Review Article - Biomedical Research (2017) Volume 28, Issue 7
Association between integrin αvβ3 expression and malignancy lymph node metastasis: A meta-analysis
Jia Chen1, Guan-Qiao Jin1, Lin-Hai Yan2 and Dan-Ke Su1*1Department of Medical Imaging Center, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, Guangxi, China
2Department of Gastrointestinal Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, Guangxi, China
- *Corresponding Author:
- Dan-Ke Su
Department of Medical Imaging Center
Affiliated Tumor Hospital of Guangxi Medical University, China
Accepted on November 28, 2016
Abstract
Background: Integrin αvβ3 (alphavbeta3) expression has recently been identified as a prognostic biomarker predicting the tumor invasion and vascularization. This study collected all relevant researches and explored the correlation of αvβ3 expression with malignant metastasis.
Methods: We searched PubMed, Web of Science, Cochrane Library, CNKI, VIP and Wanfang databases with a series of inclusion and exclusion criteria to address the level of αvβ3 expression (accessed May 2016). Nine researches in regard to αvβ3 expression in malignant tumor patients with Lymph Node Metastasis (LNM) and without LNM. Five researches in regard to αvβ3 expression in malignancy and normal control patients. Statistical analysis was conducted by using RevMan5.2 software.
Results: A total of 9 researches (7 studies in Chinese and 2 studies in English) were included in this study, comprising 425 patients with tumor metastasis, 570 without metastasis, and 382 normal control. Immunohistochemistry detection was used in all the researches. The odds ratio, expressed as group with LNM versus group without LNM, was 5.54 (95% CI: 3.72-8.24). The results also revealed that the positive expression rates of αvβ3 in malignant tumor patients were higher than those in normal control patients. The odds ratio was 12.37 (95% CI: 8.77-17.43).
Conclusions: This meta-analysis demonstrated that galectin-3 may become a potentially useful immune marker to distinguish between LNM and non-LNM patients. In addition, αvβ3 expression in malignancies was higher than that in normal control in China.
Keywords
αvβ3, Immunohistochemistry, Metastasis, Malignancy, Meta-analysis.
Introduction
It is generally known that malignant tumors are of great harm to human health, and its incidence rate and death rate is increasing year by year. Metastasis is primary cause of mortality in cancer patients, and the appearance of Lymph Node Metastasis (LNM) is a crucial reporter for distant metastasis and prognosis in numerous cancers. We noted earlier that cancer cell adhesion receptors are conducive to cancer spreading. The integrin family are heterodimeric transmembrane cell adhesion receptors that recognize extracellular matrix proteins and may exist in high or low affinity states. The affinity can determine ligand recognition and signals that impact cell adhesion, survival, invasion, and migration [1-6].
Activation and upregulation of integrin have been proven in the induction of cell metastasis in a lot of solid tumors, like colon carcinoma, melanoma, lung cancer, prostate cancer and so on [7,8]. The αvβ3 integrin is the most prominent molecule in the family plays an important role of angiogenesis [9], which recognizes the arginine-glycine-aspartic (RGD) tripeptide sequence specifically in many extracellular matrix proteins [10], involving in the metastatic cascade and affect tumor cell survival [11,12]. Previous studies have shown that αvβ3 which is generally low expression in normal epithelial cells, but high expression in tumor-like endothelial cells, also in some tumor cells [4,11,13-16]. Moreover, Integrin αvβ3 is required for anchorage-independent proliferation of cancer cells [17]. Expression of integrin αvβ3 is especially expressed on the majority of aggressive tumor cells that invade normal tissue, in majority of solid tumors such as melanoma and pancreas cancer [18]. The expression of integrin αvβ3 is closely related to prostate cancer metastasis [19], poor prognosis of patients with cervical carcinoma [11,15]. Integrin αvβ3 expression also contributes breast cancer cell migration and metastasis since exogenous expression of integrin αvβ3 in breast cancer cells rescues the invasiveness and migration that are suppressed by MYC [12]. The antagonists of integrin αvβ3 obviously inhibited the malignant cell aggressiveness by apoptosis-inducing of proliferative angiogenic vascular cells [17].
