Review Article - Environmental Risk Assessment and Remediation (2017) Novel approaches to Chemical Risk Assessment
Assessment of ecological risks of agrochemicals requires a new framework.
Francisco Sánchez-Bayo1*, Henk A Tennekes2
1School of Life & Environmental Sciences, The University of Sydney, Eveleigh NSW 2015, Australia
2Experimental Toxicology Services (ETS) Nederland BV, Zutphen, The Netherlands
- *Corresponding Author:
- Francisco Sánchez-Bayo
The University of Sydney
Eveleigh NSW 2015
Australia
Tel: +61-2-8627 1046
E-mail: sanchezbayo@mac.com
Accepted date: August 01, 2017
Abstract
The widespread contamination of a significant proportion of the planet’s land and water with pesticides is undeniable. While this takes place, innumerable species of animals associated with agricultural landscapes are declining at rates that may put them on the brink to extinction in the span of a lifetime. It is evident, therefore, that our current risk assessment of agrochemicals has failed to protect the environment. A new framework is proposed here that combines the mandatory introduction of new toxicity endpoints with a more logical assessment of risks within the existing tiered approach. Chronic toxicity tests designed to detect time-cumulative effects should be a requirement for assessing delayed mortality as well as population endpoints that are crucial for the recovery and survival of species, such as fecundity. Exposure assessments should ensure that field monitoring data integrate both the highest and average levels of residues, as the former levels determine the main ecological impacts. The first tier of the risk assessment should comprise an evaluation of short-term mortality (as currently done by a hazard quotient on acute toxicity) and of the lethality after chronic exposure to sublethal levels. Chemicals that pass this tier should be assessed in a second tier whereby the crucial population endpoints are evaluated. Unless there is evidence of recovery with no negative effects on reproduction of the species tested, a chemical should not be registered. Further tiers of assessment, including sublethal effects, community effects tested in model ecosystems (i.e. microcosms, mesocoms) and field trials can still be used as supporting evidence, as it is currently done.
Keywords
Endpoints, time-cumulative toxicity, populations, fecundity, recovery, pesticides regulation.
Introduction
Modern agriculture relies heavily on the use of chemicals. It has been estimated that every year 150 million tons of fertilizers and 6 million tons of pesticides are routinely applied to fields and crops with the only objective of increasing agricultural production [1]. While there is evidence that the use of herbicides can increase yields in many crops [2], there is also evidence that most fungicides and insecticides do not help increase such yields [3]. On the other hand, the ecological risks of these chemical inputs to the environment are often ignored by the general public, when not dismissed by those who assert that a growing human population needs to be fed at all costs [4], including health, economic and environmental costs [5,6].
Concerns about the massive use of pesticides, in particular insecticides, in agriculture were raised half a century ago by Rachel Carson [7], sparking an environmental movement that has lasted to this day. Regulations about the safety of individual pesticides were enacted in the United States and other developed countries in the 1970s, while most developing and underdeveloped countries remained oblivious to their negative effects [8,9] until their routine misuse impacted on human health [10-12] and brought about other negative environmental consequences [13-15]. Since then, assessment of the risks to humans and the environment has been carried out before a new agrochemical product is launched to the market. However, while impacts on human health are carefully scrutinized, the assessment of impacts on the environment is performed using methodologies that are either inappropriate or lack sound scientific basis. Not surprisingly, the loss of biodiversity in aquatic ecosystems has been correlated with pesticide residues in waters and sediments [16,17], which change the structure and function of invertebrate communities [18]. At the same time, the entomofauna is collapsing in developed countries [19-23] while populations of many vertebrate species that depend on them [24-26] have been declining as well. The evidence at hand attest to our inability to properly assess the risks that insecticides and other agrochemicals have on the natural environment. Some authors have proposed a post-registration monitoring in an effort “to identify unexpected direct and indirect impacts on organisms by accounting for multiple propagation routes and exposures” [27]. This approach assumes that pesticides that are already registered may be later found to cause unforeseen effects in the environment, when the damage is already done. It does not prevent the use of a new product and does not guarantee its withdrawal from the market either.
We previously evaluated the methods and shortcomings of the current approaches used for assessing the ecological risks of agrochemicals [28] and pointed out their deficiencies, which stem mostly from our poor understanding of toxicological effects at the population and ecosystem levels. In this paper, we suggest a new framework for making the ecological risk assessments (ERA) more realistic, using examples from the past and current failures to illustrate and justify our standpoint. Using the current framework for ERA, we will describe below what we consider essential for a reform of the current risk assessment system. It is expected that the implementation of the new framework may help prevent the registration of dangerous agrochemicals before they are launched to the agricultural market.
Toxicity assessment
The main flaw in the current ecological risk assessments stems from an inadequate understanding of the toxicity of chemicals to populations of organisms. The entire framework is based upon the acute toxicity of a poisonous substance to a small set of non-target species representative of major taxa-the so-called surrogate species in ecotoxicity testing-together with the chronic toxicity to mammals only. This framework is derived from our knowledge of human toxicology, which focuses exclusively on the effects at the individual level and regards effects such as carcinogenicity or mutagenicity very highly, even if they are for the most part irrelevant to animal species in the wild environment; this is because, by their very nature, pesticides are highly poisonous chemicals designed to kill either animals (e.g. insects, worms, snails, rodents) or plants and fungi. They act upon a biochemical or physiological mechanism specific to the target taxa, so the individual organisms usually die before they can develop any long-term effects such as cancer. Teratogenic effects and malformations are very rarely, if at all, caused by pesticides. Other substances, such as dioxins and heavy metals, are typically to be blamed for those aberrations [29,30]. Obviously, testing for carcinogenic and mutagenic effects is relevant only to human health, not the environment.
