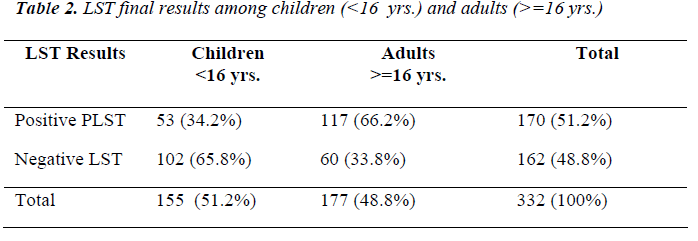- Biomedical Research (2011) Volume 22, Issue 4
Assessment of demographic and nutritional factors on Leishmaniasis in Sudan
Amani Ali Mohamed Osman
Department of Family and Community Medicine, College of Medicine, King Khalid University, Abha, P.O. Box 641, Kingdom of Saudi Arabia
- Corresponding Author:
- Amani Ali Mohamed Osman
Family and Community Medicine Department
King Khalid University
P.O. Box 641, Abha
Kingdom of Saudi Arabia
Accepted date: june 28 2011
Abstract
The objective of this study was to evaluate the magnitude of le ishmaniasis in the study area and to assess the role of demographic and nutritional status in the disease transmis-sion. It was a prospective cohort study on leishmaniasis. It was conducted in 3 villages in West of Sudan. Leishmanin skin test (LST) was assessed as an epidemiological tool in a sub-clinical focus of leishmania in Sudan. The total number of the population in all vil-lages is 332. Methods used in this study, Demographic data were taken and for assessment of nutritional status of children in regard to their type of infection, height and weight were measured using health meter, leishmanin skin test was done to all villagers in first visit. The study was conducted in three field visits, during raining season in 2008 to 2009. In the last two visits, the test was performed to those who had negative LST results in the first visit, so as to detect LST conversion. The results of the positive cases in the three visits were as follows: 42.7%, 46.3% and 51.2%. Almost half of the population had the disease and these subsequently increasing rates indicate continuous transmission. Evaluation the nutritional status which based on weight-for-age and height-for-age The frequency of seriously low weight for age is signifi-cantly higher among the children with clinical visceral leishmaniasis than children with subclinical infection.
Keywords
Leishmania, leishmanin skin test “LST”, Sudan.
Introduction
Sudan is a largest country in Africa with variable climates and environments. The Savna and Sub-Savna occupy a large belt crossing from east to west of Sudan, providing suitable inhabitant for many tropical diseases including leishmaniasis [1,2,3,4,5]..
Balanitis and Accacia forests provide ecological areas for vector {sand fly}[6] and reservoir {zoonosis} [7,8] hence the perfect medium is available for the disease to be en-demic.
Worldwide Leishmania classified as a multifactorial dis-ease and a lot of Micro and Macro environmental factors may be responsible for development of the disease. In the endemic areas like in India, Tunisia and Brazil many studies done to elicit those factors. (i.e., age, sex cytotoxic immune responses, living in same geographical region, way of living , Water lodging, Plantation, Fumigation product and way of making food) [9,10,11]. Most of these studies related malnutrition with outcomes of Leishmania infection . Since the disease predominantly affects the people of low income group in whom the nutritional status is very poor and malnutrition has been considered as a major risk factor for the development of VL. Chil-dren are at great risk of developing VL when they are malnourished. The relationship between malnutrition and VL is poorly understood especially among children. The various nutritional status and laboratory related tests have been carried out in different categories of malnourished VL patients. i.e., cholesterol level, vitamin A level . all test showed sever malnutrition with leishmaniasis and revel low level of previous elements with leishmania in-fection .by comparing children with VL to relatives. [11,12].
LST test is considered to represent an evaluation in vivo of cell mediated immunity (CMI) in leishmaniasis [13,14, 15, 16, 17]. Together with the determination of circulating antibodies, it is a useful tool in epidemiological studies [18]. During active infection, the test is usually negative, but it has been reported to be positive in some cases of sub-clinical or very early visceral leishmanisis (VL) and after successful treatment [19]. Since VL in its active stage is associated with both specific and non-specific suppression of CMI, a previously positive tuberculin reaction may become negative during active VL. After successful treatment, the tuberculin reaction may revert to positive earlier than the leishmanin reaction [20].