It is reported that αvβ3 expression may serve as a useful prediction biomarker in the incidence of metastasis in many malignancies. However, most research examining the impacts of αvβ3 expression are limited by small sample size. Therefore, we performed a meta-analysis to explore the exact association of αvβ3 expression with LNM in human malignancies, furthermore, to prove whether αvβ3 can be a potential tumor marker for LNM.
Materials and Methods
Publication search
Online electronic databases (PubMed, Web of Science, Cochrane Library, CNKI, VIP and Wanfang) were searched with the key terms: (alphavbeta3 or αvβ3) and (metastasis) (update to May 2016). No publication data restriction was applied, and we also checked out the reference lists of all retrieved studies and relevant reviews manually for important cross-references. The citation lists of the retrieved articles were manually screened to ensure the sensitivity of the search strategy.
Inclusion and exclusion criteria
Published studies were included in our meta-analysis if they met all of the following criteria: 1) The study inclusion of patients with LNM; 2) The study must evaluation of the αvβ3 protein positive expression rates in malignancies; 3) Sufficient data, especially αvβ3 positive expression in LNM patients and non-metastatic controls, have been provided to calculate Risk Ratios (RR) and 95% confidence interval (95% CI); 4) Number of cases in enrolled studies should be more than 50; 5) The study must be published in a peer-reviewed journal; 6) The study must be independent from other studies. The exclusion criteria were as follows: 1) The studies did not conform to the inclusion criteria; 2) Reviews, case reports, editorials, guidelines and comments were excluded; 3) Articles published in a language other than English or Chinese; 4) In case of duplicated publications or studies with overlapping data, the study with largest data was selected. 5) Repeated studies were based on non-human subjects.
Data extraction and qualitative assessment
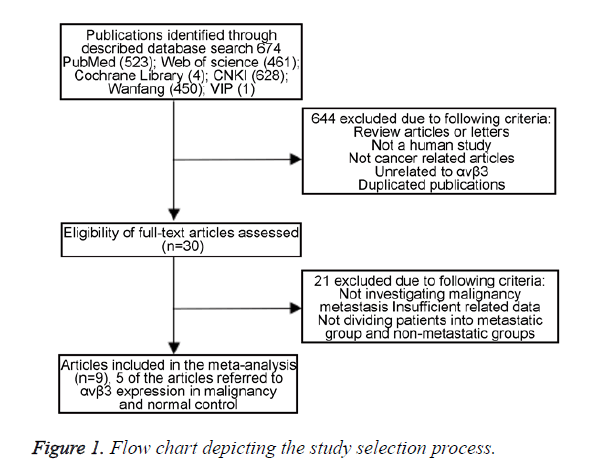
The following data were collected from all the included studies: first author, publication year, country, ethnicity of participants, language, tumor type, numbers of participants, number of high αvβ3 expression group and low αvβ3 expression group, detection method and number of patients with lymph node metastasis in each group. Two investigators extracted data from the selected studies independently, depending on the inclusion and exclusion criteria above. Potential discrepancy was resolved by discussions or by consulting the original report. A flowchart describing the identifying process of qualifying studies is shown in Figure 1.
Sensitivity analysis
In the presence of heterogeneity, sensitivity analysis was performed by omitting one result in each turn and performing statistical analysis again. The new result was compared with the original to explore the effects of omitting the result on the overall estimate. If no difference was observed, the results of the meta-analysis were considered reliable.
Statistical analysis
This meta-analysis was performed by using Review Manager (RevMan) Version 5.2 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Statistical heterogeneity among the studies was assessed by using the I2-statistic. If I2>50%, studies were considered to exhibit severe heterogeneity. If there was a significant statistical heterogeneity among the studies, random-effects model was applied, otherwise fixed-effects model was used. The potential publication bias was assessed by using Begg’s funnel plots. A P-value of less than 0.05 was considered statistically significant.
