Research Article - Biomedical Research (2017) Volume 28, Issue 5
Anti-proliferative and apoptotic action of sophocarpine in human prostate cancer cell lines
Dong SF*, Yang JX, Lv GY and Fu QZDepartment of Urinary Surgery, Affiliated Zhong Shan Hospital of Dalian University, PR China
- *Corresponding Author:
- Dong SF
Department of Urinary Surgery
Affiliated Zhong Shan Hospital of Dalian University, PR China
Accepted date: October 10, 2016
Abstract
Sophocarpine is an herbal compound obtained from foxtail-based sophora seeds and herbs, and is found to have various clinical applications in Chinese herbal medicine. This study was aimed to determine the anticancer properties of sophocarpine against human prostate cancer cells PC93, TSU-Pr1 and their molecular mechanisms involved in anticancer activity. The cytotoxicity assay indicated that sophocarpine markedly inhibited cell proliferation against prostate cancer cells in a concentration and time-dependent manner. Further, TUNEL and flow cytometry results demonstrated that sophocarpine treatment may trigger apoptosis in a concentration dependent way. In addition, the western blot experiments revealed a significant increase in expression of Bax and p53 and significant reduction in Bcl-2 expression in a concentration dependent manner. In vivo studies determined that sophocarpine hinders tumour progression following subcutaneous injection of cancer cells into nude mice. The results indicated that the anticancer properties of sophocarpine are through the inhibition of cell proliferation and inducing apoptosis mediated through the regulation of apoptosis related protein expressions. Hence, the results may pave for new approach in prostate cancer therapy using sophocarpine.
Keywords
Sophocarpine, Apoptosis, PC93, TSU-Pr1, Prostate cancer.
Introduction
Prostate cancer is the second most common type of cancer which can cause death among men world-wide [1,2]. At present, the observation and recognition of prostate cancer growth are performed with the help of digital rectal inspection, evaluating prostate-specific antigens and examination of prostate needle biopsies using histopathology [3,4]. Treatment of prostate cancer with the available drugs is not that effective. Hence, the exploration of potential and novel effective drugs to cure prostate cancer is need of the hour [5]. It has been reported that androgen suppression therapy is used for prostate cancer patients in metastatic phase but they always develop progressive ailments [6]. Based on the duration of survival rate of the patient when administered with combination of mitoxantrone and prednisone in metastatic prostate cancer patients with castration resistance [7-9], a mixture of prednisone and docetaxel is considered as classic chemotherapeutic treatment. Unites States Food and Drug Administration (FDA) did not approve this combination of drugs, because the cancer cells start to grow again for patients after the treatment of docetaxel.
Sophocarpine, a tetracyclic matrine-type quinolizidine moeity belongs to the sophorae alkaloid. It can be isolated from foxtail like herbs of sophora and their seeds. The structure of sophocarpine is identical with Kushen alkaloids and is used widely in Chinese traditional medicines. The plants with genus name Sophora can be found from both tropic and temperate regions in the world. Only few of these herbs are used as essential sources in Chinese medicine. Sophocarpine is found to contain various therapeutic, pharmacological and biological properties such as anti-cancer, anti-nociceptive, antiinflammatory, anti-virus and immunoregulating agent [10-14]. Several reports showed that sophocarpine is participated in the maintenance of TLR4 [15], cytokine balance [16] and pathways of NF-κβ [17,18].
