- Biomedical Research (2016) Volume 27, Issue 3
Application of solid Carbon dioxide as a novel hemostatic agent on a hepatectomy model in rats.
Ibrahim Ali Ozemir1*, Cagri Bilgic1, Erman Aytac2, Sinan Aslan1, Baris Bayraktar1, Banu Isbilen3, Ebru Zemheri4, Duygu Kösemetin4, Rafet Yigitbasi1 and Orhan Alimoglu11Department of General Surgery, Goztepe Education and Research Hospital, Istanbul Medeniyet University, Istanbul, Turkey
2Department of General Surgery, Cerrahpasa Medical Faculty, Istanbul University, Istanbul, Turkey
3Department of Biochemistery , Goztepe Education and Research Hospital, Istanbul Medeniyet University, Istanbul, Turkey
4Department of Pathology, Goztepe Education and Research Hospital, Istanbul Medeniyet University, Istanbul, Turkey
- *Corresponding Author:
- Ibrahim Ali Ozemir
Department of General Surgery
Istanbul Medeniyet University
Turkey
Accepted date: March 31, 2016
Abstract
Background: Blood loss and the prolonged operative time for haemostasis is still a great problem after injury or during elective surgery of the liver. Various topical hemostatic agents were introduced to reduce the blood loss. The aim of this study is to evaluate the afficacy of solid carbon dioxide (SoCO2) as a hemostatic agent on hepatectomy model.
Materials and methods: Thirty-two Wistar albino female rats were included in this study. They were divided in four groups of 8 rats. Haematocrit levels were determined preoperatively. Non-anatomic hepatectomy was performed to the left lobe. Hemostasis was provided with gauze tampons in Group-1, with surgical sutures in Group-2 and with asistance of SoCO2 in Group-3. Hemostasis time and blood loss were recorded. Serum haematocrit, liver function tests were determined postoperatively. SoCO2 assisted hemostasis was provided and relaparotomy was performed after 7 days to determine the late effects of SoCO2 in Group-4.
Results: Blood loss in Group-1 and Group-2 were statistically significant higher than the Group-3 (119.1 ± 22,4 mg; 80.6 ± 18.9 mg; 36.3 ± 11.1 mg, respectively) (p<0.01). Therewithal hemostasis time in Group-3 was statistically significant shorter than Group-1 and Group-2 (p<0.01). Serum ALT and AST values were increased in first three groups, but did not reveal significant difference between groups (p: 0.045 and p:0.163). ALT and AST values were significantly decreased in Group-4 compared to other groups (p:0.001 and p:0.005, respectively).
Conclusions: SoCO2 assisted hemostasis provide bloodless surgical site, reduced blood loss and also shortened hemostasis time. Although further studies are requires, this study reveals that hepatectomies can be performed safer with SoCO2 assistance.
Keywords
Hemostasis, Solid carbon dioxide, Hepatectomy.
Introduction
Sinusoidal structure of liver parenchyma lacks smooth muscle fibers providing vasoconstriction. Thus, when hepatic tissue integrity is distrupted, hard-to-control, significant bleeding is noted [1]. Mortality secondary to liver traumas is 10-15%, the most important factor being bleeding [2,3]. Likewise, in elective surgical resections performed for primary or metastatic liver tumor, bleeding and its secondary complications, continue to be one of the most important trouble [4,5]. In order to perform liver surgery in a safer fashion and to shorten hemostasis time, bipolar and monopolar electro-cauters, ultrasonic dissectors, radiofrequency equipments and various hemostatic agents are utilized [6].
In cryosurgery, the purpose is to produce vascular stasis and microcirculation defect by exposing the tissue to freezing and liquefaction temperatures [7,8]. In recent years, Argon (-186°C) and Nitrous oxide (-89.5°C) are used as cooling agents in cryosurgery. Response of tissues to cold differs according to degree, duration of exposure and tissue type [9]. Mildly freezing short-term cold exposure produces inflammatory response; whereas severe freezing cold results in cell necrosis secondary to circulatory disruption [7,8]. Carbon dioxide (CO2) is a chemical in gaseous state under normal conditions liquidates under high pressure and solidified under laboratory conditions. Surface temperature of solid carbon dioxide is -78.5°C. It can directly proceed to gaseous from solid state (sublimation). Therefore, not causing carbonization or leaving remnant material on the tissue it comes in contact with having a high cooling capacity (152 Kcal/kg) during sublimation. The aim of this study is to evaluate the hemostatic effect of solid CO2 (SoCO2), a low-cost, easily available agent, on the hepatectomy model that was generated using rats.
