Research Article - Biomedical Research (2017) Volume 28, Issue 15
Assessment of cooling effect on neonatal pain during heel prick blood sampling: A randomized clinical trial
Arash Malakian1*, Masoud Dehdashtiyan1, Mohammad Raza Aramesh1, Seyyed Mohammad Hassan Aletayeb1 and Fatemeh Ghazanfari2
1Department of Paediatrics, Imam Khomeini Hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
2Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- *Corresponding Author:
- Arash Malakian
Department of Paediatrics, Imam Khomeini Hospital
Ahvaz Jundishapur University of Medical Sciences
Ahvaz, Iran
Accepted date: July 12, 2017
Abstract
Background: Heel prick blood sampling is a painful and stressful procedure performed in NICUs (Neonatal Intensive Care Unit) to collect blood samples for some diagnostic laboratory tests. The local cold is a simple, effective and safe analgesia in newborns. Due to the side effects of pharmacological methods of analgesia and also the relative impact of non-drug methods available, it can be a good alternative for them.
Methods: The current study was a double-blind clinical trial, the infants were born in Imam Khomeini Ahvaz in 2016 and randomly assigned to the case and control groups. In the case group, 20 s after exposure to cold compress on the heels, samples were taken and the control group without exposure to cold compress samples were taken. Pain assessment was performed by CRIES (Pain Scales and Pain Assessment) scale. Data collected by SPSS software version 22 and T-test were analyzed statistically.
Results: The current study was conducted on 68 infants assigned to the case and control groups. Study participants included more boys (60.95%) than girls. All infants enrolled in the current study were 1 d old with Apgar scores of 9-10. In the case group, 90.62% of the subjects had scores<4, and accordingly, 9.37% had scores>4. In the control group, 40.62% of the subjects had scores<4 and 59.37% had >4. There was a statistically significant difference in CRIES score between the groups (P-value<0.001) (according to the CRIES score a total score>4 indicates severe pain). There was no significant difference between the case and control groups in the level of arterial oxygen saturation.
Conclusions: This study showed that local cold can reduce the level of pain caused by heel prick blood sampling and can decrease early and late complications of pain in infants.
Keywords
Pain, Local cold, Infant
Introduction
At 25th week of pregnancy, fetal functional Central Nervous System (CNS) activity starts, which indicates the fetus’s ability to feel pain [1]. Pain affects CNS development, causes changes in behaviour, and enables response to painful stimuli. Therefore, painful procedures, such as drawing blood, can cause significant psychological damage to infants [2]. Inhibition or reduction of pain caused by diagnostic/therapeutic procedures can reduce physiological and behavioural distress in infants [3].using a sterile lance
Infants admitted to the Neonatal Intensive Care Unit (NICU) experience about 14 painful procedures each day, such as drawing blood samples from the veins, arteries, and heel [4]. Acute pain intensifies stress and causes physiological responses such as difficulty in breathing, changes in arterial blood pressure or pressure in the chest, and vasoconstriction in the vital organs [5,6]. Experimental models indicate that failure to alleviate pain and its associated distress, which can cause tissue damage, results in pain disorders, a low pain threshold, increased response to painful procedures, and finally chronic pain later in life [7-9].
Heel prick blood sampling is a painful and stressful procedure performed in NICUs to collect blood samples for some diagnostic laboratory tests [10]. Response to pain following blood drainage from the plantar forefoot is greater in infants [11].
The Canadian Paediatric Society and the American Academy of Paediatrics recommend NICUs to have a specific program of pain management for infants [12]. Effective pharmacological and non-pharmacological methods are available to control pain, which can be administered singly or in combination [13].
Pharmacological pain control methods are as follows: Local ointments such as lidocaine-prilocaine cream, 4% tetracaine gel, liposomal lidocaine, injectable drugs including narcotic drugs (morphine, fentanyl, and remifentanil), and non-narcotic drugs including acetaminophen and ketamine. Routine non-pharmacological pain control methods are giving oral sucrose, swaddling, skin to skin contact, and non-nutritive sucking [14-20].