LST specificity in healthy controls is nearly 100% for all antigens. Sensitivity increased minimally with increasing dose. Side effects such as vesiculation and ulceration at the site of LST application increased with antigen dose. Storage under hard conditions decreased LST potency but not sensitivity, while storage at 2-8 °C affected neither potency nor sensitivity. 85% of parasitologically diagnosed, LST-positive cases of leishmaniasis remained LST-positive when retested six months to three years later [14].
The LST, which measures the delayed-type cutaneous hypersensitivity (0TH) response to Leishrnania-derived antigens, is useful tool for both clinical diagnosis and epidemiological studies [21]. However, it fails to distinguish current from prior leishmanial infection, a characteristic most problematic in areas where the prevalence of LST reactivity is high [22].
The relative pathogenicity (probability of illness, given infection) in specific endemic foci have been estimated by the fraction of LST reactive individuals who manifest signs or a history of prior leishmanial lesions [23].
Aim of the study
The objective of this study was to evaluate the magnitude of leishmaniasis in the study area.
Specific objectives
To assess the role of demographic characteristic in accordance to leishmaniasis To assess the role of nutritional status in the disease transmission
Rational of the study
This area was not subjected to previous studies for leishmaniasis [24, 25] although it is situated within the leishmania belt which extends across Sudan between two hyperendemic areas on the east for visceral and on the west for cutaneous type of leishmaniasis. The study area is characterized by Unique natural, geographic and ethinic forms. Leishmaniasis was thought to be hyperendemic in this area during the seventies with high mortality among the villagers that led the inhabitants to desert their village for more than 15 years, only few years ago they return back to their village.
Materials and methods
It was prospective cohort epidemiological study, held in three visits during pre-raining, raining and post raining seasons of year 2008 to 2009 (July, September and February ) which reflect the transmission load according to breading of sand flies( leishmania vector).
Study Area
The study was conducted in three Villages in Rashad Province, West of Sudan.
Sample size
All inhabitants of the three villages were included. They were 332 person.
Clear explanation of this study has been provided to all villagers, so the human right have been preserved according to the research ethical requirement of the Gezira university Sudan.
Demographic data were collected using a special questionnaire using personal interviewed to all villagers visiting them in their cottages. For assessment of nutritional status of children in regard to their type of infection, height and weight were measured using health meter.
Analysis of nutritional status was evaluated on the basis o f weight-for-age and height-for-age. The standards from the National Centre for Health Statistics, for expected weight for age and height for age were used in the nutritional status calculations. Nutritional values were assessed using the age, height and weight using health-meter apparatus and depending on Gomes classification system. All children were classified into one of three categories; normal, mild and moderately to severe malnutrition. Normal children those were above 90% for mean weigh for age (WAM) and above 95% for median height to age (HAM). Mild malnutrition describes those who fall between 75% and 90% of median of weight for age or between 90% and 95% of median of height for age. Moderate to severe malnutrition describes individuals of < 75% of the median weight for age or < 90% of the median height for age.
Leihmanin Skin Test - LST (Montenegro Test)
Skin test (delayed-type hypersensitivity [DTH]) antigens used in this study for all villagers . The antigens were prepared from washed suspension of L infanturn promastigotes (Roma), grown in blood agar medium. The promastigotes were resuspended in 0.5% phenol-saline to reach a concentration of 108-1010 cells/ml. A total of 0.1 ml of the antigen was injected intradermally into the alcohol- cleansed volar surface of the patient’s right forearm. The diameter of the induration was measured 48 hrs later by outlining the indurated border with a ball-point pen. The induration with a diameter of 5 mm or bigger has been reported as positive [26]. Grade of induration of LST was measured. It was classified to 4 grades < 5cm is negative, from5 to 8 cm was grade I&II, grade III is more than 8cm. While grade IV is more than8cm with skin bolus reaction. Data collection and measuring height and weight were done by the author who is medical pro-fessional. Leihmanin Skin Test was done by lab techni-cian
Results
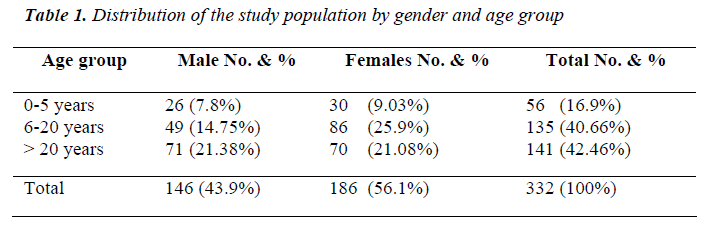
In our study population females were 186 and males were 146 (56.1% and 43.9%, respectively). Population under 20 years of age were 40.6 %. Many mobile, nomadic tribes are settled in this area from various ethnicity such as Massaleet, Arabs and Nuba they mainly work as shep-herds and farmers. Results showen in Table 1.