Material and Methods
This study was carried out at Marmara University Animal Experiments and Research Laboratories under the June 17, 2011 no."36.2011. Mar” permit of “Ethical Association of Marmara University Animal Experiments”. Thirty-two Wistar albino female adult rats, weighing 250-300 grs were randomly divided into 4 groups of 8. Four rats were placed in each cage, fed ad libitum, and followed in 18-23°C room temperature of 50-55°F humidity for 12 hours/day and 12 hours/night.
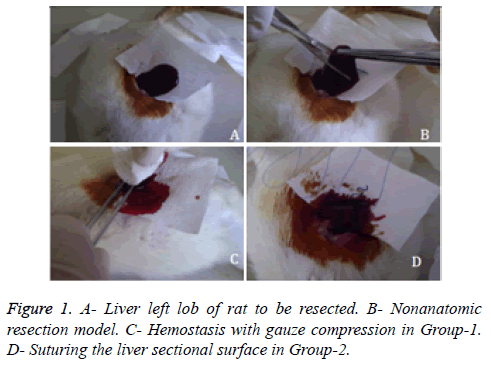
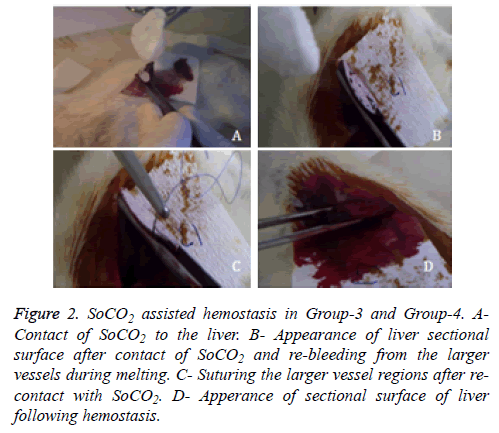
Under general anesthesia with ketamin hydrochloride (100 mg/kg) and xylazine hydrochroride (10 mg/kg), blood sample was drawn from caudal vein to determine pre-operative hematocrite levels of rats. Laparotomy was performed via an abdominal midline incision and suspension ligaments of liver were released. Blotting papers were used to calculate the amount of bleeding. Prior to the resection, weight of the blotting papers were weighed on a precision scale and placed under the liver left lobe (Figure 1A). Consequently nonanatomic surgical resection was performed to the left lobe of liver (Figure 1B). The area of the sectional surface of liver were measured using milimetric paper and also weight of resected liver tissue were measured on analytic scale. In Group-1 gauze tampon press was applied to resected surface until hemostasis was achieved (Figure 1C). In Group-2, sectional surface hemostasis was achieved by 5/0 prolene continuous sutures (Figure 1D). In Group-3, sectional surface of liver was contacted with SoCO2 for 20 seconds (Figure 2A). Bleeding was controlled after spontaneous melting of frozen surface (Figure 2B). Re-bleeding from the hepatic vessels was observed after melting. Sectional surface was re-frozen with SoCO2 and tip of the vessels were ligated with 5/0 prolene in a bloodless environment (Figure 2C). At the end of each freezing/melting period, blotting papers were re-weighed to calculate the total bleeding amount (Figure 2D). Hemostasis was observed for 10 minutes in each group, 5cc intracardiac blood sample was drawn to evaluate the blood tests and haematocrit levels. Rats were sacrificed under general anesthesia. Remnant liver tissue was resected and fixated with 10% formaldehyde for pathologic research. Procedure processing time was recorded.
Figure 2: SoCO2 assisted hemostasis in Group-3 and Group-4. A-Contact of SoCO2 to the liver. B- Appearance of liver sectional surface after contact of SoCO2 and re-bleeding from the larger vessels during melting. C- Suturing the larger vessel regions after re-contact with SoCO2. D- Apperance of sectional surface of liver following hemostasis.