Currently, ecotoxicity assessments of agrochemicals are based on the median lethal dose (LD50) or concentration (LC50) of a particular chemical to the non-target surrogate species that are presumably present in a given environment. As mentioned above, such endpoints refer to acute lethality, usually within a short time frame: from 24 to 96 hours for most organisms, although 1 or 2 weeks are typical with earthworms. Chronic toxicity is only tested in experimental mammals (e.g. rats, mice or rabbits) because of its relevance to human health. Recent regulations have proposed also chronic toxicity tests with bees [31,32], but lasting only 10 days while forager bees usually live 30 days and winter bees up to three months.
Knowing the lethal potency of a pesticide is very important, but it is not the only way to assess its impact on populations of organisms. Animals, plants and fungi reproduce, which means they overcome their individual losses, caused by either pesticides or any other factor, by producing new individuals. This is very obvious in the case of insecticide resurgence, whereby an insect pest that has been decimated by an insecticide application reacts by mass-producing more progeny, as the insect pest struggles to cope with a threat to its own survival. A similar outcome is expected in populations of non-target species that may be affected by the toxic effects of the insecticide: it is called recovery, and it allows the populations affected by the toxic chemical to survive in the long-term [33,34]. Therefore, no matter how deadly a pesticide may be in the short-term, the survival of a few individuals may be sufficient to restore the impacted populations to their former levels. It also implies that toxicity endpoints that consider only the acute effects of a substance are not suitable to predict its long-term impacts on populations.
Our planet, however, is recording an unprecedented loss of populations of species that live in agricultural landscapes and yet are not the target of the pesticides applied in those areas [35]. While pesticides are not the only factor involved in such declines, as habitat and food losses are also to blame, they are the major contributors to the population collapses [36]. These declines are observed with insect pollinators [37], in particular bees [38,39] and butterflies [40,41], as well as insectivore vertebrates such as frogs [42,43], fish [14], small birds [44,45] and bats [46,47]. Sound ecological theory states that a population of organisms will decline whenever its rate of increase is lower than its rate of mortality [48,49], while a continuous downward trend will eventually cause its extinction [50]. The demise of a population, therefore, is more important in ecological terms than the temporary loss of a few individuals that can be offset by recovery. Furthermore, if the declining populations mentioned above are linked to pesticides, a toxicity assessment should be able to explain the physiological mechanisms involved in the long-term declines. In this context, three explanations are possible:
i) there is complete eradication of all individuals in a given population by direct toxic effect of the pesticide;
ii) the recovery of the population is hampered by some reproduction impairment in the affected species; and
iii) the decline results from indirect effects due to insufficient food resources or another factor.
Because of the recovery process explained above, the first mechanism is rarely observed with most pesticides. However, there are chemicals that can achieve a complete elimination of local populations, including pest species as well as nontarget species. Examples of this kind are the neonicotinoid insecticides, which are very efficient in eradicating mango hoppers (Idioscopus spp.) in fruit trees [51], aphids and thrips in ornamental flowers [52,53], citrus pests such as the Asian citrus psyllid (Diaphorina citri), and the citrus leafminer caterpillar (Phyllocnistis citrella) [54], mealybugs (Pseudococcus spp.) in vineyards [55] and the hemlock woolly adelgid (Adelges tsugae) in forests [56,57], to mention a few. However, similar level of efficacy applies to non-target insects such as mirid and veliid bugs, both natural enemies of rice pests, with 100% mortalities recorded in 24 h [58]. In experimental rice mesocosms, epibenthic ostracods, larvae of chironomids, mayflies nymphs and some aquatic predators were wiped out or significantly reduced in numbers after planting of rice seedlings treated with imidacloprid, and while the recovery of most species occurred two or three months later, some ostracod species did not recover at all [59]. Also, when rice paddies were treated with a mixture of ethiprole + imidacloprid (125 g/ha), the predatory mirid bugs and spiders also suffered an initial set back, and their recovery was slow and never attained the densities found in the nontreated plots [60]. In stream mesocosms, residues of thiacloprid in water eliminated several species of aquatic insects, and only those species that had short life cycles were able to recover after 10 weeks, whereas those that had long life cycles did not [61]. Such long-term toxic effects occurred despite the insecticide residues in leaves or water having declined to undetectable levels, suggesting a delayed toxic effect [62].
Delayed mortality, also called time-cumulative toxicity or reinforced toxicity, results in increased mortality rates with time of exposure and it applies to toxicants that act irreversibly or slowly reversibly on a receptor while producing an irreversible effect [63]. It can be detected by estimating the acute/chronic ratios of a toxic substance, which in this case may span two or three orders of magnitude [64], but the best way to assess it is by conducting time-to-event (TTE) toxicity tests. Instead of conducting simple toxicity tests under fixed-time constraints, as is the standard ecotoxicity practice, TTEs provide information on the concentrations (or doses for terrestrial organisms) as well as the time to achieve levels of mortality upon exposure to a chemical [65]. This should be a mandatory requirement for testing chemicals from now on.