Results
Description of studies
A total of 9 articles including a total of 1377 patients were enrolled from a search of the above databases using the search strategy as described above [20-29] (Figure 1). Eight studies are in Chinese and two articles are in English. All the research objects were Asians. Among the nine studies, two focused on colorectal cancer, two on non-small cell lung cancer, two on gastric cancer, one on hepatocellular carcinoma, one on breast cancer, and one on epithelial ovarian cancer. The expression of αvβ3 was measured by Immunohistochemistry (IHC) in all normal or malignant tissue. No patients received chemotherapy or radiotherapy before surgery. All the diagnoses of lymph node metastasis were based on pathology. Among these patients, there are 635 with αvβ3 upregulated (63.8%) and 360 with αvβ3 downregulated (36.2%). The clinical characteristics of these nine studies eligible for the meta-analysis are summarized in Tables 1 and 2.
| Year | Surname | Ethnicity | Language | Tumor type | Total number | Detection method | αvβ3 expression | |||
|---|---|---|---|---|---|---|---|---|---|---|
| With LNM | Without LNM | |||||||||
| + | - | + | - | |||||||
| 2014 | Yi Jin | Asian | English | HCC | 305 | IHC | 85 | 6 | 151 | 63 |
| 2012 | Yi Jin | Asian | English | NSCLC | 208 | IHC | 63 | 5 | 95 | 45 |
| 2016 | Kang Li-Xia | Asian | Chinese | GC | 80 | IHC | 26 | 6 | 28 | 20 |
| 2013 | Zhang Ming-Kun | Asian | Chinese | CRC | 53 | IHC | 22 | 4 | 14 | 13 |
| 2011 | Jiang Xue-Qin | Asian | Chinese | EOC | 50 | IHC | 21 | 2 | 11 | 16 |
| 2010 | Hu Chun-Yan | Asian | Chinese | NSCLC | 52 | IHC | 27 | 1 | 15 | 9 |
| 2010 | Shen Qing-Lin | Asian | Chinese | CRC | 73 | IHC | 37 | 20 | 5 | 11 |
| 2007 | Yu Shou-Jian | Asian | Chinese | GC | 105 | IHC | 11 | 59 | 2 | 33 |
| 2006 | Zheng Wei | Asian | Chinese | BC | 69 | IHC | 16 | 14 | 6 | 33 |
| Notes: HCC: Hepatocellular Carcinoma; NSCLC: Non-Small Cell Lung Cancer; GC: Gastric Cancer; CRC: Colorectal Cancer; EOC: Epithelial Ovarian Cancer; BC: Breast Cancer. | ||||||||||
Table 1. Characteristics of the eligible studies about αvβ3 expression in cancer patients with or without LNM in this meta-analysis.
| Year | Surname | Ethnicity | Language | Tumor type | Total number | Detection method | αvβ3 expression | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | Non-cancer | |||||||||
| + | - | + | - | |||||||
| 2014 | Yi Jin | Asian | English | HCC | 610 | IHC | 236 | 69 | 66 | 239 |
| 2016 | Kang Li-Xia | Asian | Chinese | GC | 130 | IHC | 54 | 26 | 23 | 27 |
| 2011 | Jiang Xue-Qin | Asian | Chinese | EOC | 60 | IHC | 32 | 18 | 0 | 10 |
| 2010 | Hu Chun-Yan | Asian | Chinese | NSCLC | 104 | IHC | 42 | 10 | 14 | 38 |
| 2010 | Shen Qing-Lin | Asian | Chinese | CRC | 88 | IHC | 42 | 31 | 2 | 13 |
Table 2. Characteristics of the eligible studies about αvβ3 expression in patients with or without cancer in this meta-analysis.
Study results report and meta-analysis
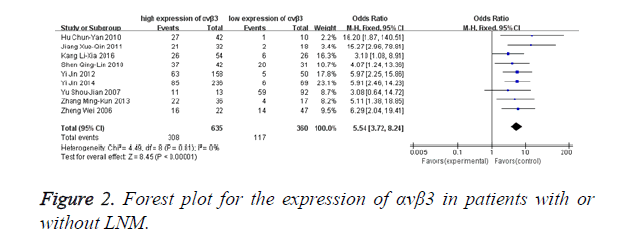
Fixed-effects model was adopted in analysing the expression rates of αvβ3 in malignant patients with lymph node metastasis since there was no significant heterogeneity among the studies (I2=0%, P=0.81). Figure 2 directly reflects significant difference in the positive expression of αvβ3 between the group with LNM and the compare group without LNM. αvβ3 was found to be a highly sensitive (308/425, 72.47%) marker in the diagnosis of LNM. The odds ratio, expressed as LNM group vs. without LNM group, was 5.54 (95% CI: 3.72-8.24, P<0.00001).