LST
The final LST results showed that 170 (51.2%) were posi-tive and 162(48.8) were negative, detail information about children and adults was shown in Table (2).
LST-conversion
In the second visit, more 12 cases became positive, the total positives cases were 46.3%. Distributed as follows; 2, 1 and 9 cases in sub-village 1, 2 and 3 consecutively. Sixty-seven percent of them were children (<l6years) and 33% adults (>=l6years). Male/female ratio was 1:1.
LST-grades were; 11 out of those 12 cases were grade 3 (>8mm.). The largest induration size was 16 mm. All 12 cases were subclinical.
In the last visit there were 16 positive cases more, their distribution as follows; 5, 4 and 7 cases in sub-village 1, 2 and 3 consecutively. Fifty percent of them were children (<16years) and 50% adults (>=l6years). Thirty-one per-cent were male and 69% were female.
LST-grades were; 14 out of 16 were grade 3 (>8mm.). The largest induration size was 20 mm. Details were shown in Table 3. All 16 cases were subclinical.
LST-pattern in studied homes during the study
Village 1 (24 cottages): All positive homes (all family members) were 7 on first visit and in the second visit in-creased to 8. While there were no total negative homes which might suggest that the infection was widely spread and involved all homes in this village. Village 2 (36 cot-tages): Positive homes (all family members) were 4 and remain constant throughout the study duration. 8 families (homes) were all negatives this might be due to a large number of absents, denoting that members of this village were not constantly settled due to internal and/or exter-nal migrations. Village 3 (17 cottages): Positive homes (all family members) were 4 in first visit raised to 5 on second one. While total negative homes were 2 reduced to one home in first visit and non on the third visit which suggest the infection transmission.
Analysis of the nutritional status
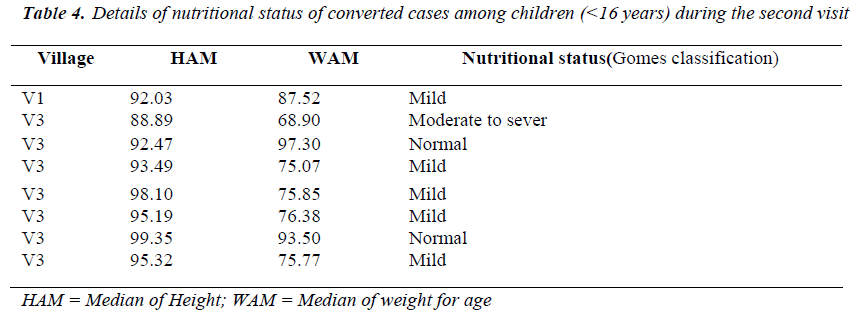
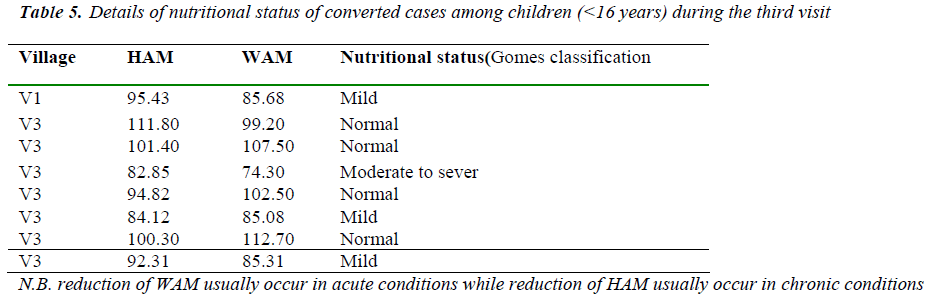
The frequency of seriously low weight for age is signifi-cantly higher among the children with clinical visceral leishmaniasis than children with subclinical infection. The effect of nutritional status on LST conversion among children (<l6yrs) in this study showed in Tables (4,5). Almost two third of children (8 children ) which their LST test converted from negative to positive had malnu-trition according to Gomes classification, in the first visit and 50% of them in the third visit.