The second mechanism is probably more common or likely to occur amongst pesticides. Even if the individual organisms exposed to a pesticide survive, it may be that they experience non-lethal side effects that may reduce their fecundity, so that the rate of growth of the population turns to be negative [48]. A multitude of impairments have been observed with many pesticides tested at sub-lethal levels under laboratory conditions, with some of them negatively affecting the reproductive abilities of the species tested. Examples include the lower fecundity of the pea aphid (Acyrthosiphon pisum) exposed to sublethal concentrations of Margosan-O extracts (active ingredient, azadirachtin) [66] and that of the cotton aphid (Aphis gossypii) exposed to cycloxaprid [67], both of which can help reduce the populations of those pests and achieve their effective long-term control. However, similar negative effects can be observed in beneficial, predatory and parasitoid arthropod species, such as the decrease in fecundity of predatory Coleomegilla maculata lady beetles after exposure to commercial formulations of 2,4-D and dicamba herbicides [68] and reduced ovipositions of Eriopis connexa lady beetles exposed to the insecticides teflubenzuron and cypermethrin [69]; the severe reduction in fecundity of predatory thrips (Scolothrips longicornis) exposed to abamectin [70]; or the reduced fecundity of the generalist predatory bug Orius armatus exposed to spinosad [71], amongst many others. Other non-target organisms also experience impaired reproductive effects; for example, the reduced fecundity of queen honey bees (Apis mellifera) exposed to sublethal doses of bifenthrin and deltamethrin [72] or to field relevant residues of imidacloprid [73,74]; the reduced spawning of Australian crimson-spotted rainbowfish (Melanotaenia fluviatilis) and medaka fish (Oryzias latipes) exposed to sublethal concentrations of esfenvalerate insecticide in water [75,76]; or the reduced population growth rate of Daphnia spp. exposed to sublethal concentrations of spinosad [77].
In rare cases, pesticides may enhance the fecundity of some species, as for example that of the snail Lymnaea palustris exposed to the fungicide hexachlorobenzene [78], and Lymnaea stagnalis exposed to the herbicide chlorotoluron [79].
Even if fecundity is not reduced under sublethal exposure, the rate of population growth can be negative for other reasons, therefore compromising the long-term viability of a species. This is the case of the lady beetle Hippodamia variegata exposed to sublethal concentrations of thiamethoxam [80]. Neonicotinoids can also negatively affect honeybee drone sperm quality [81,82] while fipronil affects drone fertility by inducing a decrease in spermatozoa quantity that is associated with an increase in spermatozoa mortality [83], so these insecticides may ultimately lead to colony failure [84].
It is our contention that sublethal effects that impair the viability of populations through reproduction should be given at least the same priority in risk assessments as the acute toxicity, as indicated in Table 1. This is because reproductive impairments, such as those mentioned above, are more likely to be the cause of population declines than the temporary losses of individuals that result from direct exposure to the acute toxicity of some insecticides.
Indirect effects through the food chain, such as those that result from lack of food resources [85], cannot be included in these tiers because they do not derive from the direct toxic effects of the pesticide, be lethal or sublethal, on the species affected. Further toxicity evaluations may be necessary for assessing the indirect effects of pesticides on communities of organisms. For that purpose, microcosms and mesocosms treated with a single pesticide or a mixture of pesticides can be carried out. This kind of information may not be necessary for regulatory assessments but it should be included in post-registration monitoring studies whenever there is evidence of negative impacts at the ecosystem level [27].
Finally, toxicity of mixtures is also important for comprehensive ERAs of pesticides, as many chemical substances are applied to the same crop during the growing season, not just one alone. Special attention must be paid to the synergism of certain fungicides (i.e. ergosterol inhibiting compounds) with insecticides, which enhance the toxicity of neonicotinoids and pyrethroids to both target and non-target organisms [86,87]. However, mixture toxicity is not included in regulatory assessments of any chemical [88], and should not be required unless two or more active ingredients are present together in the same product.
Exposure assessment
Current exposure assessments are performed using a variety of models [28]. The models are necessary for assessing the likely scenarios of exposure to a pesticide during the registration process, since the products are not released into the environment yet. For agrochemicals that are already in use, the data obtained from modeling must be validated with actual measurements done through monitoring under different conditions, locations and crop situations.
Bioaccumulation in tissues, degradability in environmental matrices and persistence are the key properties to watch out. Agrochemicals that bioaccumulate should not be considered for registration in the first place, given the problems they cause, as the dark history of organochlorines and chlorfluazuron insecticides has demonstrated. Such chemicals are still present in agricultural soils [89] and are being transferred to animal tissues [90-92].
The analytical methods available nowadays are sufficiently good to measure any pesticide residues that can be found in the environment. In many surveys, however, the highest residue levels are missed due to using inappropriate sampling procedures. This introduces a bias in the monitoring data gathered, as the worst case scenarios that may well be the cause of population declines in some species are ignored. Passive samplers deployed in water or air can obtain integrated measurements of residues over a period of time, including peaks and troughs, so are found to produce better data than simple grab samples [93]. Whatever the case, the monitoring residue data must be evaluated for the highest peaks as well as the average or the median concentrations of residues in the matrices considered, whether plant products (e.g. pollen, nectar, fruit), soil, water or air.
The only requirement for this assessment is to obtain a comprehensive set of values that can be useful for the ERA. In this regard, the only obstacle is the high cost of the analyses, which often impedes or reduces the monitoring efforts necessary for a correct assessment of risks. Cheaper alternatives exist (e.g. ELISA kits), but they are mainly used for screening purposes in routine quality tasks (e.g. to discard negative detections in food or environmental matrices) and are not a substitute for instrumental analyses.
Risk assessment
The existing ERA framework attempts to integrate the toxicity and exposure assessments into a single evaluation that will be used to either register a new product or to assess the ecological impact of the pesticide(s) under consideration in a particular area or region.
Many shortcomings are present in the current ERA of pesticides, most of which have been explained in our previous publication [28], so they won’t be dealt with here. Our emphasis now is in applying a rational and logical framework based on the new ecotoxicity data outlined above.