Local cold can reduce pain by inhibiting sensory receptors and reducing conduction time and synaptic activity in the peripheral nerve [21]. Saeki revealed a significant association between the reduction in skin conductance levels, reduced blood flow, and local cold, which can inhibit damaging stimuli at the spinal level [22].
The current study aimed to evaluate the effectiveness of local cold on reducing the severity of pain caused by heel prick blood sampling.
Materials and Methods
Study participants
The current study was a double-blind clinical trial (registration no. IRCT2016081329336N1). Sixty-eight new born term infants admitted to the NICU in Imam Khomeini Hospital in Ahvaz, Iran, were enrolled in the study. All parents signed written consent for participation of their infants in the study and control of their blood glucose.
Inclusion criteria
One-day-old newborns with a gestational age ≥ 37 weeks who were normally delivered and had Apgar scores>8, weight ≥ 2500 g, and >95% arterial oxygen saturation, had not received sedatives for 6-8 h prior to the procedure, and had no respiratory distress were enrolled into the study.
Exclusion criteria
Infants from whom a blood sample could not be obtained the first time and infants who had hypoglycaemia (blood glucose<47 mg/dL) were excluded from the study.
Study measurements
Infants were assigned to two groups based on a computer-generated random numbers table. Infants were enrolled into the study one hour after feeding when they were alert and calm with a dry diaper.
First, each infant was placed on a bed in a calm room, and then the pulse oximetry probe was placed on the infant’s right hand.
In the case group, a 1 × 1 cm ice pack was placed on the side of infant’s foot (blood drainage area) for 20 s. In the control group, a similar 1 × 1 cm room-temperature pack was placed.
The infant’s Achilles tendon and ankle were fixed with thumb and fingers, respectively [23]. Heel prick samples were drawn by a trained nurse using a sterile lancet.
Then, the infant’s response was assessed, based on the CRIES tool, by a trained nurse who was unaware of the nature of the study, and the results were recorded. The CRIES parameters were also recorded for all participants before the intervention.
CRIES is a 10-score observer-rated pain assessment tool. Its letters stand for five physiological and behavioural parameters associated with pain: crying, requires oxygen, increased vital signs, expression (facial), and sleeplessness (Table 1) [12]. Each parameter is scored 0-2 and a total score>4 indicate severe pain.
| Evaluate | 0 Point | 1 Point | 2 Points |
|---|---|---|---|
| Cry | Absent | High pitch | Inconsolable |
| SpO2>95% | 0.21 | 0.21 to 0.30 | >0.30 |
| HR and/or BP (Compare with preoperative values) | No increase | Increase of up to 20% | ≥ 20% |
| Facial expression | Relaxed | Occasional grimace | Contracted |
| Sleep | Normal | Short intervals | Absent |
Table 1: CRIES scale (Observer-rated pain assessment tool).
Statistical analysis
To compare quantitative and qualitative variables between the groups, the t-test and Chi-square test were used.
Intervening variables were evaluated by analysis of covariance; all analyses were conducted by SPSS version 22. P<0.05 was considered as the level of significance.
Results
In the current study, 2 subjects from the control group and 2 subjects from the case group were excluded from the study because of failure to obtain a blood sample the first time, according to the exclusion criteria.Analysis of demographic characteristics showed that the study participants included more boys (60.95%) than girls.
All infants enrolled in the current study were 1 d old with Apgar scores of 9-10.
There was no significant difference between the groups regarding the above-mentioned variables (Table 2).
| Variables | Case | Control |
|---|---|---|
| Girls-Boys | 14 (43.8%)-18 (56.3%) | 11 (34.4%)-21 (65.6%) |
| Gestational age (38 weeks/37 weeks) | 64%/36% | 52%/48% |
| Apgar score | 9-10 | 9-10 |
| Age (d) | 1 | 1 |
Table 2: Demographic characteristics of the study participants.