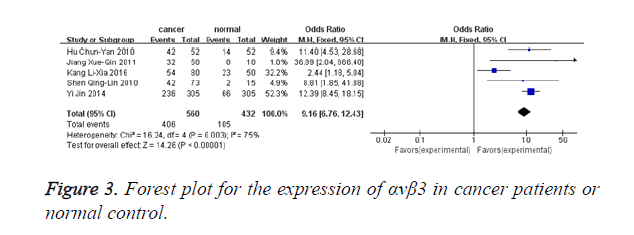
Figure 3 shows that 5 studies provided αvβ3 expression level in malignant patients and normal controls (480 malignant patients and 382 normal controls). Heterogeneity test revealed the existence of heterogeneity in those 5 trials, thus a random-effects model was used (I2=75.0%, P=0.003). Meta-analysis result revealed that αvβ3 expression in malignant patients was significantly higher when compared with normal controls (OR=8.19, 95% CI: 3.53-19.00, P<0.00001).
Sensitivity analysis and publication bias
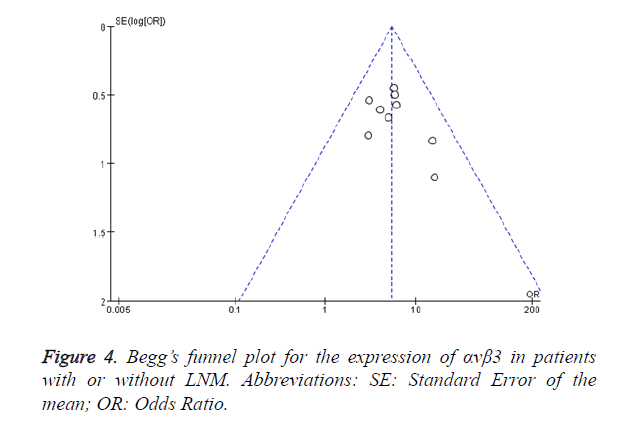
Begg’s test was used to assess the publication bias. Figure 4 shows the Begg’s funnel plot (pseudo 95% CI) for the expression of αvβ3 in malignant patients with or without LNM. No significant publication bias was observed. This result showed that αvβ3 was helpful for the diagnosis of LNM for malignant patients and the conclusion of this meta-analysis had high credibility.
The results showed that heterogeneity existed in investigating the correlation of αvβ3 expression level in malignant patients and normal controls (Figure 3). Then, a sensitive analysis was used to find the heterogeneous study. After removal of the study by Kang et al. the heterogeneity was reduced from I2=75% (P=0.003) to I2=0% (P=0.86), suggesting it might be the heterogeneous study [29]. The odds ratio, was 12.23 (95% CI: 8.69-17.22, P<0.00001).
Discussion
The metastasis of cancer cells from the primary tumor is the main cause of the failure of tumor therapy. Metastasis is a combinative cascade event that involves numerous sequential processes by cancer cells migrate to distant organs through lymphatic channels and/or the circulation and attack new sites to form new tumors [30]. A range of incorrect but precisely coordinated cellular activities participate in metastasis such as migration, invasion, survival, apoptosis and proliferation. Effective control of cell attachment and detachment are needed in all of these steps, which require integrin involve in these metastatic cascades [7]. At least 24 integrins contain a α- subunit and a β-subunit. They lack the key components of the Extracellular Matrix (ECM), which are the endogenous kinase, regulating localization and activity of proteases, and the cell will migrate on and adhere to diverse matrices [31-34].