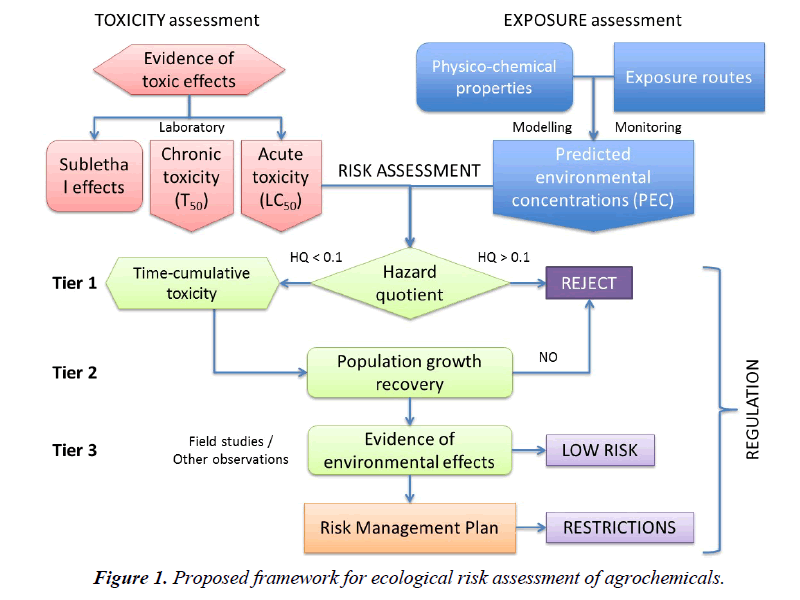
The first tier of an ERA aims at screening chemicals that pose an unacceptable risk to the surrogate test species. The standard hazard quotient (HQ) ratio used for this purpose typically evaluates the acute toxicity of the pesticide (e.g. LC50, LD50, NOEL) against the expected environmental concentrations in several media (e.g. air, water, soil), accepting any chemical that produces values below 0.1. There are three reasons for setting this threshold value: i) the acute toxicity data evaluated in the first tier refers to a representative species of a taxon, but we know that differences in sensitivity among species in any one taxon range at least one order of magnitude [94] therefore, to account for the sensitivity of other species the HQ threshold should be some 10 times lower; ii) the infamous history of DDT and cyclodiene insecticides revealed that populations of predatory birds affected by eggshell thinning started to decline when these insecticide residues in their bodies were 10 times lower than the median effective doses that produced such an effect [95]; iii) a large number of mesocosm studies with insecticides have shown that recovery of aquatic invertebrate populations tends to occur when residue concentrations in water are about 0.1 x EC50 values [34]. As a result, values of 1 for HQ ratios that use LC50 or LD50 data are not protective and have to be lowered by a factor of ten.
In the current system of pesticide registration, if the resulting HQ exceeds 0.1 for a given surrogate species, the chemical must undergo a second tier evaluation that considers further laboratory toxicity tests (e.g. acute toxicity to more species, microcosms) and trials in semi-field (e.g. mesocosms) or field conditions, under the assumption that such conditions usually reduce the exposure of the organisms and, therefore, the effects may not be as pronounced as predicted by the original HQ values. It should be noted that these evaluations are done using only acute, short-term toxicity data. Whenever the data available are inconclusive to make a decision, a third tier may be considered to evaluate further impacts due to sublethal effects (e.g. endocrine disruption and others). Indirect effects are not considered in any case.
In site-specific ERA of pesticides that are currently used in agriculture, the risk assessment may consider species sensitivity distributions (SSD) of acute toxicity values to a range of species in the first tier instead of using HQ values, although more often SSDs are used in the second tier assessment. In that case, SSD data are used in probabilistic risk assessment (PRA) to determine the proportion of species that would be negatively affected by the highest or normal levels of residues predicted in a given environment [96].
Up to now risk assessments have been mostly, if not exclusively, based on acute toxicity data, ignoring any other toxicity effects that may be due to chronic exposure but are more relevant to the long-term viability of a species in the natural environment. A new order of priorities is proposed here, which considers mortality under acute or chronic exposure in the first tier, population growth endpoints in the second tier and sublethal effects such as endocrine disruption and other impairments in the third tier (Table 1).
| Effects | Exposure time | Concentration | Endpoints* | Current ERA | Proposed ERA |
|---|---|---|---|---|---|
| Mortality | 24-96 h (acute) | Range | LC50, LD50 | 1st tier | 1st tier |
| >96 h (chronic) | Range | T50 | Not | 1st tier | |
| Population growth | >96 h (chronic) | Sub-lethal | Recovery rate | Not | 2nd tier |
| >96 h (chronic) | Sub-lethal | Intrinsic rate of growth | Not | 2nd tier | |
| >96 h (chronic) | Sub-lethal | Fecundity | 2nd tier | 2nd tier | |
| Endocrine disruption | 24-96 h (acute) | Range | LOEC | 3rd tier | 3rd tier |
*median lethal concentration (LC50) or dose (LD50); median time to death (T50); lowest observed effect concentration (LOEC)
Table 1. Current and proposed toxicity tests used for ecological risk assessment (ERA) of agrochemicals.
The assessment of acute mortality endpoints can still be done as up to now, but with an additional difference: even if the HQ value is below 0.1, the chemical should not be approved until it is evaluated for its time-cumulative toxicity under sublethal chronic exposures (Figure 1). All agrochemicals should be tested using TTE assays to determine whether or not the chemical has delayed, time-cumulative mortality, whereas chemicals that produce HQ>0.1 should be exempt of further evaluations because they must be rejected. The rationale for acting like this is based on our experience with the novel class of neonicotinoid insecticides, which produce values of HQ below 0.1 for most aquatic and terrestrial species when the acute 24 or 48-h LC50 or LD50 data are used, and yet produce a large proportion of mortality when the same species are exposed to much lower concentrations for a prolonged period of time [97-99]. Screening for such chemicals is deemed essential – thus, its inclusion in the first tier.