To determine the presence and amount of acute hepatocellular destruction aspartate aminotransferase (AST), alanin aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), gamma glutamil transferase (GGT), total bilirubin, direct bilirubin, albumin, prothrombin time (PT), INR and haematocrit values were established.
SPSS software version 20.0 (SPSS Inc., Chicago, IL) was used to analyze the data of this study. Kolmogorov-Smirnov distribution test was used for related statistical methods (Frequency, Percentage, Average, and Standard Deviation) and to examine the normal distribution. Quantitative data were compared with Mann Whitney U and Kruskal Wallis tests. Wilcoxon test was used to compare the parameters within the group. Results were reviewed in a confidence interval of 95%, and a value of p<0.05 was accepted as statistically significant.
Homeostasis model used in Group-3, was also used for Group-4. In Group-4 rats were not sacrificed after hemostasis and abdominal incision was closed with 4/0 prolene. They observed for 7 days in unrestricted conditions. At the end of the 7th day, re-laparotomy was performed via abdominal midline under general anesthesia that provided with ketamine hydrochloride (100 mg/kg) and xylazine hydrochloride (10 mg/ kg). Early stage effects of SoCO2 on the contact surface of liver tissue and the presence of intraabdominal abcess, seroma, biloma or adhesion were observed. Euthanasia was applied after drawing 5cc intracardiac blood.
Results
The mean weight of rats was 234.5 ± 9.3 gr there was no significant difference between the groups with regard to weight of rats (p:0.725).
In comparison of groups with regard to weight of resected liver tissue and sectional surface areas, there was no statistically difference (p:0.637, p:0.931, respectively) (Table 1).
| Group-1 (Mean ± SD) | Group-2 (Mean ± SD) | Group-3 (Mean ± SD) | P vlue | |
|---|---|---|---|---|
| Rat weights (gr)a | 234.4 ± 10.5 | 236.3 ± 8.7 | 232.5 ± 8.5 | 0.725 |
| Liver section surface (mm2)a | 74.1 ± 6.2 | 76.2 ± 10.7 | 72.1 ± 16.1 | 0.637 |
| Resected segment weight (mg)a | 0.35 ± 0.02 | 0.34 ± 0.21 | 0.35 ± 0.06 | 0.931 |
| Mean blood loss (mg)a | 119.1 ± 22.4 | 80.6 ± 18.9 | 36.4 ± 11.1 | 0.001* |
| Pre-op hematocrita, b | 54.2 ± 3.2 | 52.7 ± 4.9 | 53 ± 3.7 | 0.709 |
| Post-op hematocrita,b | 47.4 ± 10.1 | 47.8 ± 4.8 | 47.7 ± 3.6 | 0.919 |
| Hemostasis time (sec.)a | 377.5 ± 56.7 | 203.7 ± 69.7 | 176.2 ± 56.8 | 0.001* |
| *: p<0.01 a: Kruskal Wallis test b: Wilcoxon test |
||||
Table 1: Comparision of experimental and surgical data between groups.
Blood samples from caudal vein drawn pre-procedure and intracardiac sample drawn post-procedure were used for determination of hematocrit (Htc) values. No significant pre or post-procedure Htc value difference was found among the groups (p:0.709, p:0.919, respectively). In Group-3 in which hemostasis was achieved with SoCO2 mean bleeding amount was 36.4 ± 11.1 mgr where as it was 119.1 ± 22.4 mgr in Group-1 and 80.6 ± 18.9 mgr in Group-2. Statistical comparison among groups showed that bleeding was significantly less in Group-3 where hemostasis was achieved with SoCO2 (p: 0.001).
The mean SoCO2 contact time was found 38.7 ± 5.8 (30-45 second) to achieve the hemostasis in Group-3 and Group-4. The length of time until hemostasis was also compared between groups. The mean hemostasis time in Group-3 was 176.2 ± 56.8 sec. whereas it was 377.5 ± 56.7 sec. in Group-1 and 203.7 ± 69.7 sec. in Group-2. Statistical comparison of hemostasis time among the groups showed that hemostasis time was significantly shorter in Group-3 compared to Groups 1 and 2 (p:0.001) (Table 1).