The anticancer property of sophocarpine was carried out by administering the patients with positive-enterovirus using sophocarpine of six to eight mg/kg a day for at least three to four hours. This was continued for three to four weeks and then compared with controls without treatment [19,20]. The analysis results indicated that concentration dependent reduction in the virus multiplication in patients treated with sophocarpine. Reports showed that low cost sophocarpine exhibited minor poisonous side-effects and broad distribution in tissues, in vivo [19]. Several studies indicated that sophocarpine has the ability to suppress inflammatory response in cells in vitro, endotoxin inactivation and regulation of inflammation [21-23]. Further, it is an efficient compound to be used as a therapeutic agent for the treatment of myocarditis and anti-hepatitis B caused by virus [20,24]. Also, therapeutic and preventive measures of sophocarpine were also revealed by experiments conducted on non-alcoholic steatohepatitis and the results showed that sophocarpine could reduce the non- alcoholic steatohepatitis [25]. Sophocarpine was utilized in regulating inflammatory functions by hindering the pathways of AMPK and NF-κβ [26,27]. However, no studies were conducted regarding the effectiveness of sophocarpine in prostate cancer to date and hence, we decided to study the anticancer properties of sophocarpine in prostate cancer cell lines.
Materials and Methods
Chemicals and reagents
Sophocarpine with purity of >98% was acquired from Abcam (ab143179, Cambridge, UK). Stock solution of sophocarpine was prepared with a concentration of 10 mg/ml using Dimethyl Sulfoxide (DMSO). It was diluted using culture medium and used for further experiments. DMSO, Dulbecco’s Modified Eagle’s Medium (DMEM), Fetal Bovine Serum (FBS), Penicillin and streptomycin were purchased from Thermo Fisher Scientific (#85190, Waltham, MA, USA).
Cell lines and Cell culture methods
Two human prostate cancer cell lines, PC93, TSU-Pr1 and one normal prostate cell line RWPE-1 were obtained from American Type Culture Collection (ATCC, USA). Cell lines were maintained in DMEM medium supplemented with heatinactivated FBS (10%), penicillin and streptomycin of 100 μg/ml in a 5% CO2 incubator at 37°C.
Cytotoxicity
MTT assay was used to determine the cell viability. Human prostate cell lines PC93, TSU-Pr1 and normal cell line RWPE-1 were cultured at 1000 cells per well in 96-well plates. After the cells were grown for 24 h, various sophocarpine concentrations (0, 1, 2, 5 and 10 mg/ml) were added and incubated overnight. After incubation, 6 mg/ml of MTT reagent was added and kept for 2 h incubation at 37°C. A 96- well microplate reader (Sunrise, Tecan, Switzerland) was used to record the absorbance at a wavelength of 480 nm.
Flow cytometry
The human prostate cancer cell lines were treated with different concentration of sophocarpine (0, 5 and 10 mg/ml) and incubated for next 72 h. The cells were trypsinized and followed by centrifugation at 1000 x g. Then, PBS was used to wash the pellets thrice. Annexin V-FITC/PI was used to detect the apoptotic cells and this was performed with the help of apoptosis detection kit based on the manufacturer’s instructions (Sigma Aldrich, St. Louis, USA).
Western blot
The extraction and separation process of proteins were carried out after the treatment of sophocarpine using Sodium Dodecyl Sulfate-Polyacrylamide Electrophoresis Gel (SDS-PAGE) and then the proteins were transferred to Polyvinylidene Difluoride (PVDF) membranes (GE Healthcare Life Sciences, USA). The membranes were blocked and incubated overnight at 4°C with the primary antibodies including human anti-p53 antibody (monoclonal; mouse; 1:1000 dilution; cat. no ab28, Abcam), human anti-Bcl2 antibody (monoclonal; mouse; 1:1000 dilution; cat. no. ab9478, Abcam), human anti-Bax antibody (monoclonal; mouse; 1:1000 dilution; cat. no. ab77566, Abcam) and human anti-GADPH antibody (monoclonal; mouse; 1:5000 dilution; cat. no. ab110305, Abcam) (Abcam, Cambridge, UK,). After incubation, the membranes were washed with Tris-buffer and Tween 20 solution and then incubated with goat anti-mouse secondary antibodies conjugated with horseradish peroxidase with a dilution of 1:10,000. Finally, chemi-luminescent reagent was used for visualization and detection Merck Millipore. (Billerica, MA, USA).