Chemicals that act agonistically upon specific receptors, such as nicotinic receptors or others, tend to produce delayed, timecumulative mortality because the continuous excitation of the receptor often leads to the death of the cell. If the cell cannot be regenerated (i.e. neurons), such an effect is irreversible, hence the resulting pattern of toxicity is not only dependent on dose but also on the time of exposure to sublethal levels [63]. For such chemicals, the risk assessment should aim at determining the time to 50% mortality (T50) in a population under the normal and worst exposure scenarios, as it has been described elsewhere [28]. In our view, chemicals that behave this way should not be approved because the long-term negative impacts they have on the populations of numerous species.
Once the first tier is passed, all chemicals should be evaluated for their effects on population growth, which are the effects that really determine whether the species will recover from exposure or not, due to the variety of mechanisms indicated above, under normal and worst case exposure scenarios. This step is also essential for making a decision about the agrochemical under assessment, so only those compounds that do not affect the fecundity of species and do not produce a negative rate of increase in the populations tested should pass this assessment or else be rejected. Again, the history of DDT and cyclodiene insecticides has shown that while the individual birds of prey that accumulated these chemicals were alive and possibly healthy, their populations were on the road to extinction simply because the hatching of their fragile, thin-shelled eggs failed; consequently, the rates of population growth declined to levels below the natural replacement threshold of the species and were unsustainable in the long-term [100]. Indeed, sublethal effects that have a serious impact on the long-term viability of populations are as important or more than lethal effects in the short-term. Lessons from the past such as this should be borne in mind when regulating the use of current systemic insecticides that are implicated on colony collapses of honey bees, and other pollinators, mainly through sublethal effects [101], and dismiss calls to the contrary [102].
Further tiers of assessment should be kept as they are now, as possible community impacts can only be detected in microcosm or mesocosm studies, while the indirect effects on non-target populations can only be detected after years of using pesticides that are apparently harmless [103]. Once again, we should learn from the hard lessons of the past so as not to repeat them [104].
Conclusions
Data collected over many years of monitoring are showing that population declines of innumerable vertebrate and invertebrate species associated with agricultural landscapes are mainly due to pesticides. Failure in protecting these environments, which are subject to the constant application of a large array of chemical pesticides year after year, strongly suggests that the current risk assessments methods used to date are inadequate to evaluate the ecological impacts of such products. Not only the methods, but the existing framework of ERA is clearly inadequate.
To correct the mistakes done so far we must embark on a serious reform of the current ERA framework or else face the extinction of a substantial number of species in the coming years. Here we have proposed some modifications to that framework in the hope that chemicals may be assessed for the long-term damage they cause rather than the short term losses they inflict. Further adjustments may be necessary to make this new framework more robust and workable, but we are confident that we have set the basis for a reform that is long overdue.
References
- Bernhardt ES, Rosi EJ, Gessner MO. Synthetic chemicals as agents of global change. Front Ecol Environ. 2017;15:84-90.
- Gianessi LP. The increasing importance of herbicides in worldwide crop production. Pest Manag Sci. 2013;69(10):1099-1105.
- Lechenet M, Dessaint F, Py G, et al. Reducing pesticide use while preserving crop productivity and profitability on arable farms. Nature Plants. 2017;3:17008.
- Jeschke P. Progress of modern agricultural chemistry and future prospects. Pest Manag Sci. 2016;72(3):433-55.
- Popp J, Pet? K, Nagy J. Pesticide productivity and food security. A review. Agron Sust Develop. 2013;33(1):243-55.
- Wilson C, Tisdell C. Why farmers continue to use pesticides despite environmental, health and sustainability costs. Ecol Entomol. 2001;39:449-62.
- Carson R. Silent Spring. Boston, MA: Houghton-Mifflin. 1962:323 p.
- Khan M, Mahmood HZ, Damalas CA et al. Pesticide use and risk perceptions among farmers in the cotton belt of Punjab, Pakistan. Crop Protection. 2015;67(0):184-90.
- Mansour SA. Pesticide exposure- Egyptian scene. Toxicology. 2004;198(1-3):91-115.
- Ecobichon DJ. Pesticide use in developing countries. Toxicology. 2001;160(1-3):27-33.
- Konradsen F, van der Hoek W, Cole DC, et al. Reducing acute poisoning in developing countries- options for restricting the availability of pesticides. Toxicology. 2003;192(2-3):249-61.
- Wesseling C, Corriols M, Bravo V. Acute pesticide poisoning and pesticide registration in Central America. Toxicol Appl Pharmacol. 2005;207(2, Supplement 1):697-705.
- Rands RW. Pesticide use on cereals and the survival of grey partridge chicks: a field experiment. J Appl Ecol. 1985;22(1):49-54.
- Scholz NL, Fleishman E, Brown L, et al. A perspective on modern pesticides, pelagic fish declines, and unknown ecological resilience in highly managed ecosystems. BioScience. 2012;62(4):428-34.
- Walker CH, Newton I. Effects of cyclodiene insecticides on the sparrowhawk (Accipiter nisus) in Britain-A reappraisal of the evidence. Ecotoxicology. 1998;7:185-89.
- Beketov MA, Kefford BJ, Schäfer RB, et al. Pesticides reduce regional biodiversity of stream invertebrates. PNAS. 2013;110(27):11039-43.
- Kellar CR, Hassell KL, Long SM, et al. Ecological evidence links adverse biological effects to pesticide and metal contamination in an urban Australian watershed. J Appl Ecol. 2014;51(2):426-39.
- Schäfer RB, Caquet T, Siimes K, et al. Effects of pesticides on community structure and ecosystem functions in agricultural streams of three biogeographical regions in Europe. Sci Total Environ. 2007;382(2-3):272-85.
