Research Article - Biomedical Research (2022) Volume 33, Issue 7
Assessment of adverse events following immunization with COVID-19 vaccination: An observational study at a tertiary care hospital.
Imran Khan*, Padmaja M, Ramanjaneyulu P, Bhuvana Sri M, Usha Rani P
Department of Clinical Pharmacology and Therapeutics, Nizam's Institute of Medical Sciences, Hyderabad, Telangana, India
- Corresponding Author:
- Imran Khan
Department of Clinical Pharmacology and Therapeutics
Nizam’s Institute of Medical Sciences
Hyderabad
India
Accepted date: 06 September, 2022
Abstract
Introduction: The COVID-19 pandemic continues to ravage the whole world, causing a dramatic impact on the health, social and economic well-being of the people. Vaccines are key tools in the battle against COVID-19. As anticipated with any drug or biological product, vaccines also may cause some adverse events. There is a need to address the safety concerns of COVID-19 vaccines in our population.
Objectives: To assess the pattern of adverse events associated in the beneficiaries receiving COVID-19 vaccination.
Methodology: The study was conducted on eligible beneficiaries receiving COVID-19 vaccine at a tertiary care hospital in south India. AEFI were collected by active and passive methods. Beneficiary details, data of the events were recorded in the case report form and analysed for the pattern, age and gender preponderance, severity, and causality with the COVID-19 vaccine.
Observations: Five hundred and fifty-four beneficiaries reported a total of 1529 events, with a mean age of 26.9 (± 7.2) years. 57.4% were female and 42.6% were male. Fever was the most reported adverse event, followed by body pains and headache 20 (3.52%) cases of AEFI were categorized as serious, which required hospitalization. Outcome of all the events was resolved within a median duration of 4 days from the day of appearance. 99% of the events were assessed to be causally related with the vaccines
Conclusion: Majority of the observed AEFI with COVID-19 vaccinations were non-serious, of mild to moderate severity, hence the COVID-19 vaccines are safe when compared to the risk of severe COVID-19 infection in non-vaccinated.
Keywords
Adverse Events Following Immunization (AEFI), COVID-19 vaccination, Causality.
Introduction
The COVID-19 pandemic continues to ravage the whole world, causing a dramatic impact on the health, social and economic well-being of the people [1]. More than a hundred million patients are affected with COVID-19 of which India contributes to more than 15 million cases [2]. Vaccines save millions of lives by preparing the body to recognize and fight against different viruses and bacteria they target and are key tools in the battle against COVID-19, scientists all over the world have been working relentlessly to develop safe and effective vaccines to save lives and end this pandemic [3]. Vaccines are rigorously tested, however, as anticipated with any drug or biological product, these also may cause some adverse events which may be local or systemic reactions of varying severity.
During the past two years, clinically available COVID-19 vaccines have been developed at an unprecedented speed. With COVID-19 vaccines for mass vaccination, one extremely important prerequisite is to illustrate their safety with confirmed clinical evidence. Their accelerated development process might raise more concerns regarding their potential safety problems and thereby deepen the vaccine hesitancy among people.
Two Indian-made vaccines were granted Emergency Use Authorization. These were ChAdOx1 CoV-19 VACCINE- Covishield (developed by Oxford-AstraZeneca and manufactured by Serum Institute of India- SII) and Covaxin (manufactured by Bharat Biotech Limited)
Adverse Event Following Immunization (AEFI) is any untoward medical occurrence which follows immunization, and which does not necessarily have a causal relationship with the usage of the vaccine. The adverse event may be any unfavourable or unintended sign, abnormal laboratory finding, symptom or disease [4]. Vaccine clinical trials generally recruit healthy or more homogenous and limited number of participants in their studies and the data thus generated may be limited. Furnishing people with evidencebased scientific data is extremely essential and beneficial [1]. There is a possibility of identifying some unexpected and unusual effects or usual effects of unexpected frequency when these vaccines are administered in large scale such as in immunization programs.