Discussion
The frequency of exposure to leishmaniasis among the inhabitant under study was 51.2%. All age group were involved and there were no gender variation. Since the disease predominantly affects the people of low income group in whom the nutritional status is compromised and malnutrition has been considered as a major risk factor for the development of VL. (12) This was clearly showed in children under study whom their LST converted from negative to positive in second visit, 62.5% of them were mild malnourished. In the third visit the percentage of children converted to positive was 8 children 50% of them were malnourished both mild and moderate accord-ing to Gomez scale. Final results according to the stan-dard LST grades (1-4 grades); 20% of children group lies in the 3rd grade, while 51% of the adult group had grade III and grade 4 (bolus reaction) which was observed only among adults. This can be explained by the immature immune system among children. The continuous conver-sion of the negatives to positive LST, is clear in both groups children and adults, this may indicate the continu-ous transmission after rain season as well it indicates in-doors transmission.
There are few reports on the use of leishmanin skin test in the Sudan. Most information comes from Kenya, where Manson- Bahr reported the test to be characteristically negative during active kala-a.zar and to become positive in 80% of cured cases after two years [19]. The first re-port from the Sudan is from the NAMRU-3 team, who found that 11 patients out of 14 treated kala-azar patients positive three or more months after treatment. Also an important epidemiological observations were made by them in Upper Nile Province, areas of active transmission of kala-azar (Palai-Tir), that 59% of individuals tested were positive while in areas where the disease was un-known Bantiu (Southern West of Sudan), only 10% showed positive reactions. While in Shilluk village (South of Sudan), an average of 44% leishmanin positive reac-tions was obtained; no clear increase with age was seen [27].
In a hospital based study 95% of patients with active dis-ease were leishmanin negative [28]. Six Americans who contracted leishmaniasis only four with skin lesions were leishmanin positive at diagnosis, the other two had kala-azar became leishmanin positive after treatment [29].
A leishmanin test survey in Sudan was done in the south-ern Blue Nile area, 42% of individuals were found posi-tive, compared with 77% in a known endemic area for cutaneous leishmaniasis (Darfour West Sudan) and 4% in Khartoum (central Sudan) where all forms of leishmani-asis are uncommon [30]. It is important to evaluate whether repeated skin testing is sensitizing or not. The sensitizing capacity of LST is of interest in setting where they will be administered repeatedly, e.g. vaccination tri-als rely on LST conversion being due to vaccination and not to repeated skin testing [31,32]. Because potency and sensitivity may vary by the patient group and clinical set-ting, LST antigens are best compared when administered simultaneously to the same individuals. Comparison of potency in contrast to the size of the induration, which accompanied the DTH response. The sensitivity of a given antigen is defined as the percent of persons with leishmaniasis in whom the antigen induced a positive leishmanin reactions. The specificity of a given antigen is defined as the percent of healthy volunteers in whom the antigen failed to induce a positive leimshmanin reaction. The duration of LST reaction is widely considered to be for whole life [16].
Conclusion
Malnutrition and associated alterations in resistance to infectious disease contribute to the poor health of popula-tions in much of the world. In fact that malnutrition is a risk factor for developing severe disease after infection by an opportunistic parasite.
The gender has no significant role in disease distribution in this study and as all ages were affected this indicates indoors transmission.
Future recommendation
Further prolong studies are highly recommended to pro-vide useful clinical and epidemiological information of the disease in this area. These clinical and epidemiologi-cal data will aid in disease control strategies.