Blood samples were studied to determine acute effects of SoCO2 on the liver. Serum ALT, AST and LDH levels were elevated in all groups (Table 2); however there was no statistically significant difference between the groups with respect to serum albumin, total bilirubin, direct bilirubin, ALP, ALT, AST, PT, INR and Htc levels. In Group-3 mean LDH level was 1066.4 ± 561.7 U/L, whereas this level in Group-1 and Group-2 was 1827.3 ± 537.6 U/L and 2074.4 ± 766.1 U/L, respectively. Based on these results, LDH value increase was statistically significant lower in Group-3 where hemostasis was provided with SoCO2 (p: 0.010) (Table 2).
| Group-1 ( Mean ± SD) | Group-2 (Mean ±SD) | Group-3 (Mean ±SD) | P value | |
|---|---|---|---|---|
| Albumin (g/dl)a | 3.2 ± 0.2 | 3,6 ± 0,3 | 3.1 ± 0.2 | 0.005* |
| Total bilirubin (mg/dl)a | 0.13 ± 0,01 | 0.16 ± 0.05 | 0.13 ± 0.06 | 0.266 |
| Direct bilirubin (mg/dl)a | 0.02 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.967 |
| ALP(U/L)a | 170.1 ± 36.7 | 155.1 ± 50.9 | 128.2 ± 39.8 | 0.090 |
| AST(U/L)a | 294.6 ± 46.1 | 249.4 ± 102.8 | 273.6 ± 172.9 | 0,163 |
| ALT(U/L)a | 174.2 ± 71.5 | 93.7 ± 32.8 | 146.4 ± 68.1 | 0.045 |
| LDH (U/L)a | 1827.3 ± 537.6 | 2074.4 ± 766.1 | 1066.4 ± 561.7 | 0.01* |
| PT (sn)a | 9.5 ± 0.7 | 9.8 ± 1.6 | 10.41 ± 1.1 | 0.158 |
| INRa | 0.78 ± 0.01 | 0.81 ± 0.14 | 0.84 ± 0.08 | 0.275 |
| *: p <0.01 a: Kruskal Wallis tests |
||||
Table 2: Comparison of biochemical data obtained from intracardiac blood samples within groups.
To examine the possible adverse effects of SoCO2 contact in the early stage, serum ALT, AST, ALP, LDH, PT and INR levels of rats were compared between Group-3 and Group-4 that observed for 1 week after SoCO2 contact. There was no statistically significant difference between Group-3 and Group-4 with respect to weight of rats, sectional surface area of liver, resected tissue amount, mean bleeding amount, pre-operative and post-operative hematocrite values. AST and ALT values that evaluated to estimate hepatocellular damage, were significantly reduced in Group-4 (p:0.005, p:0.001) (Table 3).
| Group-3 (Mean ± SD) | Group-4 (Mean ± SD) | p value | |
|---|---|---|---|
| Rat weights (gr)a | 232.5 ± 8.4 | 235 ± 10.3 | 0.591 |
| Liver section surface (mm2)a | 72.1 ± 161.1 | 70 ± 4.5 | 0.156 |
| Resected segment weight (mg)a | 0.35 ± 0.06 | 0.34 ± 0.03 | 0.561 |
| Mean blood loss (mg)a | 363.8 ± 11.1 | 353.7 ± 17.1 | 0.156 |
| Pre-op hematocrita | 53 ± 3.7 | 54 ± 2.6 | 0.449 |
| Post-op hematocrita | 47.8 ± 3.6 | 48.5 ± 2.5 | 0.667 |
| Hemostasis time (sec.)a | 176.3 ± 56.8 | 138.1 ± 23.6 | 0.1 |
| SoCO2 Total contact time (sn)a | 38.8 ± 5.8 | 37.5 ± 4.6 | 0.507 |
| Albumin (g/dl)a | 3.1 ± 0.2 | 3.2 ± 0.3 | 0.431 |
| Total bilirubin (mg/dl)a | 0.12 ± 0.06 | 0.16 ± 0.03 | 0.181 |
| Direct bilirubin (mg/dl)a | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.546 |
| ALP(U/L)a | 128 ± 39.9 | 158.5 ± 58.5 | 0.059 |
| AST(U/L)a | 273.6 ± 172.9 | 147.8 ± 19.2 | 0.005* |
| ALT(U/L)a | 146.4 ± 68.1 | 54 ± 10.9 | 0.001* |
| LDH (U/L)a | 1066.4 ± 561.7 | 909 ± 418.7 | 0.753 |
| PT (sn)a | 10.4 ± 1.12 | 9.8 ± 0.64 | 0.225 |
| INRa | 0.84 ± 0.08 | 0.80 ± 0.06 | 0.267 |
| *:p <0.01 a: Mann Whitney U tests |
|||
Table 3: Comparison of rats features and biochemical values in group-3 and group-4 which undergo SoCO2 assisted hemostasis.
