Research Article - Journal of Clinical Ophthalmology (2020) Volume 4, Issue 2
Aqueous humor vascular endothelial growth factor concentration before and after intravitreal injection of Bevacizumab for diabetic retinopathy
Nguyen Tuan Thanh Hao1, Vu Tuan Anh2, Dao Ngoc Mai1*, Mai Quoc Tung1, Nguyen Thi Phuong1, John Grigg3, Peter McCluskey3, Pham Trong Van11Hanoi Medical University, Hanoi, Vietnam
2Vietnam National Institute of Ophthalmology, Hanoi, Vietnam
3The University of Sydney, Sydney, Australia
- Corresponding Author:
- Dr. Dao Ngoc Mai
Hanoi Medical University
Hanoi
Vietnam
E-mail: ndao4887@uni.sydney.edu.au
Accepted date: 06 May, 2020
Abstract
Aim: To study the concentration of aqueous humor vascular endothelial growth factor (VEGF) before and after intravitreal injection of Bevacizumab in eyes with proliferative diabetic retinopathy (PDR) and correlate this with area of retinal ischemia and neovascularization.
Methods: In this prospective, interventional case series; 1.25 mg of Bevacizumab was injected into the vitreous cavity in 48 PDR eyes of 29 consecutive patients. Aqueous humor samples were obtained before intravitreal Bevacizumab injection and 1 week after, or before starting cataract surgery in the controls. The controls were 15 consecutive non-diabetic patients undergoing cataract surgery (15 eyes).
Results: Aqueous VEGF level was higher in eyes with PDR than in control eyes (474.2 ± 361.3 vs. 120.65 ± 45.05 pg/mL) and this level reduced to 16.96 ± 18.1 pg/ mL 1 week after Bevacizumab injection (p<0.001). There was significant correlation between aqueous VEGF level and retinal ischemia-neovascularization area. No correlation between aqueous VEGF level and clinical markers such as blood glucose levels, HbA1C lever and duration of diabetes.
Conclusion: VEGF levels correlate with the area of retinal ischemia and presence of neovascularization.
Keywords
Proliferative diabetic retinopathy, VEGF, Bevacizumab
Introduction
Retinal ischemia and abnormal vasoproliferation occurs in advanced diabetic retinopathy. Vascular endothelial growth factor (VEGF) is an endothelial mitogen and vasopermeability factor which breaks down the blood retina barrier (BRB) leading to exudation, edema and vessel development [1,2]. Humans express alternatively spliced isoforms of 121, 145, 165, 183, 189 and 206 amino acid in length [3-5]. VEGF165 is the most abundant and potent isoform, followed by VEGF121 and VEGF189 [5]. Studies have demonstrated increased VEGF levels in the vitreous and aqueous humor fluid of patients with active proliferative diabetic retinopathy [6-9].
Bevacizumab (Avastin, Genentech Inc.) is an antiVEGF monoclonal antibody that is widely used intravitreally to treat vision threatening diabetic retinopathy by reducing intraocular VEGF levels [10-13]. Normal, clinical practice uses a standardized dose (1.25mg) and treatment regimen [14]. Titrating the treatment regimen to retinopathy severity may permit decrease the drug dose and frequency, thus reducing drug related adverse effects [8,15]. This may be achieved by using the aqueous VEGF concentration as a surrogate maker to determine the optimal dose of intravitreal Bevacizumab to treat proliferative diabetic retinopathy.
Although several studies have analyzed the effect of intravitreal Bevacizumab on aqueous humor VEGF levels, few studies have quantitatively compared aqueous VEGF concentration with severity of proliferative diabetic retinopathy. This study will compare aqueous humor VEGF levels before and after intravitreal Bevacizumab injection in patients with proliferative diabetic retinopathy and correlate VEGF concentration with the clinical features. The hypothesis for this study is that aqueous humor VEGF levels can be estimated by measuring clinical parameters such as the area of ischemia or neovascularization. Larger, subsequent studies will determine and validate a formula to estimate the aqueous VEGF concentration based on clinical features that will allow individualised dosing of intravitreal Bevacizumab for proliferative diabetic retinopathy.
Materials and Methods
Subjects
Consecutive patients undergoing intravitreal Bevacizumab injection for proliferative diabetic retinopathy or undergoing surgery for age related cataract in the Da Nang Eye Hospital from January 2016 to May 2018 formed the study groups. Informed consent was obtained from subjects following an explanation of the purpose and potential adverse effects of the procedures.