Acknowledgements
I would like to thank Professor Mohd. Yunus Khan in reviewing the manuscript. Our gratefulness to Dr. Barker DC and Dr. Lambson B. (Cambridge University.), Prof Miles M. and Mauricio I. (London Schobi of Tropical and Hygiene.) for their technical supports. Also my special thank to Drs. Y. Ozbel, S. Ozensoy, M. Z. Alkan, Profs. A. Kuman and M. A. Ozcel fo their valuable help during my visit to Department of Parasitology Ege University Medical School.
References
- Archibald RG. A preliminary report on some further investigations on kala-azar in the Sudan. J Research Army Med Crops, 1914; 23 :479-495.
- Abdalla NM. Leishmania Parasites: Comparative study of variable immune-diagnostic tools with Polymerase Chain Reaction uses in sub-clinical leishmaniasis iso-lates. 2011. J Medicine. 12: 34-39. ISSN 1997-9797 (print), ISSN 2075-5384 (online).
- El Hassan AM, Hashim FA, Ali MS, Chalib HW, Zijlstra EE. Kala-azar in western Upper Nile province in the southern Sudan and its spread to a nomadic tribe from the north. Trans R Soc Trop Med Hyg, 1993; 87: 395-98.
- El-Safi SH, Peters W, Evans DA. Studies on the leishmaniases in the Sudan. 3. Clinical and parasi-tological stutlies on visceral and mucosal leishmaniasis. Trans R Soc Trap Med Hyg,1991; 85:4
- Perea W, Ancelle T, Nagelkerke M, Sondorp E. Vis-ceral leishmaniasis in southern Sudan. Trans R Soc Trap Med Hyg, 1991; 85: 48-53.
- Schorscher JA, Cons M. Incrimination of Phlebotor-nus meeting of the (Larroussius) orientalis as a vector of visceral leishmaniasis in Leishmaniasis western Up-per Nile Province, southern Sudan. Trans R Soc Trap Med Hyg 1992; 86: 622-3.
- Berrahal F, Mary C, Roze M, Berenger A, Escoffier K, Lamouroux D, Dunan S. Canine leishmaniasis: Identi-fication of a symptomatic carrier by polymerase chain reaction and immunoblotting. AmJTrap Med Hyg, 1996; 55(3): 273-7.
- Evans TG Vasconcelos AB, Lima JW, Teixeira JM, McAullife IT, Lopes UG, Pearson RD, Vasconcelos AW. Canine visceral leishmaniasis in Northeast Brazil: Assessment of serodiagnostic methods. Am J Trop Med Hyg,1990; 42: 118-23.
- Anshuman M., Vijay K.P., Sanjana M., Kamlesh G., Madhukar R., Shyam S. Risk Factors For Visceral Li-eshmaniasis In India: Case-Control Study. 4th world congress on leishmaniasis 2009. Page 228.
- Nissaf A, Hechmi L, Sadok C, Amine T, Mourad M, Koussay D, Afif S. Pasteur Institute of Tunis. Risk Factors of Severe Cutaneous Leishmaniasis In Tunisia. 4th world congress on leishmaniasis 2009. Page 506
- Selma M J, Iraci D, Bruna M, Gloria R., Monteiro E S, Nbia NP, Hênio GL, Paula Q, Richard DP, Jenefer B. Risk Factors For The Outcome Of Leishmania Chagasi Infection In A Brazilian Population. 4th world congress on leishmaniasis 2009.P 712.
- Bruna L. Lima M, Hênio GL, José WQ, Juliana G, Núbia NP, Roberto D, Stephen E. M, Lúcia FC, Selma MB. Association of Nutritional Status with the Response to Infection with Leishmania chagasi. The American Society of Tropical Medicine, 2008 vol. 79 no. 4 591-598
- Carvalho EM, Teixcira RS, Warren JD. Cell mediated immunity in American visceral leishmaniasis: re-versible immunosuppression during acute infection. In-fect Immun, 1981; 33:498-502.
- Weigle KA, Valderrama L, Arias AL:, Santrich C, Saravia NG. Leishmanin skin test standardization and evaluation of safety, dose, storage, longgevity of reac-tion and sensitization. Am J Trap Med Hyg,1991; 44(3):260-71.