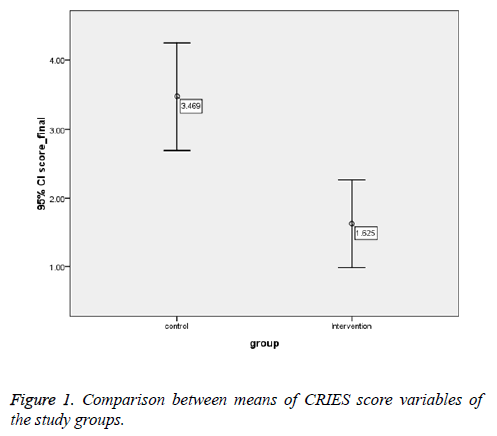
Means of CRIES score variables were significantly lower in the case group (Table 3).
| Variables | Control Mean ± SD |
Case Mean ± SD |
Mean difference | P-value |
|---|---|---|---|---|
| Crying | 0.619 ± 0.94 | 0.619 ± 0.56 | 0.38 | 0.018 |
| Heart rate | 0.499 ± 0.59 | 0.246 ± 0.06 | 0.53 | <0.001 |
| Facial expression | 0.504 ± 0.94 | 0.621 ± 0.47 | 0.47 | 0.002 |
| Sleeplessness | 1.00 ± 0.842 | 0.491 ± 0.22 | 0.78 | <0.001 |
| Total score | 3.47 ± 2.155 | 1.625 ± 0.06 | 2.16 | <0.001 |
Table 3: Comparison between means of variables of the case and control groups.
There was no significant difference between the case and control groups in the level of arterial oxygen saturation.
In the case group, 90.62% of the subjects had scores<4, and accordingly, 9.37% had scores>4. In the control group, 40.62% of the subjects had scores<4 and 59.37% had >4.
There was statistically significant difference in CRIES score between the groups (P-value<0.001) (Table 1 and Figure 1).
Discussion
The current study was conducted on 68 infants assigned to the case and control groups. The mean of the CRIES score in the control and case groups was 3.469 and 1.625, respectively, and this difference was statistically significant (P-value<0.001). Many studies have evaluated methods to reduce pain in infants; some methods were excluded because of their high rate of side effects, such as using analgesic and narcotic drugs, or because they were not useful for routine procedures on infants such as venous/arterial blood sampling, subcutaneous injections, and heel prick blood sampling. Giving 30% oral sucrose, swaddling, skin to skin contact, and non-nutritive sucking are common methods used to reduce infant’s pain.
Slater et al. reported that although administration of sucrose before heel prick blood sampling reduced the Premature Infant Pain Profile (PIPP) score in infants in the case group compared to the control group, it had no effect on reducing the severity or response to pain (P=0.4) [24].
Saeki evaluated the performance of the autonomic nervous system, skin conductance level, and blood flow after painful stimulation with a needle in children before and after local application of cold. They indicated a significant association of reduced skin conductance and lower blood flow with local cold application, in other words, local cold inhibits damaging stimuli at the spinal level [22].
Application of local cold can prevent early and late complications of pain and the impact of pain on the neurodevelopmental evolution of infants by inhibiting damaging stimuli at the spinal level and directing them to the cerebral cortex [3].
Kiran et al. evaluated the effect of cold compression on the level of pain caused by venous blood sampling on 50 children aged 3-7 y old in India. They reported a significant difference in the severity of pain between the control and case groups [21].
Bastami et al. also evaluated the effect of cold compression on the level of pain caused by arterial blood sampling on 61 subjects in Iran. They found a significant difference between the control and case groups in the pain score, which is consistent with the results of the current study [25].
Conclusions
Local cold can reduce the level of pain caused by heel prick blood sampling and can decrease early and late complications of pain in infants.
Acknowledgement
The authors thank the research deputy of Jundishapur University of Medical Sciences for the financial support. The authors also wish to thanks the personals of nursery of Ahvaz Imam Hospital for their assistance.