- Colla S, Packer L. Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodivers Conserv. 2008;17(6):1379-91.
- Conrad KF, Warren MS, Fox R, et al. Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol Conserv. 2006;132(3):279-91.
- Forister ML, Jahner JP, Casner KL, et al. The race is not to the swift: Long-term data reveal pervasive declines in California's low-elevation butterfly fauna. Ecology. 2011;92(12):2222-35.
- Sorg M, Schwan H, Stenmans W, et al. Ermittlung der Biomassen flugaktiver Insekten im Naturschutzgebiet Orbroicher Bruch mit Malaise Fallen in den Jahren 1989 und 2013. Proceedings of the Krefeld Entomological Society. 2013;1:1-5.
- Woodcock BA, Isaac NJB, Bullock JM, et al. Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nature Communications. 2016;7:12459.
- Fuentes-Montemayor E, Goulson D, Park KJ. Pipistrelle bats and their prey do not benefit from four widely applied agri-environment management prescriptions. Biol Conserv. 2011;144(9):2233-46.
- Hallmann CA, Foppen RPB, van Turnhout CAM, et al. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature. 2014;511:341-43.
- Sparling DW, Fellers GM, McConnell LL. Pesticides and amphibian population declines in California. USA. Environ Toxicol Chem. 2001;20(7):1591-95.
- Vijver MG, Hunting ER, Nederstigt TAP, et al. Postregistration monitoring of pesticides is urgently required to protect ecosystems. Environ Toxicol Chem. 2017;36(4):860-65.
- Sánchez-Bayo F, Tennekes HA. Environmental Risk Assessment of Agrochemicals- A Critical Appraisal of Current Approaches. In: Larramendy ML, editor. Toxicity and Hazard of Agrochemicals. Rijeka, Croatia: InTech Open Science. 2015:p 1-37.
- Dickman M, Rygiel G. Chironomid larval deformity frequencies, mortality, and diversity in heavy-metal contaminated sediments of a Canadian Riverine Wetland. Environ Int. 1996;22:693-703.
- White DH, Seginak JT. Dioxins and furans linked to reproductive impairment in wood ducks. The Journal of Wildlife Management. 1994;58(1):100-06.
- Hesketh H, Lahive E, Horton AA, et al. Extending standard testing period in honeybees to predict lifespan impacts of pesticides and heavy metals using dynamic energy budget modelling. Sci Rep. 2016;6:37655.
- OECD. Honey bee (Apis mellifera), chronic oral toxicity test 10 day feeding in the laboratory. In: OECD, editor. Paris, France. 2016:p8.
- Van den Brink PJ, Wijngaarden RPAV, Lucassen WGH, et al. Effects of the insecticide Dursban 4E (active ingredient chlorpyrifos) in outdoor experimental ditches: II. Invertebrate community responses and recovery. Environ Toxicol Chem. 1996;15(7):1143-53.
- Wijngaarden RPAV, Brock TCM, van den Brink PJ. Threshold levels for effects of insecticides in freshwater ecosystems: a review. Ecotoxicology. 2005;14(3):355-80.
- Chamberlain DE, Fuller RJ. Local extinctions and changes in species richness of lowland farmland birds in England and Wales in relation to recent changes in agricultural land-use. Agric Ecosyst Environ. 2000;78:1-17.
- Mineau P, Whiteside M. Pesticide acute toxicity is a better correlate of U.S. grassland bird declines than agricultural intensification. PLoS One. 2013;8(2):e57457.
- Cane JH, Tepedino VJ. Causes and extent of declines among native North American invertebrate pollinators: detection, evidence, and consequences. Conservation Ecology. 2001;5(1):31-38.
- Cameron SA, Lozier JD, Strange JP, et al. Patterns of widespread decline in North American bumble bees. PNAS. 2011;108(2):662-67.
- Kosior A, Celary W, Olejniczak P, et al. The decline of the bumble bees and cuckoo bees (Hymenoptera: Apidae: Bombini) of Western and Central Europe. Oryx. 2007;41(1):79-88.
- Forister ML, Cousens B, Harrison JG, et al. Increasing neonicotinoid use and the declining butterfly fauna of lowland California. Biol Lett. 2016;12(8):20160475.
- Gilburn AS, Bunnefeld N, Wilson JM, et al. Are neonicotinoid insecticides driving declines of widespread butterflies? PeerJ. 2015;3:e1402.
- Fellers GM, Drost CA. Disappearance of the cascades frog Rana cascadae at the southern end of its range, California, USA Biol. Conserv. 1993;65(2):177-81.
- Lips KR. Decline of a tropical montane amphibian fauna. Conserv Biol. 1998;12(1):106-17.
- Fuller RJ, Gregory RD, Gibbons DW, et al. Population declines and range contractions among lowland farmland birds in Britain. Conserv Biol. 1995; 9(6):1425-41.
- Hart J, Milsom T, Fisher G, et al. The relationship between yellowhammer breeding performance, arthropod abundance and insecticide applications on arable farmland. J Appl Ecol. 2006;43(1):81-91.
- Clark DR. DDT and the decline of free-tailed bats (Tadarida brasiliensis) at Carlsbad Cavern, New Mexico Arch. Environ Contam Toxicol. 2001;40(4):537-43.
- Stahlschmidt P, Brühl CA. Bats at risk? Bat activity and insecticide residue analysis of food items in an apple orchard. Environ Toxicol Chem. 2012;31(7):1556-63.
- Sibly RM, Hone J. Population growth rate and its determinants: an overview. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2002;357(1425):1153-70.
- Walthall WK, Stark JD. A comparison of acute mortality and population growth rate as endpoints of toxicological effect. Ecotoxicol Environ Saf. 1997;37(1):45-52.