In our country, the currently available COVID-19 vaccines are known to cause a spectrum of mild to moderate adverse events such as injection site pain and swelling, headache, nausea, vomiting, diarrhoea, fever, weakness, myalgia, rash, anaphylaxis, chest pain, chest discomfort, etc. Most of the adverse events resolve on their own within few days, however, more serious, or long-lasting adverse events are possible [5].
Hence, this study was planned to assess the adverse events following COVID-19 vaccination and to establish the causality assessment.
Materials and Methods
Settings and design
This study was a prospective observational study done from 16 January to 15 April 2021, conducted in a tertiary care hospital of Southern India. Ethics approval was obtained from the institutional ethics committee. Beneficiaries receiving COVID-19 vaccine at our centre were included in our study.
Reporting of adverse events
At the vaccination centre, details of beneficiaries including their contact number were noted and beneficiaries were observed for AEFI for a period of 30 minutes after receiving the vaccination. Details of the beneficiaries developing AEFI at the centre were recorded as per the case safety report form and they were followed up telephonically to know the outcome and medical management of the event.
All the vaccine beneficiaries were instructed to call a designated number to self-report any AEFI which they had experienced in the following days of receiving vaccination. In addition, the beneficiaries after 8 days of vaccination contacted and enquired from them as per a predefined checklist of AEFI whether they developed any of the events. Such reports of AEFI were recorded as per the Suspected Adverse Drug Reaction Reporting Form. The beneficiaries reporting AEFI were followed up telephonically every week till the resolution of the events or for a maximum duration of 4 weeks.
Analysis
Data pertaining to beneficiary demographics, detailed medical and current drug history, details of vaccination, details of the adverse events including date and time of onset, duration, and event outcomes were recorded in the form and data was analysed subsequently.
Assessment was done to know the most reported AEFI with COVID-19 vaccines, age and gender preponderance, organ class involved, severity of adverse event, seriousness according to World Health Organization (WHO) criteria and causality using WHO UMC causality scale.
Observations
A total of 554 (N) vaccine beneficiaries reported 1529 (n) AEFI with an average of 3 events per case.
Demographics
Majority of the reported events were in the age group of 18 – 30 years which amounts to 253 of 554 (45.6%) cases of AEFI. Gender wise there is female preponderance 318 of 554 (57.4%). Demographic details are presented in Table 1.
| Demographic details of the AEFI cases (N=554) | ||
|---|---|---|
| Sub-group | Number | |
| Age (years) | 18-30 | 253 (45.6%) |
| 31-45 | 175 (31.5%) | |
| 46-60 | 119 (21.4%) | |
| >61 | 7 (1.2%) | |
| Gender | Female | 318 (57.4%) |
| Male | 236 (42.6%) | |
| Profession | Doctors | 173 (31.2%) |
| Nurses | 194 (35.0%) | |
| Employees | 61 (11.0%) | |
| Students | 90 (16.4%) | |
| Others | 36 (6.4%) | |
Table 1. Demographic distribution based on age, gender, and profession of the AEFI cases.
Pattern of AEFI
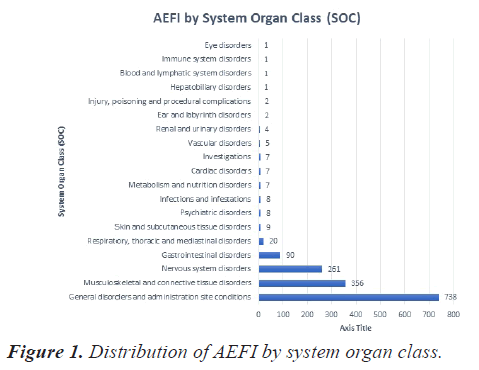
Of the reported 1529 AEFI, 738 (48%) belong to the System Organ Class (SOC) of General disorders and administration site conditions, followed by musculoskeletal and connective tissue disorders 356 events (23.2%), Nervous system disorders 261 events (17%) and Gastrointestinal disorders 90 events (5.8%). SOC wise distribution of AEFI is depicted in Figure 1.