Inclusion criteria were patients with type 1 or type 2 diabetic mellitus with PDR as defined by the modified Early Treatment Diabetic Retinopathy Study (ETDRS) [16]. Exclusion criteria included: patients with the history of cardiovascular disease or stroke, allergy to Bevacizumab, acute eye infectionand intraocular injection of Triamcinolone, use of other VEGF inhibitors, retinal laser and vitreous surgery within 3 months of the study. Control subjects were age-matched patients with a normal retina undergoing cataract surgery.
Ophthalmic examination
All patients were evaluated by careful biomicroscopic examination using a 90 D Volk super-field Lens. Fundus findings were confirmed by standardized fundus color photography and fundus fluorescein angiography, which was performed with a Topcon TRC- 50 EX fundus camera and an IMAGNET system. The severity of diabetic retinopathy was graded according to modified ETDRS [16].
Measurement of retinal ischemia and neovascularization area from fluorescein angiography [17]
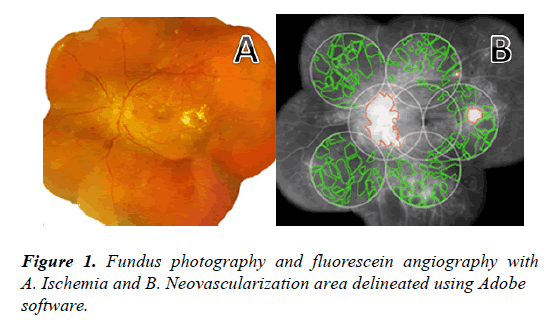
Representative fundus images were obtained and digitally captured during the venous phase (45 seconds to 2 minutes) of fluorescein angiography. The images were transferred to Adobe software. The 30o angiogram images were combined to create 7 standard fields (7 SF) using the ETDRS protocol [18]. Ischemic retina was defined as dropout of the retina capillary bed. Active neovascularization on the disc or retina surface was defined by leakage from the abnormal blood vessels. The boundaries of the ischemic retina and area of neovascularization were outlined and the total area in 7 SF was calculated in pixels and converted into number of disc areas using Adobe measurement software (Figure 1).
Treatment with Intravitreal Bevacizumab
All PDR patients received an intravitreal injection of Bevacizumab (Avastin) 1.25mg/0.05 ml using 1 mL syringe and 30 G needle via the pars plana at a site 3.5 mm posterior to the limbus. Topical Betadine 5% was used before the procedure. There were no complications following Bevacizumab injection other than short duration of mild ocular discomfort, epiphora and subconjunctival hemorrhage.
Sample collection
Aqueous humor was collected by performing a limbal paracentesis using a 30 gauge needle attached to a 1 ml insulin syringe, immediately prior to intravitreal bevacizumab injection and 1 week following intravitreal therapy in the PDR group, and immediately before beginning cataract surgery in the control group. Samples were immediately frozen and stored at -80oC until analysis.
Measurement of VEGF levels
Aqueous humor VEGF165 and VEGF121 levels were measured with the Quantikine Human VEGF enzyme - linked immunosorbent assay (ELISA) kit (Code DVE00 - R&D System Inc, Minneapolis, MN, USA).
Statistical Analyses
Statistical analysis was performed with SPSS 20.0. Data was presented as mean ± SD. To assess the relationship, Mann Whitney U test and Spearman’s correlation co-efficient were used to compare VEGF level across the range of retinal ischaemia values.
Results
We study 15 normal individuals (15 eyes) with cataract surgery and 29 patients (48 eyes) with proliferative diabetic retinopathy. Clinical characteristic data of all patients are summarized in Table 1.
| Characteristics | Controls (n=15) | Diabetic patients(n=29) |
|---|---|---|
| Mean age ± SD (years) | 57.40 ± 9.49 | 53.17 ± 7.9 |
| p=0.458 | ||
| Gender (male/female) | 7/8 | 14/15 |
| Diabetes (type 1/type 2) | - | 4/25 |
| Duration of diabetes (year) | - | 12.24 ± 6.40 |
| Fasting glucose level (mmol/l) | - | 8.15 ± 2.41 |
| HbA1C (%) | - | 7.76 ± 2.16 |
| Central Retinal Thickness µm (mean ± SD) | 213.53 ± 18.71 | 402.04 ± 134.17 |
| p < 0.001 | ||
| Total Macula Volume mm3 (mean ± SD) | 7.21 ± 0.45 | 10.34 ± 1.43 |
| p < 0.001 | ||
Table 1. General characteristics of controls and diabetic patients.