References
- Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J. Cortical pain responses in human infants. J Neurosci 2006; 26: 3662-3666.
- Gabriel MÁM, de Mendoza BdRH, Figueroa LJ, Medina V, Fernández BI, Rodríguez MV. Analgesia with breastfeeding in addition to skin-to-skin contact during heel prick. Archiv Dis Childhood Foetal Neonatal Ed 2013; 98: 499-503.
- Walker SM. Neonatal pain. Paediatr Anaesth 2014; 24: 39-48.
- Bajic D, Commons KG, Soriano SG. Morphine-enhanced apoptosis in selective brain regions of neonatal rats. Int J Dev Neurosci 2013; 31: 258-266.
- Bosenberg A, Flick RP. Regional anesthesia in neonates and infants. Clin Perinatol 2013; 40: 525-538.
- Taddio A, Katz J. The effects of early pain experience in neonates on pain responses in infancy and childhood. Paediatr Drugs 2005; 7: 245-257.
- National Academies Press. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Mil Med 2016; 181: 397-379.
- Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth 2010; 105: 69-85.
- Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain 2009; 141: 79-87.
- Wilson D, Wong DL, Hockenberry MJ, Wilson D. Wong’s nursing care of infants and children. Mosby/Elsevier 2011.
- Ogawa S, Ogihara T, Fujiwara E, Ito K, Nakano M, Nakayama S. Venepuncture is preferable to heel lance for blood sampling in term neonates. Arch Dis Child Foetal Neonatal Ed 2005; 90: 432-436.
- American Academy of Pediatrics Committee on Foetal and Newborn. Prevention and management of pain in the neonate: an update. Pediatr 2006; 118: 2231-2241.
- Anand KJ. International evidence-based group for neonatal p: consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med 2001; 155: 173-180.
- Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 2004; 363: 1673-1682.
- Anand KJ, Johnston CC, Oberlander TF, Taddio A, Lehr VT, Walco GA. Analgesia and local anesthesia during invasive procedures in the neonate. Clin Ther 2005; 27: 844-876.
- Aranda JV, Carlo W, Hummel P, Thomas R, Lehr VT, Anand KJ. Analgesia and sedation during mechanical ventilation in neonates. Clin Ther 2005; 27: 877-899.
- Saarenmaa E, Huttunen P, Leppaluoto J, Fellman V. Alfentanil as procedural pain relief in new born infants. Arch Dis Child Foetal Neonatal Ed 1996; 75: 103-107.
- Hopchet L, Kulo A, Rayyan M, Verbesselt R, Vanhole C, de Hoon JN. Does intravenous paracetamol administration affect body temperature in neonates? Arch Dis Child 2011; 96: 301-304.
- Oklu E, Bulutcu FS, Yalcin Y, Ozbek U, Cakali E, Bayindir O. Which anesthetic agent alters the hemodynamic status during pediatric catheterization? Comparison of propofol versus ketamine. J Cardiothorac Vasc Anesth 2003; 17: 686-690.
- da Motta Gde C, da Cunha ML. Prevention and non-pharmacological management of pain in newborns. Rev Bras Enferm 2015; 68: 123-127.
- Kiran N, Kaur S, Marwaha R. Effect of ice pack application at the site prior to Venipuncture on intensity of pain among children. Nursing Midwifery Res J 2013; 4: 160-167.
- Saeki Y. Effect of local application of cold or heat for relief of pricking pain. Nurs Health Sci 2002; 4: 97-105.
- MacDonald MG, Ramasethu J, Rais-Bahrami K. Atlas of procedures in neonatology. Lippincott Williams Wilkins 2012.
- Slater R, Cornelissen L, Fabrizi L, Patten D, Yoxen J, Worley A. Oral sucrose as an analgesic drug for procedural pain in newborn infants: a randomised controlled trial. Lancet 2010; 376: 1225-1232.
- Bastami M, Azadi A, Mayel M. The use of ice pack for pain associated with arterial punctures. J Clin Diagn Res 2015; 9: 7-9.