Frequency of AEFI
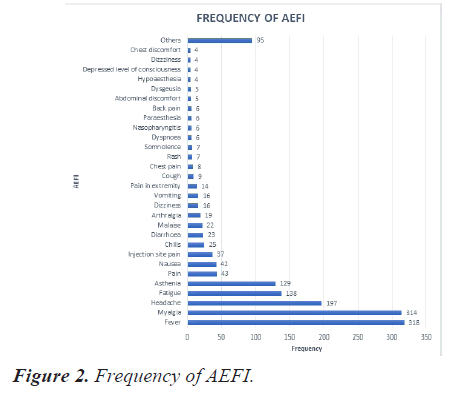
The most reported event was fever (20.8%), followed by myalgia (20.5%), headache (12.9%), fatigue (09%) and asthenia (8.4%). Figure 2 depicts the frequency of observed AEFI. Events with frequency less than 3 were categorized as others that constitute to 6% of the AEFI.
Onset latency of the events
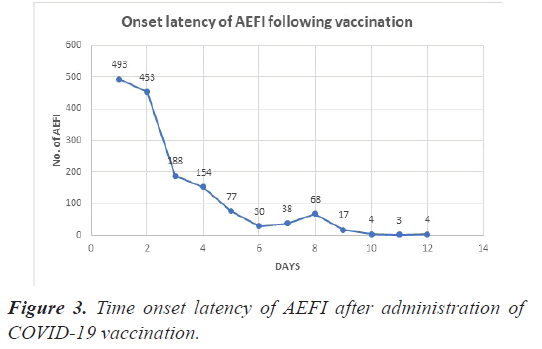
Four hundred and ninety-three (32.2%) and 453 (29.6%) of the 1554 AEFI appeared within 1 and 2 days of vaccine administration respectively, in the following days there is a decreasing trend in the number of AEFI as depicted in Figure 3.
Serious AEFI cases
Of the 554 cases, 20 (3.6%) were categorized as serious, according to the seriousness criteria and the major criterion for seriousness being hospitalization due to AEFI as represented in Table 2. All the serious cases improved and were discharged, no deaths were recorded. 534 (96.4%) cases were non-serious, most of which resolved with home based symptomatic treatment.
| Seriousness of the cases (N=554) | ||
|---|---|---|
| No. of cases (%) | Seriousness criteria | |
| Serious | 20 (3.6%) | Hospitalization |
| Non-serious | 534 (96.4%) | - |
Table 2. Division of serious and non-serious cases.
Outcome of the events
Outcome of 1476 (96.5%) AEFI were recovered/resolved and 47 (3%) were recovering/resolving at the time of last follow up. The median time for resolution of the events is 4 days from the time of appearance.
Severity of the events
Distribution as per the severity of the events is depicted in Figure 4, show that majority of the AEFI 945 of 1554 (62%) were of mild severity, 512 of 1554 (33%) were of moderate severity, and the rest 5% were severe.
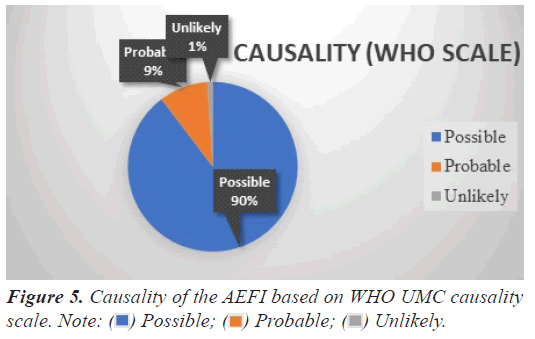
Causality of the AEFI
AEFI were assessed taking into consideration of beneficiary medical history, onset latency, concomitant medications, and consistency with two doses. Majority of events were assessed as possibly [1371(89.6%)] and probably [142(9.2%)] related with the COVID-19 vaccination according to WHO UMC causality scale 16 (1%) of the events were assessed as unlikely to be related with vaccine as these events had alternate explanation. Figure 5 depicts the causality of the AEFI.