ALT values were regressed to normal levels, however LDH and AST values were still remain above the normal limits after a week`s follow-up in Group-4. Group-3 and Group-4, did not show statistically significant difference in albumin, total/direct bilirubin, ALP, LDH, PT and INR values.
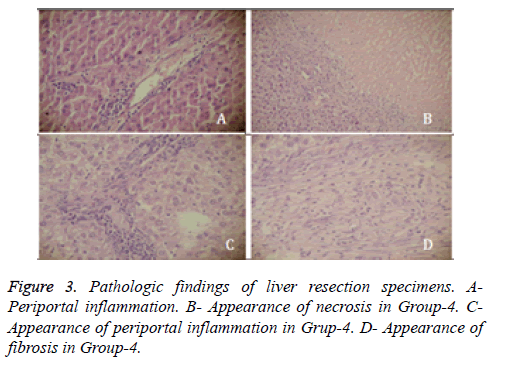
Following euthanasia, liver tissue which came in contact with SoCO2 was resected and fixated in 10% formaldehyde for pathologic analysis. Tissue specimens stained with hematoxylin & eosin were evaluated under light microscope by “Histologic Activity Index (HAI)” scoring for presence of portal inflammation, fibrosis and necrosis [10]. Rats in Group-1 and Group-2 showed slight portal inflammation (score-1) in the contact surface of liver, in Group-3 portal inflammation was moderate (score-2) in contact surface, and mild (score-1) in a distance of 1 cm from surface. None of the rats in these 3 groups showed symptoms of fibrosis or necrosis. Rats in Group-4 showed 240 ± 60 micron deep necrosis in liver contact surfaces. Compared to rats in Group-3 portal inflammation was more severe (score-3) in Group-4 and sporadic fibrosis areas had developed (Figure 3).
Discussion
Achievement of hemostasis in hepatic surgery continues to be one of the most crucial issues, performed either for traumatic lacerations or for benign or malignant diseases of the liver. Sinusoidal structure of the liver that enveloped in a thin capsule makes it difficult to control the bleeding originated from liver parenchyma. Uncontrollable bleedings especially seen in trauma patients is one of the most important causes of mortality [11]. Therefore quick and effective controlling of bleeding during hepatic surgery, reduces the amount of blood loss and operative complications. This also provides reduction in the need for transfusion, shorten the operation time and length of stay in hospital. In traumatic injuries of the liver, some patients are followed without surgery according to protocols whereas for some bleedings topical hemostatic agents, intrahepatic balloon tamponades, suturing and packing maybe required [12,13]. Therefore, in this study gauze tampon compress were applied for bleeding in Group-1 and hemostasis was achieved by continuous sutures in Group-2. Findings from these conventional techniques were compared with hemostasis achieved with SoCO2. Today several devices and hemostatic agents are used to simplify hemostasis, to shorten hemostasis time and provide a more bloodless field for the surgeon [14-19]. In recent years use of topical hemostatic agents has increased [20]. Therefore, significant decrease is noted in morbidity and mortality rates in surgeries of the liver [21]. Some evidence from randomized controlled trials exists regarding the use of fibrin sealants [22,23] or combined with a carrier matrix [24,25]. However, the routine use of topical hemostatic agents has to be judged against noticeable additional costs. It was shown in two studies [24,26] to be superior in obtaining intraoperative hemostasis over argon plasma coagulation in liver resection by reducing the time to hemostasis significantly. Amount of bleeding, simplicity of material use, cost-benefit ratio, storing conditions are also important in addition to surgeon`s preference for choice of hemostasis method [27,28].