- Leeuwenburg J, Bryceson ADM, Mbugua GG, Arap Siongok TK. The use of the leishmanin skin-test to de-fine transmission of leishmaniasis in Baringo District, Kenya East Afr Med J 1983; 60:81-84.
- Reed SG, Badaro R, Masur N, Carbalho EM, Lorenco R, Lisboa A, Teixeira R, Johnson WD Jr, Jones TC. Selection of a skin test antigen for American visceral leishmaniasis. Am j Trop Hyg 1986; 35: 79085
- Pampiglione, S, Manson-Bahr, P.E.C, La Placa, M, Borgatti, M, Musumeci S. Studies in Mediterranean Leishmaniasis III. The leishmanin skin test in kala-azar. Trans R Soc Trap Med Hyg, 1985; 69, 60-8.
- Manson-Bahr PEC, Heisch RB, Granham PCC. Stud-ies in leishmaniasis in East Africa IV. The montenegro test in kala-azar in Kenya. Trans R Soc Trop Med Hyg, 1959; 53:380-83.
- Abdalla NM. Evaluation of Gene Targeted PCR and Molecular Hybridization Used in Diagnosis of Human Leishmania Isolates. 2010. J. of Biotechnology 9 (2): 212-217. ISSN 1682-296X
- Rees, PH, Kager, PA, Murithi, MR, Wambua, PP, Shah, SD. & Butterworth, AE. Tuberculin sensitivity in kalaazar. Trans R Soc Trap Med Hyg, 1981; 75:630-31.
- Zijlstra EE, El-Hassan AM. Leishmanin and tuberculin sensitivity in leishmaniasis in the Sudan with special reference to kala-azar. Trans R Soc Trop Med Hyg, 1993; 87: 825-7.
- Kerdell-Vegas F. American Leishmaniasis. Intern J Dermatol, 1982; 291-303.
- Aston DL, Thorley AP. Leishrnaniasis in Central Bra-zil: Results of Montenegro skin test survey among amerindians in the Xingu National Partk. Trans R Soc Trap Med Hyg, 1970; 64: 671-678.
- Abdalla NM, Eldosh MA, Osman OF, Daifalla NS, Magzoub MM. Sero epidemiological Study on Leishmaniasis in The Nuba Mountain - Sudan. Ada Parasitologica Turcica, 2001; 24(3): 228-33.
- Abdalla NM, Ibrahim ME, El-Hassan AM, Osman OF, Daifalla NS, Barker DC, Lambson B, Miles M, Mauri-cio I, Magzoub MM. Clinico-epidemiological Study On Leishmaniasis In The Nuba Mountain - Sudan. Ada Parasilologica Turcica, 2002; 26(1): 23-30.
- Sati MM. Early phases of an outbreak of kala-azar Southern Fung. Sudan Med J, 1958; 1: 98-111
- El Safi SH, Peters W, El Toam B, El-Kada row A, Ev-ans DA. Studies on the leishmaniasis in the Sudan. 2-Clinical and Parasitological studies on cutaneous leishmaniasis. Trans R Soc Trap Med Hyg, 1991; 85: 457-64.
- Van Peenen PFD, Gutekuost RR, Dietlein DR, Reid TP. Serological and skin test survey in a Shilluk vil-lage of Central Sudan. Trans R Soc Trop Med Hyg 1963; 57: 297-305.
- Cahill KM. Leishmaniasis in the Sudan Republic. XXI. Infection in American personnel. Am J Trap Ivfed, 1964; 13:794-9.
- Abdalla RE, Ali M, Wasfi Al, El-Hassan AM. Cutane-ous leishmaniasis in the Sudan Trans RSoc Trop Med Hyg 1973; ; 67: 549-559.
- Antunes CMF, Mayrink W, Magalhaes PA, Costa P, Oliveira Lirna A, Vicira JBF, Schettini APM. Con-trolled field trials of a vaccine against New World Cu-taneous leishmaniasis. Inter J Epid 1986; 15: 572-580.
- Sokal JE. Measurement of delayed skin-test responses. Engl J Med,1984; 93: 501-5022