Some studies have revealed that integrin αvβ3 is overexpression in both tumor cells, angiogenic endothelial cells and highly vascularized tumors, which is a main regulator of angiogenesis and tumor growth [17,35]. Overexpression of αvβ3 has been reported in many cancers including melanoma, prostate, breast cancer and so on [36-38]. Studies also showed that its overexpression correlates with angiogenesis go up and metastasis in many cancers [39,40]. The integrin αvβ3 allows tumor cells to attach ECM, which generates signals for support cell proliferation, survival, and invasion [5,40,41]. For example, inhibiting the activation of FAK/Src pathway, that depends on integrin αvβ3, not merely reduce tumor metastasis, but also suppresses new blood vessel formation in metastases [39,42].
Several cancer models that affect tumor metastases by reducing angiogenesis has made αvβ3 a factor brightening prospects for preclinical cancer models therapy, such as glioma, breast cancer and multiple myeloma [43,44]. Moreover, a humanized anti-αvβ3 MoAb used in phase I and II clinical trials exerted the anti-proliferative effect with less toxicity and more stability in a few of patients with advanced cancer like ovarian cancer and breast cancer [45]. Although αvβ3 has received great attention for its utility in anti-angiogenesis and represents one of the most promising molecular candidate for the diagnosis of metastasis, using anti-αvβ3 therapies alone have provided unsatisfied results in additional clinical research.
The objective of this meta-analysis is to explore the relationship between overexpression of αvβ3 and LNM of multiple malignancies. Our research combined the results of 995 cancer patients from 9 individual researches, showing that αvβ3 positive expression can predict a high incidence of lymph node metastasis in malignant tumors patients (OR 5.54, 95% CI 1.15-3.28). And αvβ3 was found to be a highly sensitive (308/425, 72.47%) marker in the diagnosis of LNM.
Our researches observed that the overexpression rates of αvβ3 in malignant patients and normal controls as well. The odds ratio, expressed as positive αvβ3 group in malignant patients versus negative αvβ3 group in normal controls, was 12.37 (95% CI: 8.77-17.43). In this review, the test for heterogeneity of the included researches was significant. We find the possible source of heterogeneity in this research and the article might have a significant influence on overall heterogeneity [29]. And when we excluded this study, the heterogeneity was disappearing (I2=0%, P=0.86). We analysed the potential source of heterogeneity for this paper might be as the following factors: 1) tumor specimen preservation methods and time were different after surgical resection. 2) In included studies, reagents for IHC, reaction condition and analysis software may different; the difference in this literature is likely to affect the results. 3) The formation of heterogeneity might link to the condition that an optimal threshold has not been estimated, the cut-off defining LNM with αvβ3 expression is arbitrary, which might create heterogeneity. 4) In this text cut-off value is responsible for part of the heterogeneity.
So far, this is the first meta-analysis performed to evaluate the relationship between αvβ3 and LNM of malignancy in human comprehensively. However, we also acknowledged our review has potential limitations: 1) it is still need to perform large-scale and better design studies to support our conclusions; 2) the major limitation of the research was that patients in our research were all of Asian descent; therefore, our study results may just represent patients from Asia; 3) tumor category is limited that reported by literature, so that we cannot actually confirm the αvβ3 overexpression influence LNM in other types of tumors; 4) a universal standard for the division of αvβ3 expression groups is crucial for our research.
In conclusion, this study revealed that the incidence of LNM in patients defined with positive expression of αvβ3 was higher than that in patients with negative expression of αvβ3 in China. The αvβ3 displayed more sensitivity and value in respect of diagnosis and prognosis of LNM in many malignancies. In addition, αvβ3 also could become a useful immune marker to distinguish between normal controls and malignant patients. The αvβ3 expression levels in cancers could become an independent and accurate candidate provide more significant contributions in predicting disease staging and prognostic for malignant patients.
Acknowledgments
This work was supported by the grants: the National Natural Science Foundation of China (No. 81460452).
References
- Arnaout MA, Goodman SL, Xiong JP. Structure and mechanics of integrin-based cell adhesion. Curr Opin Cell Biol 2007; 19: 495-507.
- Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol 2005; 17: 509-516.
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol 2007; 25: 619-647.
- Felding-Habermann B, Otoole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, Mueller BM. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA 2001; 98: 1853-1858.
- Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci 2002; 115: 3729-3738.
- Lim ST, Mikolon D, Stupack DG, Schlaepfer DD. FERM control of FAK function: implications for cancer therapy. Cell Cycle 2008; 7: 2306-2314.
- Ganguly KK, Pal S, Moulik S, Chatterjee A. Integrins and metastasis. Cell Adh Migr 2013; 7: 251-261.
- Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol Sci 2012; 33: 405-412.
- Ravelli C, Mitola S, Corsini M, Presta M. Involvement of αvβ3 integrin in gremlin-induced angiogenesis. Angiogenesis 2013; 16: 235-243.
- Horton MA. The alpha v beta 3 integrin vitronectin receptor. Int J Biochem Cell Biol 1997; 29: 721-725.
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 2010; 10: 9-22.
- Liu H, Radisky DC, Yang D, Xu R, Radisky ES, Bissell MJ, Bishop JM. MYC suppresses cancer metastasis by direct transcriptional silencing of alphav and beta3 integrin subunits. Nat Cell Biol 2012; 14: 567-574.
- Hosotani R, Kawaguchi M, Masui T, Koshiba T, Ida J, Fujimoto K, Wada M, Doi R, Imamura M. Expression of integrin alphaVbeta3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas 2002; 25: e30-35.
- Danen EH, Ten Berge PJ, Van Muijen GN, Van t Hof-Grootenboer AE, Brocker EB, Ruiter DJ. Emergence of alpha 5 beta 1 fibronectin- and alpha v beta 3 vitronectin-receptor expression in melanocytic tumour progression. Histopathology 1994; 24: 249-256.
- Gruber G, Hess J, Stiefel C, Aebersold DM, Zimmer Y, Greiner RH, Studer U, Altermatt HJ, Hlushchuk R, Djonov V. Correlation between the tumoral expression of beta3-integrin and outcome in cervical cancer patients who had undergone radiotherapy. Br J Cancer 2005; 92: 41-46.
- McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene 2007; 26: 6238-6243.
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994; 79: 1157-1164.
- Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N, Tarin D, Shattil SJ, Cheresh DA. An integrin alpha (v) beta (3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med 2009; 15: 1163-1169.
- van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Pelger RC, van der Pluijm G. Integrin alphav expression is required for the acquisition of a metastatic stem/progenitor cell phenotype in human prostate cancer. Am J Pathol 2011; 179: 2559-2568.
- Hu CY, Zhou JH, Deng ZH, Fu CY, Yang XJ, Chen C. Expression of ADAM23 and avß3 in non-small cell lung cancer and its clinicopathologic significance. Chin J Clin Exp Pathol 2010; 26: 433-437, 441.
- Zhang MK, Wang FJ, Yang MP, Li SL, Wang CJ. ADAM23 and avß3 expression in colorectal cancer and their relations with liver metastases. Chin J Gen Srug 2013; 22: 1297-1301.
- Jin Y, Tong DY, Chen JN, Feng ZY, Yang JY, Shao CK, Li JP. Overexpression of osteopontin, alphavbeta3 and Pim-1 associated with prognostically important clinicopathologic variables in non-small cell lung cancer. PLoS One 2012; 7: e48575.
- Jin Y, Chen JN, Feng ZY, Zhang ZG, Fan WZ. OPN and αvβ3 expression are predictors of disease severity and worse prognosis in hepatocellular carcinoma. PLoS One 2014; 9: e87930.
- Liu P, Li YF, Li Q, Xia CF, Cheng XS, Yang ZB. Expression and clinical significance of EGFR, αvβ3 and ADAM23 in Colorectal Cancer. Prac J Cancer 2016; 1-3.
- Shen QL, Ma JL, Pu HW, Bo XH, Chen X. Expressions and its clinical significance of avß3-integrin and MMP-11 in colorectal carcinoma tissues. Med Res Edu 2010; 27: 3-6.