In the past, cryosurgery was used for different purposes such as hemostasis or tumor ablasion [29]. Especially “Argon” and “Nitrous Oxide” were used for cryohemostasis; however, with development of ultrasonic and electromagnetic energy devices, they were not considered enough appropriate for clinical applications [11,30]. Purpose of cryosurgery is to obliterate circulation of damaged tissue by freezing, thus provide coagulation. Coagulation is formed as a result of vascular endothelium damage during freezing. However vascular structures can re-bleed secondary to liquefaction of blood clots during the melting period [31,32]. In this study, re-bleeding of relatively large vessels in the liquefaction process following freezing was also encountered. However the surgeon is able to work in a clean and bloodless field and has opportunity to ligate the re-bleeding areas during melting which can be extended with repeated SoCO2 applications.
Strong cryogenicity effect of SoCO2 was shown in patients who were accidentally in direct contact with SoCO2. In normal conditions carbondioxide is found in gaseous state, liquefies under high pressures. It is then converted into solid state under laboratory conditions. Surface temperature of SoCO2 is -78.5°C. It changes directly to gaseous state from solid state, bypassing the liquid phase (sublimation) in normal conditions. As a result it does not leave sediment on the contact tissue. Carbondioxide gas procured from several sources is purified and frozen to -20°C. And liquid CO2 freezes itself to solid state under atmospheric pressure of 20 bars. Sublimation provides a high cooling capacity (152 Kcal/kg) in very low temperatures (-78.5°C) [33].
Kopelman et al. applied cryosurgery -160°C for 10 minute on traumatic grade III-IV wounds they have created in pig livers. They have demonstrated that cryohemostasis significantly reduced the blood loss and substantially attenuated hemorrhagic shock [11]. Likewise in our study, in the Group-3 and Group-4 in which hemostasis was achieved with SoCO2, statistically significant reduced bleeding and shorten hemostasis time were established.
Garanchini et al. [34], compared bipolar vascular sealing devices and classic clamp-crush technique in liver parenchyma transection. They used transaminase values as indication of parenchymal damage which showed statistically significant increase in the group bipolar vascular sealing device was used. There was no statistically significant difference between groups in terms of the serum transaminase levels in our study.
Matsumoto et al. reported histologic outcomes during cryosurgery in the liver. According to this study tissue edema and central necrosis was detected after a ∼3hrs follow up and fibrosis was detected after a ∼1 week follow up after cryosurgery [35]. In our study that consistent with this article, tissue inflammation and edema was observed in the early stage in Group-3 and sporadic areas of fibrosis were observed in Group-4 that presents findings one week after SoCO2 contact.
Recently, thermal energy devices were developed for hepatic resections that causing heat coagulation necrosis in the liver parenchymal. These devices produce a necrotic zone thus enables resection. Stavrou et al. utilized this technique in 28 cases and found the mean blood loss to be less than 100 ml.
Disadvantages of these devices were, probability of producing thermal damage in main vessels where it is close and its use in the hilar area is also limited. It also produces 1 cm area of necrosis in the parenchyma secondary to heat and causing excessive parenchyma loss in cases requiring wide resections. Several complications, including intrahepatic or subhepatic abscess, was noted in 20% of the patients [36]. In our study, necrosis area was deteted within 240 ± 60 micron depths from the surface in Group-3 and Group-4. Cryohemostasis with SoCO2 is considered to be an appropriate agent due to both creating necrosis in a limited area and do not cause further increasing in the biochemical parameters compared to the other groups. Cryohemostasis with SoCO2 is considered to be an appropriate agent due to create necrosis in a limited area and do not cause differences in terms of biochemical parameters compared to the other groups. Thermal energy devices raise the tissue temperature to 70°C, resulting in reactions causing inflammation, edema and carbonization. Adhesions and increased infection problems may arise due to tissue carbonization. SoCO2 assisted cryohemostasis provides hemostasis without residue on the contact surface of liver, and creating smaller necrosis area can result in decline of infection risk.
