarding SCH are variable. For instance, Negro et al. reported a significantly higher miscarriage rate in TPOAb- women with TSH levels between 2.5 and 5.0 mIU/ L compared with those with TSH levels below 2.5 mIU/L [23]. However, Cleary-Goldma et al. reported no adverse effect from subclinical maternal hypothyroidism in a cohort of 10,990 pregnant women [5]. In our study, SCH in pregnant women was not associated with adverse maternal and foetal outcomes, but we have to be cautious that the small number of SCH women in our study may not be sufficient for us to draw such conclusion.
OH in pregnancy has been shown to be associated with an increased risk of adverse pregnancy complications, and these risks included premature birth, low birth weight, and miscarriage [12]. Allan and colleagues reported an increased risk of foetal death among pregnant women with OH [24], and Abalovich et al. demonstrated OH patients carry an estimated 60% risk of foetal loss [8]. Consistently, our study further demonstrated that OH patients had a higher rate of premature delivery.
Furthermore, we also found that women with OH received LT4 treatment had a lower rate of premature delivery compared that with OH received no treatment, which were consistent with previous studies demonstrating the benefits of LT4 treatment in pregnant women with SCH or OH [13,15,25-28]. Studies also proposed that thyroid diseases have been associated with pulmonary hypertension, and hypothyroidism can impair the vascular relaxation process mediated by adenosine [29]. Taddei et al. also observed a reduction in the availability of the nitric oxide in SCH, which can be partially restored by LT4 treatment [30].
Moreover, in our study, only one SCH women from our study received LT4 treatment, and the treated SCH women had no premature delivery. Future studies may recruit more pregnant women with SCH received LT4 treatment to confirm that effect of LT4 treatment on maternal and foetal outcomes.
In conclusion, our findings demonstrated that pregnancy with different clinical classifications of hypothyroidism is correlated with the rate of premature delivery. In pregnant women with newly diagnosed OH, it is strongly recommended to begin substitutive treatment with LT4 as soon as possible. The present provided support for improved LT4 supplemental replacement treatment for pregnancy in the future and the effects of LT4 treatment on maternal and foetal outcomes in SCH women may require further investigation.
Acknowledgements
This work was supported by Science and Technology Project of Nanjing City (No. 201402002)
References
- Lazarus JH. Thyroid disorders associated with pregnancy:etiology, diagnosis, and management. Treat Endocrinol 2005; 4: 31-41.
- Syrenicz A, Syrenicz M, Sworczak K, Garanty-Bogacka B, Zimnicka A, Walczak M.Hashimoto disease and hypothyroidism in child-bearing period-essential problem for woman and her child. Endokrynol Pol 2005; 56: 1008-1015.
- Rendina D, De Palma D, De Filippo G, De Pascale F, Muscariello R, Ippolito R, Fazio V, Fiengo A, Benvenuto D, Strazzullo P. Prevalence of simple nodular goitre and Hashimotos thyroiditis in current, previous, and never smokers in a geographical area with mild iodine deficiency. HormMetab Res 2015; 47: 214-219.
- Abalovich M, Mitelberg L, Allami C, Gutierrez S, Alcaraz G, Otero P, Levalle O. Subclinical hypothyroidism and thyroid autoimmunity in women with infertility. GynecolEndocrinol 2007; 23: 279-283.
- Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, Canick J, Porter TF, Luthy D, Gross S, Bianchi DW, DAltonME. Maternal thyroid hypofunction and pregnancy outcome. ObstetGynecol 2008; 112: 85-92.
- Gyamfi Bannerman C. Basic science and clinical evidence regarding treatment of subclinical hypothyroidism during pregnancy. ClinObstetGynecol 2011; 54: 488-492.
- Gyamfi C, Wapner RJ, DAlton ME. Thyroid dysfunction in pregnancy:the basic science and clinical evidence surrounding the controversy in management. ObstetGynecol 2009; 113: 702-707.
- Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid 2002; 12: 63-68.
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J ClinEndocrinolMetab 2010; 95: 44-48.