- Jiang XQ, Kong FD, Dong HL, Liu FL. Expression of OPN,avß3 and VEGF in EOC and their relationship with invasion and metastasis. Chin J Woman Child Health Res 2011; 22: 428-431.
- Yu SJ, Tan G, Pan SH, Sun XY. Expression of integrin aVß3 in gastric cancers and its clinical significance. Chin J Curr Adv Gen Surg 2007; 10: 416-419.
- Zheng W, Wu ZY, Zhen LL, Wang HJ, Zhu X. Expression of integrin αvβ3 in breast cancer and the relationship between integrin αvβ3 and bone marrow micrometastasis. Acta Univ Med Nanjing 2006; 26: 417-420, 430.
- Kang LX, Zheng H, Wang L, Feng YL. Expression and significance of integrin avß3 mRNA and its proteins in gastric carcinoma. Chin J Health Lab Tech 2016; 984-985, 988.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646-674.
- Conroy KP, Kitto LJ, Henderson NC. αvβ3 integrins: key regulators of tissue fibrosis. Cell Tissue Res 2016; 365: 511-519.
- Missan DS, Dipersio M. Integrin control of tumor invasion. Crit Rev Eukaryot Gene Expr 2012; 22: 309-324.
- Xiong J, Balcioglu HE, Danen EH. Integrin signaling in control of tumor growth and progression. Int J Biochem Cell Biol 2013; 45: 1012-1015.
- Ricard-Blum S, Vallet SD. Matricryptins network with matricellular receptors at the surface of endothelial and tumor cells. Front Pharmacol 2016; 7: 11.
- Versleijen-Jonkers YM, Vlenterie M, van de Luijtgaarden AC, van der Graaf WT. Anti-angiogenic therapy, a new player in the field of sarcoma treatment. Crit Rev Oncol Hematol 2014; 91: 172-185.
- Kageshita T, Hamby CV, Hirai S, Kimura T, Ono T, Ferrone S. Differential clinical significance of alpha(v)Beta(3) expression in primary lesions of acral lentiginous melanoma and of other melanoma histotypes. Int J Cancer 2000; 89: 153-159.
- Wechsel HW, Petri E, Feil G, Nelde H, Bichler K. Renal cell carcinoma. Immunohistological study to the expression of the inactive form of the pyruvate kinase. Urologe A 1999; 38: 583-585.
- Stucci S, Tucci M, Passarelli A, Silvestris F. αvβ3 integrin: Pathogenetic role in osteotropic tumors. Crit Rev Oncol Hematol 2015; 96: 183-193.
- Liu GX, Xi HQ, Sun XY, Wei B. Role of periostin and its antagonist PNDA-3 in gastric cancer metastasis. World J Gastroenterol 2015; 21: 2605-2613.
- Weber MR, Zuka M, Lorger M, Tschan M, Torbett BE, Zijlstra A, Quigley JP, Staflin K, Eliceiri BP, Krueger JS, Marchese P, Ruggeri ZM, Felding BH. Activated tumor cell integrin alphavbeta3 cooperates with platelets to promote extravasation and metastasis from the blood stream. Thromb Res 2016; 140: 27-36.
- Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM. The integrin-growth factor receptor duet. J Cell Physiol 2007; 213: 649-653.
- Lee YJ, Kim IS, Park SA, Kim Y, Lee JE. Periostin-binding DNA aptamer inhibits breast cancer growth and metastasis. Mol Ther 2013; 21: 1004-1013.
- Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer 2004; 90: 561-565.
- Mulgrew K, Kinneer K, Yao XT, Ward BK, Damschroder MM. Direct targeting of alphavbeta3 integrin on tumor cells with a monoclonal antibody. Abegrin Mol Cancer Ther 2006; 5: 3122-3129.
- Posey JA, Khazaeli MB, Delgrosso A, Saleh MN, Lin CY, Huse W, Lobuglio AF. A pilot trial of Vitaxin, a humanized anti-vitronectin receptor (anti alpha v beta 3) antibody in patients with metastatic cancer. Cancer Biother Radiopharm 2001; 16: 125-132.