Conclusion
In conclusion, our study showed that the SoCO2 assisted hemostasis decreases the blood loss, shortens the hemostasis time, enabling the surgeon to work in a bloodless surgical site. SoCO2 also does not leave a residue due to its sublimation feature, easily procurable and low-cost. Although further studies are required, we believe SoCO2 is an effective hemostatic agent in liver surgery.
References
- Clark WR Jr, Leather RP. Hemostasis during liver resections. Surgery 1970; 67: 556- 557.
- Romano F, Franciosi C, Caprotti R, Uggeri F. Hepatic surgery using the LigaSure vessel sealing system. World J Surg 2005; 29: 110-112.
- Carmona RH, Lim RC Jr, Clark GC. Morbidity and mortality in hepatic trauma. A 5 year study. Am J Surg 1982; 144: 88-94.
- Laurent C, Blanc JF, Nobili S, Sa Cunha A, Le Bail B, Bioulac-Sage P, Balabaud C, Capdepont M, Saric J. Prognostic factors and long-term survival after hepatic resection for hepatocelluler carcinoma originating from noncirrhotic liver. J Am Coll Surg 2005; 201: 656-662.
- Jamagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1.803 consecutive cases over the past decade. Ann Surg 2002; 236: 397-406.
- Ruitenbeek K, Ayez N, Verhoef C, De Wilt JH, Bottema J, Rijken AM, van Rij M, Koopman J, Zuckerman LA, Frohna P, Porte RJ. Safety and efficacy of a novel, dry powder fibrin sealant for hemostasis in hepatic resection. Dig Surg 2014; 31: 422-427.
- Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology 1998;37:171-186.
- Baust JG, Gage AA. The molecular basis of cryosurgery. BJU Int 2005; 95: 1187-1191.
- Permpongksol S, Nicol TL, Link RE, Varkarakis I, Khurana H, Zhai QJ, Kavoussi LR, Solomon SB. Differences in ablation size in porcine kidney, liver, and lung after cryoablation protocol. Am J Roentgenol 2007; 188: 1028-1032.
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22: 696-699.
- Kopelman D, Klein Y, Zaretsky A, Ben-Izhak O, Michaelson M, Hashmonai M. Cryohemostasis of Uncontrolled Hemorrhage from Liver Injury Cryobiology 2000; 40: 210-217.
- Meyer AA, Crass RA, Lim RC Jr, Jeffrey RB, Federle MP, Trunkey DD. Selective nonoperative Management of blunt liver injury using computed tomography. Arch Surg 1985; 120: 550-554.
- Pachter HL, Feliciano DV. Complex hepatic injuries. Surg Clin North Am 1996; 4: 763-782.
- Romano F, Franciosi C, Caprotti R, Uggeri F, Uggeri F. Hepatic surgery using the LigaSure vessel sealing system. World J Surg 2005; 29: 110-112.
- Strasberg SM, Drebin JA, Linehan D. Use of a bipolar vessel-sealing device for parenchy- mal transection during liver surgery. J Gastrointest Surg 2002; 6: 569–574.
- Constant DL, Slakey DP, Campeau RJ, Dunne JB. Laparoscopic nonanatomic hepatic resection employing the LigaSure device. JSLS 2005; 9: 35-38.
- Saiura A, Yamamoto J, Koga R, Sakamoto Y, Kokudo N, Seki M, Yamaguchi T, Muto T, Makuuchi M. Usefulness of LigaSure for liver resection: analysis by randomized clinical trial. Am J Surg 2006; 192: 41-45.
- Davidson BR, Burnett S, Javet MS, Seifalian A, Moore D, Doctor N. Experimental study of a novel fibrin sealent for achieving haemostasis following partial hepatectomy. Br J Surg 2000; 87: 790-795.
- Costasis Multi-center Collaborative Writing Committee. A novel collagen-based composite offers effective hemostasis for multiple surgical indications: Results of a randomized controlled trial. Surgery 2001; 129: 445-450.