- Tanaka Y. Ecological risk assessment based on extinction probability of populations. Nihon Seitai Gakkaishi. 1998;48:327-35.
- Verghese A. Effect of imidacloprid on mango hoppers, Idioscopus spp. (Homoptera: Cicadellidae). Pest Manage Hort Ecosyst. 1998;4(2):70-74.
- Abanowski G, Soika G. Usefulness of neonicotinoid insecticides in control of ornamental plant pests Przydatnosc preparatow neonikotynoidowych do zwalczania szkodnikow roslin ozdobnych. Progress in Plant Protection. 2001;41(1):161-69.
- Maienfisch P, Angst M, Brandl F, et al. Chemistry and biology of thiamethoxam: a second generation neonicotinoid. Pest Manag Sci. 2001;57(10):906-13.
- Sétamou M, Rodriguez D, Saldana R, et al. Efficacy and uptake of soil-applied imidacloprid in the control of Asian citrus psyllid and a citrus leafminer, two foliar-feeding citrus pests. J Econ Entomol. 2010;103(5):1711-19.
- Lo PL, Walker JTS. Soil applications of two neonicotinoid insecticides to control mealybugs (Pseudococcidae) in vineyards. New Zealand Plant Protection. 2011;64:101-06.
- Cowles RS, Montgomery ME, Cheah CASJ. Activity and residues of imidacloprid applied to soil and tree trunks to control hemlock woolly adelgid (Hemiptera: Adelgidae) in forests. J Econ Entomol. 2006;99(4):1258-67.
- Frank SD, Lebude A. Season-long insecticide efficacy for hemlock woolly adelgid, Adelges tsugae (Hemiptera: Adelgidae), management in nurseries. Fl Entomol. 2011;94(2):290-95.
- Lakshmi VJ, Krishnaiah NV, Katti GR. Potential toxicity of selected insecticides to rice leafhoppers and planthoppers and their important natural enemies. J Biol Control. 2010;24(3):244-52.
- Sánchez-Bayo F, Ahmad R, Goka K. Evaluation of the standard quotient and EcoRR methodologies based on field monitoring from rice fields. In: Kennedy IR, Solomon KR, Gee SJ, Crossan AN, Wang S, Sánchez-Bayo F, editors. Rational Environmental Management of Agrochemicals. Washington, DC: American Chemical Society. 2007:p 66-86.
- Kumar BV, Boomathi N, Kumaran N, et al. Non target effect of ethiprole+imidacloprid 80 WG on predators of rice planthoppers. Madras Agricultural Journal. 2010;97(4/6):153-56.
- Beketov M, Schäfer RB, Marwitz A, et al. Long-term stream invertebrate community alterations induced by the insecticide thiacloprid: Effect concentrations and recovery dynamics. Sci Total Environ. 2008;405:96-108.
- Beketov MA, Liess M. Acute and delayed effects of the neonicotinoid insecticide thiacloprid on seven freshwater arthropods. Environ Toxicol Chem. 2008;27(2):461-70.
- Tennekes HA, Sánchez-Bayo F. The molecular basis of simple relationships between exposure concentration and toxic effects with time. Toxicology. 2013;309:39-51.
- Sánchez-Bayo F, Goka K, Hayasaka D. Contamination of the aquatic environment with neonicotinoids and its implication for ecosystems. Front Environ Sci. 2016;4:71.
- Newman MC, McCloskey JT. Time-to-event analyses of ecotoxicity data. Ecotoxicology. 1996;5(3):187-96.
- Stark JD, Rangus TM. Lethal and sublethal effects of the neem insecticide formulation, Margosan-O, on the pea aphid. Pestic Sci. 1994;41(2):155-60.
- Yuan HB, Li JH, Liu YQ, et al. Lethal, sublethal and transgenerational effects of the novel chiral neonicotinoid pesticide cycloxaprid on demographic and behavioral traits of Aphis gossypii (Hemiptera: Aphididae). Ins Sci. 2016.
- Freydier L, Lundgren JG. Unintended effects of the herbicides 2,4-D and dicamba on lady beetles. Ecotoxicology. 2016;25(6):1270-77.
- Fogel MN, Schneider MI, Rimoldi F, et al. Toxicity assessment of four insecticides with different modes of action on pupae and adults of Eriopis connexa (Coleoptera: Coccinellidae), a relevant predator of the Neotropical Region. Environ Sci Pollut Res. 2016;23(15):14918-26.
- Pakyari H, Enkegaard A. Sublethal and transgenerational effects of abamectin on the biological performance of the predatory thrips Scolothrips longicornis (Thysanoptera: Thripidae). J Econ Entomol. 2015;108(2):559-65.
- Broughton S, Harrison J, Rahman T. Effect of new and old pesticides on Orius armatus (Gross)- an Australian predator of western flower thrips, Frankliniella occidentalis (Pergande). Pest Manag Sci. 2014;70(3):389-97.
- Dai P-L, Wang Q, Sun J-H, et al. Effects of sublethal concentrations of bifenthrin and deltamethrin on fecundity, growth, and development of the honeybee Apis mellifera ligustica. Environ Toxicol Chem. 2010;29(3):644-49.
- Williams GR, Troxler A, Retschnig G, et al. Neonicotinoid pesticides severely affect honey bee queens. Sci Rep. 2015;5:14621.
- Wu-Smart J, Spivak M. Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Sci Rep. 2016;6:32108.
- Barry MJ, O'Halloran K, Logan DC, et al. Sublethal effects of esfenvalerate pulse exposure on spawning and non-spawning Australian crimson-spotted rainbow fish (Melanotaenia fluviatilis). Arch Environ Contam Toxicol. 1995;28:459-63.