VEGF level is higher in eyes with proliferative diabetic retinopathy than in control eyes (474.2 ± 361.3 vs. 120.65 ± 45.05 pg/ mL). VEGF level decreased to 16.96 ± 18.1 pg/ mL one week after Bevacizumab injection (Table 2).
| VEGF level | Controls group | Diabetic group | ||
|---|---|---|---|---|
| (n=15) | Before IVB (n=48) | After IVB (n=48) | ||
| Mean ± SD | 120.65 ± 45.05 | 474.2 ± 361.3 | 16.96 ± 18.1 | |
| p | < 0.001 | |||
| < 0.001 | ||||
Table 2. Aqueous level of VEGF (pg/ml) in controls versus diabetic group.
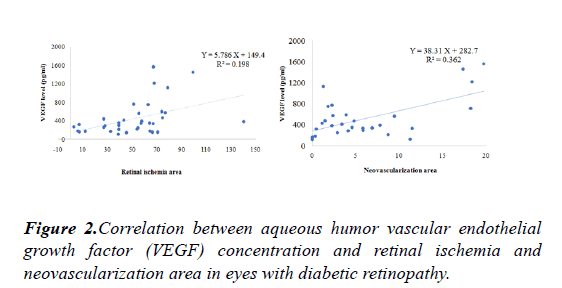
No correlation between aqueous VEGF level and fasting glucose level, HbA1C level and the duration of diabetes.Correlation between aqueous VEGF level and area of retinal ischemia and proliferation is shown Figure 2.
To evaluate relationships among the variables, simple and multiple linear regression analysis with stepwise variable selection method were applied.Aqueous VEGF level correlated positively with the area of retinal ischemia(p=0.001, R2=0.198) and neovascularization area (p<0.001, R2=0.362)
Multiple linear regression analysis was used to adjust for the area of retinal ischemia and neovascularization. After adjusting for area of retinal ischemia, aqueous VEGF level correlated significantly with neovascularization area (β=0.580, R2=0.454, p<0.001). Even after adjustment for neovascularization area, aqueous VEGF level correlated significantly with the area of retinal ischemia (β=0.304, R2=0.454, p<0,001). On the basic of the results of multiple linear regression analysis, the estimated aqueous VEGF level of patients with PDR could be calculated as follow:
Estimated aqueous VEGF level (pg/ml)=46.646+4.349 × Area of retinal ischemia+36.902 × Area of neovascularization.
Discussion
Current evidence indicates that VEGF plays a critical role in the development of retinal neovascularization [3,19]. Intraocular anti-VEGF therapy effectively controls pathologic neovascularization [3,20]. Prolonged duration of therapy may result in an increased risk of cerebrovascular accidents [21].
Lip et al. (2000) have demonstrated that elevated plasma VEGF is related to advanced diabetic retinopathy and this level decreases within 4 months of pan retinal photocoagulation [23]. Funatsu et al. (2005) reported that aqueous VEGF level correlates with the severity of diabetic retinopathy [8] but not complications such as vitreous haemorrhage, tractional retinal detachment and macular edema [22].
The extent of retinal ischemia/neovascularization was determined in the present study by using the 7SF method of the ETDRS protocol. The aqueous VEGF level in the PDR group correlated significantly with the extent of retinal ischemia/ neovascularization in the 7SF, similar to previous reports [3,17,24]. Because ischemic retina releases high levels of VEGF, the aqueous VEGF levels also may represent the severity of retinal ischemia.
Stepwise multiple linear regression analysis showed that the extent of retinal ischemia/neovascularization were significant determinants of the aqueous VEGF level. Estimated aqueous VEGF level (pg/ml)=46.646+4.349 × Area of retinal ischemia +36.902 x Area of neovascularization. This finding suggests that the severity of retinal ischemia/neovascularization in PDR on fluorescein angiography can be used to calculate the aqueous VEGF level without risk of morbidity related aqueous humour sampling. Using the aqueous VEGF concentration as a surrogate marker to determine the optimal dose of intravitreal Bevacizumab has been mentioned to in some studies [5,22]. The correlation between aqueous VEGF level and the extent of ischemia/neovascularization may reduce the dose and the frequency of intravitreal Bevacizumab by using fluorescein angiography imaging and software calculation. This may reduce the frequency of intravitreal Bevacizumab and other anti-VEGF therapy. However, this was a cross-sectional study and our sample size was relatively small, so futher studies need to be carried out to confirm the appropriate formula to calculate the aqueous VEGF level.