- Nakajima Y, Shimamura T, Kamiyama T, Matsushita M, Sato N, Todo S. Control of intraoperative bleeding during liver resection: analysis of a questionaire sent to 231 Japanese hospitals. Surg Today 2002; 32: 48-52.
- Akarsu C, Kalaycı MU, Yavuz E, Özkara S, Gokcek B, Özdenkaya Y, Yalçın O. Comparison of the hemostatic efficiency of Ankaferd Blood Stopper and fibrin glue on a liver laceration model in rats. Ulus Travma Acil Cerrahi Derg 2011; 17: 308-312.
- Jackson MR. New and potential uses of fibrin sealants as an adjunct to surgical hemostasis. Am J Surg 2001; 18: 36-39.
- Schwartz M, Madariaga J, Hirose R, Shaver TR, Sher L, Chari R, Colonna JO, Heaton N, Mirza D, Adams R, Rees M, Lloyd D. Comparison of a new fibrin sealant with standard topical hemostatic agents. Arch Surg 2004; 139: 1148-1154.
- Fischer L, Seiler CM, Broelsch CE, de Hemptinne B, Klempnauer J, Mischinger H-J, Gassel H-J, Rokkjaer M, Schauer R, Larsen PN, Tetens V, Büchler MW. Hemostatic efficacy of TachoSil in liver resection compared with argon beam coagulator treatment: an open, randomized, prospective, multicenter, parallel-group trial. Surgery 2011; 149: 48-55.
- Koea JB, Batiller J, Patel B, Shen J, Hammond J, Hart J, Fischer C, Garden OJ. A phase III, randomized, controlled, superiority trial evaluating the fibrin pad versus standard of care in controlling parenchymal bleeding during elective hepatic surgery. HPB(Oxford) 2011; 15: 61-70.
- Frilling A, Stavrou GA, Mischinger H-J, de Hemptinne B, Rokkjaer M, Klempnauer J, Thörne A, Gloor B, Beckebaum S, Ghaffar MFA, Broelsch CE. Effectiveness of a new carrier-bound fibrin sealant versus argon beamer as haemostatic agent during liver resection: a randomised prospective trial. Langenbecks Arch Surg 2005; 390: 114-120.
- Berrovet F, de Hemptinne B. Clinical application of topical sealants in liver surgery: does it work? Acta Chir Belg 2007; 107: 504-507.
- Tsugawa K, Koyanagi N, Hashizume M, Ayukawa K, Wada H, Tomikawa M, Ueyama T, Sugimachi K. Anatomic resection for severe blunt liver trauma in 100 patients: significant differences between young and eldery. World J Surg 2002; 26: 544-549.
- Serra P, Brunschwig A. Freezing of liver parenchyma with liquid nitrogen for hemostasis in excisional liver surgery. An experimental study. Cancer 1955; 8: 1234-1238.
- Rabin Y. Key issues in bioheat transfer simulations for the application of crysurgery planning. Cryobiology 2008; 56: 248-250.
- Mazur P. Theoretical and experimental effects of cool- ing and warming velocity on the survival of frozen and thawed cells. Cryobiology 1966; 2: 181-192.
- Ravikumar TS, Steele GD Jr. Hepatic cryo-surgery. Surg. Clin. North Am. 1989; 69: 433-439.
- Escribano RM, Caro GM, Cruz-Diaz GA, Roriguez-Lazcano Y, Mate B. Crystallization of CO2 ice and the absence of amorphous CO2 ice in space. Proc Nati Acad Sci USA 2013; 110: 12899-12904.
- Garancini M, Gianotti L, Mattavelli I, Romano F, Degrate L, Caprotti R, Nespoli A, Uggeri F. Bipolar vessel sealing system vs. clamp crushing technique for liver paranchyma transection. Hepatogastroenterology 2011; 58: 127-132.
- Matsumoto R, Selig AM, Colucci VM, Jolesz FA. MR monitoring during cryotherapy in the liver: predictability of histologic outcome. J Magn Reson Imaging.1993; 3: 770-776.
- Stavrou GA, Donati M, Fruehauf NR, Flemming P, Oldhafer KJ. Liver resection using heat coagulative necrosis: indications and limits of a new method. ANZ J Surg 2009; 79: 624-628.