- Werner I, Geist J, Okihiro M, et al. Effects of dietary exposure to the pyrethroid pesticide esfenvalerate on medaka (Oryzias latipes). Marine Environmental Research. 2002;54:609-14.
- Duchet C, Coutellec MA, Franquet E, et al. Population-level effects of spinosad and Bacillus thuringiensis israelensis in Daphnia pulex and Daphnia magna: comparison of laboratory and field microcosm exposure conditions. Ecotoxicology. 2010;19:1224-37.
- Baturo W, Lagadic L, Caquet T. Growth, fecundity and glycogen utilization in Lymnaea Palustris exposed to atrazine and hexachlorobenzene in freshwater mesocosms. Environ Toxicol Chem. 1995;14(3):503-11.
- Nélieu S, Bonnemoy F, Bonnet JL, et al. Ecotoxicological effects of diuron and chlorotoluron nitrate-induced photodegradation products: Monospecific and aquatic mesocosm-integrated studies. Environ Toxicol Chem. 2010;29(12):2644-52.
- Rahmani S, Bandani AR. Sublethal concentrations of thiamethoxam adversely affect life table parameters of the aphid predator, Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae). Crop Protection. 2013;54(0):168-75.
- Ciereszko A, Wilde J, Dietrich GJ, et al. Sperm parameters of honeybee drones exposed to imidacloprid. Apidologie. 2017;48(2):211-22.
- Straub L, Villamar-Bouza L, Bruckner S, et al. Neonicotinoid insecticides can serve as inadvertent insect contraceptives. Proc R Soc B. 2016;283:20160506.
- Kairo G, Poquet Y, Haji H, et al. Assessment of the toxic effect of pesticides on honey bee drone fertility using laboratory and semifield approaches: A case study of fipronil. Environ Toxicol Chem. 2017.
- Pettis JS, Rice N, Joselow K, et al. Colony failure linked to low sperm viability in honey bee (Apis mellifera) queens and an exploration of potential causative factors. PLoS One. 2016;11(2):e0147220.
- Boatman ND, Brickle NW, Hart JD, et al. Evidence for the indirect effects of pesticides on farmland birds. Ibis. 2004;146:131-43.
- Pilling ED, Bromley-Challenor KAC, Walker CH, et al. Mechanism of synergism between the pyrethroid insecticide λ-cyhalothrin and the imidazole fungicide prochloraz, in the honeybee (Apis mellifera L). Pestic Biochem Physiol. 1995;51(1):1-11.
- Sgolastra F, Medrzycki P, Bortolotti L, et al. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manag Sci. 2016.
- Lydy M, Belden J, Wheelock C, et al. Challenges in regulating pesticide mixtures. Ecology in Society. 2004;9(6):1-15.
- Shivaramaiah HM, Odeh IOA, Kennedy IR, et al. Mapping the distribution of DDT residues as DDE in the soils of the irrigated regions of northern New South Wales, Australia using ELISA and GIS. J Agric Food Chem. 2002;50(19):5360-67.
- Braune BM, Malone BJ. Organochlorines and trace elements in upland game birds harvested in Canada. Sci Total Environ. 2006;363:60-9.
- Minh TB, Watanabe M, Nakata H, et al. Contamination by persistent organochlorines in small cetaceans from Hong Kong coastal waters. Mar Pollut Bull. 1999;39:383-92.
- Nag SK, Raikwar MK. Persistent organochlorine pesticide residues in animal feed. Environ Monit Assess. 2011;174(1-4):327-35.
- Schäfer RB, Pettigrove V, Rose G, et al. Effects of pesticides monitored with three sampling methods in 24 sites on macroinvertebrates and microorganisms. Environ Sci Technol. 2011;45(4):1665-72.
- Kooijman SALM. A safety factor for LC50 values allowing for differences in sensitivity among species. Water Research. 1987;21(3):269-76.
- Walker CH. Organic Pollutants. Glasgow, UK: Taylor & Francis. 2001:282 p.
- Shi Y, Burns M, Ritchie RJ, et al. Probabilistic risk assessment of diuron and prometryn in the Gwydir River catchment, Australia, with the input of a novel bioassay based on algal growth. Ecotoxicol Environ Saf. 2014;106:213-19.
- Alkassab AT, Kirchner WH. Impacts of chronic sublethal exposure to clothianidin on winter honeybees. Ecotoxicology. 2016;25(5):1000-10.
- Roessink I, Merga LB, Zweers HJ, et al. The neonicotinoid imidacloprid shows high chronic toxicity to mayfly nymphs. Environ Toxicol Chem. 2013;32(5):1096-1100.
- U?urlu P, Ünlü E, Satar E?. The toxicological effects of thiamethoxam on Gammarus kischineffensis (Schellenberg 1937) (Crustacea: Amphipoda). Environ Toxicol Pharmacol. 2015;39(2):720-26.
- Sibly RM, Newton I, Walker CH. Effects of dieldrin on population growth rates of sparrowhawks 1963-1986. J Appl Ecol. 2000;37(3):540-46.
- Smagghe G, Deknopper J, Meeus I, et al. Dietary chlorantraniliprole suppresses reproduction in worker bumblebees. Pest Manag Sci. 2013;69(7):787-91.
- Blacquière T, van der Steen JJM. Three years of banning neonicotinoid insecticides based on sub-lethal effects: can we expect to see effects on bees? Pest Manag Sci. 2017;73(7):1299-1304.
- Poulin B, Lefebvre G, Paz L. Red flag for green spray: adverse trophic effects of Bti on breeding birds. J Appl Ecol. 2010;47(4):884-89.
- Krebs JR, Wilson JD, Bradbury RB, et al. The second Silent Spring? Nature. 1999;400:611-12.