Conclusion
Aqueous humor VEGF concentration is increased in eyes with diabetic retinopathy and correlates with the extent of retinal ischemia and neovascularization. Intravitreal Bevacizumab injection significantly decreases VEGF levels in aqueous humor. The relationship between VEGF concentration and clinical parameters such as area of retinal ischemia may help to reduce the frequency and dose of intraocular Bevacizumab injection as well as reducing the risk of drug related adverse effects.
Acknowledgments
The authors thank Hanoi Medical University, Danang Eye Hospital for the help during this study.
References
- Tolentino MJ MJ, Gragoudas ES, et al. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a non-human primate. Arch Ophthalmol. 1996;114:964-70.
- Ved N, Hulse RP, Bestall SM, et al. Vascular endothelial growth factor-A165b ameliorates outer-retinal barrier and vascular dysfunction in the diabetic retina. Clin Sci (Lond). 2017;131:1225-43.
- Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480-7.
- Stevens M, Star E, Lee M et al. The VEGF-A exon 8 splicing-sensitive fluorescent reporter mouse is a novel tool to assess the effects of splicing regulatory compounds in vivo. RNA Biol. 2019;16:1672-81.
- Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853-65.
- Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445-50.
- Funatsu H, Yamashita H, Noma H, et al. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133:70-7.
- Funatsu H, Yamashita H, Noma H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243:3-8.
- Semeraro F, Cancarini A, Morescalchi F, et al. Serum and intraocular concentrations of erythropoietin and vascular endothelial growth factor in patients with type 2 diabetes and proliferative retinopathy. Diabetes Metab. 2014;40:445-51.
- Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. 2016;123:1351-9.
- Virgili G, Parravano M, Evans JR, et al. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2017;6:CD007419.
- Tsubota K, Usui Y, Wakabayashi Y, et al. Effectiveness of prophylactic intravitreal bevacizumab injection to proliferative diabetic retinopathy patients with elevated preoperative intraocular VEGF in preventing complications after vitrectomy. Clin Ophthalmol. 2019;13:1063-70.
- Kartasasmita A, Harley O. Evaluation Of The Timing Of Intravitreal Bevacizumab Injection As Adjuvant Therapy To Panretinal Photocoagulation In Patients With Diabetic Macular Edema Secondary To Diabetic Retinopathy. Clin Ophthalmol. 2019;13:1921-6.
- Gunther JB, Altaweel MM. Bevacizumab (Avastin) for the treatment of ocular disease. Surv Ophthalmol. 2009;54:372-400.
- Hattori T, Shimada H, Nakashizuka H, et al. Dose of intravitreal bevacizumab (Avastin) used as preoperative adjunct therapy for proliferative diabetic retinopathy. Retina. 2010;30:761-4.
- Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:766-85.
- Kwon SH, Shin JP, Kim IT, et al. Aqueous Levels of Angiopoietin-like 4 and Semaphorin 3E Correlate with Nonperfusion Area and Macular Volume in Diabetic Retinopathy. Ophthalmology. 2015;122:968-75.
- Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786-806.
- Mohamed QA, Fletcher EC, Buckle M. Diabetic retinopathy: intravitreal vascular endothelial growth factor inhibitors for diabetic macular oedema. BMJ Clin Evid. 2016;2016:0702.
- Mehanna CJ, Abdul Fattah M, Haddad S, et al. Anti-VEGF Therapy for Persistent Neovascularization after Complete Panretinal Photocoagulation in Proliferative Diabetic Retinopathy. Ophthalmol Retina. 2019;3:473-7.
- Avery RL, Gordon GM. Systemic Safety of Prolonged Monthly Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2016;134:21-9.
- Sawada O, Kawamura H, Kakinoki M, et al. Vascular endothelial growth factor in aqueous humor before and after intravitreal injection of bevacizumab in eyes with diabetic retinopathy. Arch Ophthalmol. 2007;125:1363-6.
- Lip PL, Belgore F, Blann AD, et al. Plasma VEGF and soluble VEGF receptor FLT-1 in proliferative retinopathy: relationship to endothelial dysfunction and laser treatment. Invest Ophthalmol Vis Sci. 2000;41:2115-9.
- Praidou A, Klangas I, Papakonstantinou E, et al. Vitreous and serum levels of platelet-derived growth factor and their correlation in patients with proliferative diabetic retinopathy. Curr Eye Res. 2009;34:152-61.