- Goel P, Radotra A, Devi K, Malhotra S, Aggarwal A, Huria A. Maternal and perinatal outcome in pregnancy with hypothyroidism. Indian J Med Sci 2005; 59: 116-117.
- Casey BM:Subclinical hypothyroidism and pregnancy. ObstetGynecolSurv 2006; 61: 415-420.
- Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21: 1081-1125.
- Cappelli C, Negro R, Pirola I, Gandossi E, Agosti B, Castellano M. Levothyroxine liquid solution versus tablet form for replacement treatment in pregnant women. GynecolEndocrinol 2015; 32:1-3.
- Nomura S, Ikegami H, Wada H, Tamai H, Funato M, Shintaku H.Role of levothyroxine supplementation in extremely low birth weight infants who have transient hypothyroidism without thyroid-stimulating hormone elevation. Osaka City Med J 2014; 60: 29-37.
- Zhang L, Zhang Z, Ye H, Zhu X, Li Y. Association between the clinical classification of hypothyroidism and reduced TSH in LT4 supplemental replacement treatment for pregnancy in China. GynecolEndocrinol 2015; 32: 374-378.
- Stagnaro-Green A, Pearce E. Thyroid disorders in pregnancy. Nat Rev Endocrinol 2012; 8: 650-658.
- Krassas GE, Poppe K, GlinoerD.Thyroid function and human reproductive health. Endocr Rev 2010; 31: 702-755.
- Mansourian AR. Thyroid function tests during first-trimester of pregnancy:a review of literature. Pak J BiolSci 2010; 13: 664-673.
- Parkes IL, Schenker JG, Shufaro Y. Thyroid disorders during pregnancy. GynecolEndocrinol 2012; 28: 993-998.
- Limanova Z. Thyroid gland and pregnancy - summary of important findings. VnitrLek 2015; 61: 862-867.
- Marwaha RK, Chopra S, Gopalakrishnan S, Sharma B, Kanwar RS, Sastry A, Singh S. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG 2008; 115: 602-606.
- Woeber KA. Subclinical thyroid dysfunction. Arch Intern Med 1997; 157: 1065-1068.
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, foetal or neonatal death. Eur J Endocrinol 2009; 160: 985-991.
- Allan WC, Haddow JE, Palomaki GE, Williams JR, Mitchell ML, Hermos RJ, Faix JD, Klein RZ.Maternal thyroid deficiency and pregnancy complications:implications for population screening. J Med Screen 2000; 7: 127-130.
- Gur EB, Karadeniz M, Inceefe H, Tatar S, Turan GA, Genc M, Guclu S. Thyroid antibodies in euthyroid and subclinical hypothyroidic pregnant women with autoimmune hypothyroidism: effects on hematological parameters and postpartum hemorrhage. Ginekol Pol 2015; 86:666-671.
- Kraut E, Farahani P. A Systematic Review of Clinical Practice Guidelines' Recommendations on Levothyroxine Therapy Alone versus Combination Therapy (LT4 plus LT3) for Hypothyroidism. Clin Invest Med 2015; 38:305-313.
- Liu P, Liu R, Chen X, Chen Y, Wang D, Zhang F, Wang Y. Can levothyroxine treatment reduce urinary albumin excretion rate in patients with early type 2 diabetic nephropathy and subclinical hypothyroidism? A randomized double-blind and placebo-controlled study. Curr Med Res Opin 2015; 31:2233-2240.
- Villagelin D, Comarella AP, Tiago DB, Ward LS. Management of gestational hypothyroidism: results of a Brazilian survey. Arch EndocrinolMetab 2015; 60: 1-20.
- Scicchitano P, Dentamaro I, Tunzi F, Ricci G, Carbonara S, Devito F, Zito A, Ciampolillo A, Ciccone MM. Pulmonary hypertension in thyroid diseases. Endocrine 2016.
- Taddei S, Caraccio N, Virdis A, Dardano A, Versari D, Ghiadoni L, Salvetti A, Ferrannini E, Monzani F. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J ClinEndocrinolMetab 2003; 88:3731-3737.