Results and Discussion
This study aimed to characterize AEFI in the beneficiaries receiving COVID-19 vaccinations, evaluating their pattern, severity, seriousness, and causality.
A total of 1529 AEFI were recorded from 554 cases with an average of 3 events per case. We observed that the time to appearance of AEFI after vaccination had a decreasing trend i.e, number of AEFI observed within 1 day of administration was the highest 493 (32%) and then there was a decrease in number of AEFI that were observed in following days after vaccination.
In terms of immunization safety, our study showed a higher number of non-serious AEFI reports. Of the 20 serious AEFI cases, 6 affected cardiovascular system, 3 affected nervous systems, 3 affected skin, 2 affected respiratory system and 8 were non-specific.
The most common AEFI related to vaccines were fever, general body pains, fatigue, and headache i.e., mild, and transient untoward medical occurrences.
Similar studies were done in Indian population by U. Kaur et al., among Health care workers receiving COVID-19 vaccine, reported 730 AEFIs in 321 beneficiaries, majority were mild to moderate severity and resolved spontaneously, serious AEFI accounting to 1 (0.1%) [6]. A study by V. Thakur et al., reported 1558 AEFIs in 981 healthcare workers, most appearing within 2 days of vaccination, were of mild to moderate severity, serious AEFI accounting to 2 (0.2%) [7].
Majority of the AEFI in this study were causally associated with the COVID-19 vaccines, which was assessed based on the onset latency of the events with the administration of the vaccine and lack of an alternate explanation for the event. 16 AEFI (1%) were assessed as unlikely to be related with vaccination as these had an alternate explanation and were regarded as coincidental events.
Conclusion
Immunization safety has become as important as the efficacy of vaccines in the national Vaccine-Preventable Disease (VPD) programs. As per this study, vaccines used against COVID-19 are safe and the AEFI observed were non-serious. Based on our observations, the risks associated with COVID-19 immunization do not favour vaccination avoidance, in contrast to the risk of developing severe COVID-19 infection if not vaccinated. Similar active multi-centric studies across the country will help to generate more safety data for the COVID-19 vaccines.
Strengths and Limitations
This study was conducted in health care workers and general population who received vaccine at our centre, so there is less possibility of reporting bias, and the participants were actively followed to mitigate the probability of underreporting.
The limitation of this study is that we did not include data according to the existing common medical conditions of the recipients, hence results of the study might not be a true representation of the incidence of adverse events and bigger multicentric studies are required to generalize the results to general population.
References
- Biasio LR, Bonaccorsi G, Lorini C, Pecorelli S. Assessing COVID-19 vaccine literacy: A preliminary online survey. Hum Vaccin Immunother 2021; 17: 1304-1312.
[Crossref] [Google Scholar] [PubMed]
- Assessing COVID-19 vaccine literacy: A preliminary online survey
- Crossref
- WHO. Adverse Events Following Immunization (AEFI). 2021.
- Google Scholar
- Kaur U, Ojha B, Pathak BK, Singh A, Giri KR, Singh A, Das A, Misra A, Yadav AK, Kansal S, Chakrabarti SS. A prospective observational safety study on ChAdOx1 nCoV-19 corona virus vaccine (recombinant) use in healthcare workers-first results from India. E Clin Med 2021; 38: 101038.
- Kamal D, Thakur V, Nath N, Malhotra T, Gupta A, Batlish R. Adverse events following ChAdOx1 nCoV-19 Vaccine (COVISHIELD) amongst health care workers: A prospective observational study. Med J Armed Forces India 2021; 77(Suppl 2): S283–S288.
[Crossref] [Google Scholar] [PubMed]
 Mild;
Mild;  Moderate;
Moderate; 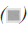 Severe.
Severe.