In vivo studies using xenografts
This experiment was performed after getting the approval from institutional ethics committee. The in vivo xenografts study was conducted using BALB/c nude mice within 6-8 weeks of about 19-21 g were maintained in a Specific-Pathogen-Free (SPF) environment. Cell lines (PC-93) at a density of 2 × 105 were suspended in PBS (100 μl). The mice were injected subcutaneously into left axilla and after five days, a total of 24 tumoral mice were classified into three groups. One group was kept as control which was treated only with PBS whereas the other two groups were given different dosages of sophocarpine of about 50 and 100 mg/kg. Sophocarpine was given to mice as intraperitoneal injections. The formula A × B2 × π/6 was used to calculate the tumour volume in which A represents the length of longest feature of the tumour and B indicates the tumour’s length perpendicular to that of A. Mice were treated for 8 weeks, sacrificed and then tumour weight was calculated.
TUNEL assay
In vivo experiments were conducted on tumour affected mouse models using BALB/c mice to demonstrate the cell apoptosis. TUNEL assay kit obtained from Roche diagnostics (Indianapolis, IN, USA) was used to perform this analysis. In this experiment, apoptotic cells were identified by noticing brown nuclei and the total apoptotic cell counts per thousand cells was measured in each group. The microscope used to view each field is IX53 from Olympus Corporation (Tokyo, Japan) at a magnification of x200.
Statistical analysis
All the obtained data are expressed as mean of ± SD. IBM SPSS Statistics (IBM Technologies, USA) was used to perform the statistical analysis. Analysis of variance was used to make the comparisons between the different sets. Values of P<0.05 was considered to point out a statistically remarkable difference.
Results
Sophocarpine inhibits cell proliferation
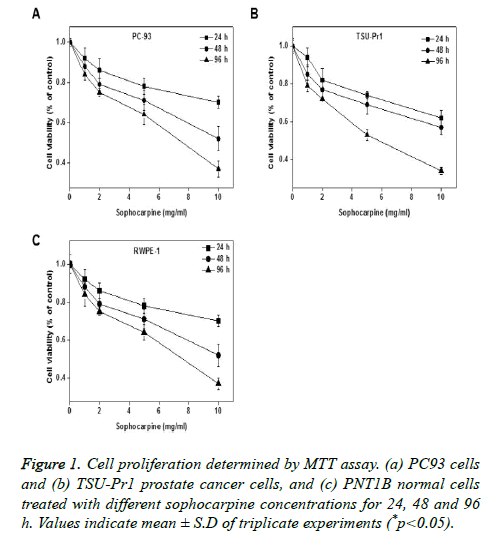
The anti-proliferation properties of sophocarpine on human prostate cancer cell lines were tested on TSU-Pr1 and RWPE-1 cells by treating it with different concentrations (0, 1, 2, 5 and 10 mg/ml) of sophocarpine and for various intervals of time such as 24, 48 and 96 h. Results of MTT assay indicated that sophocarpine showed notable inhibition on the proliferation of prostate cancer cells PC93 and TSU-Pr1 in a concentration and time dependent manner. The results are shown in Figures 1A and 1B. The cell proliferation in normal prostate cells was uninhibited upon sophocarpine treatment as shown in Figure 1C.
Sophocarpine promotes cell apoptosis
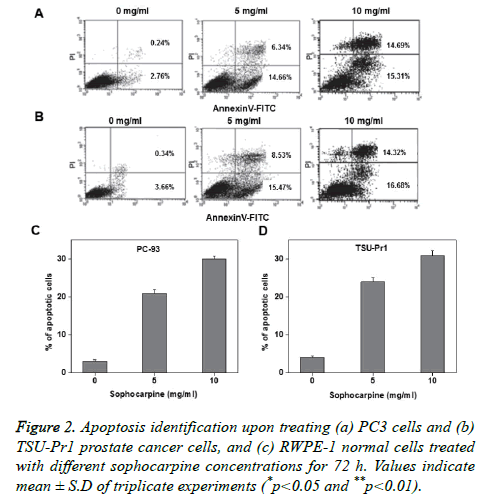
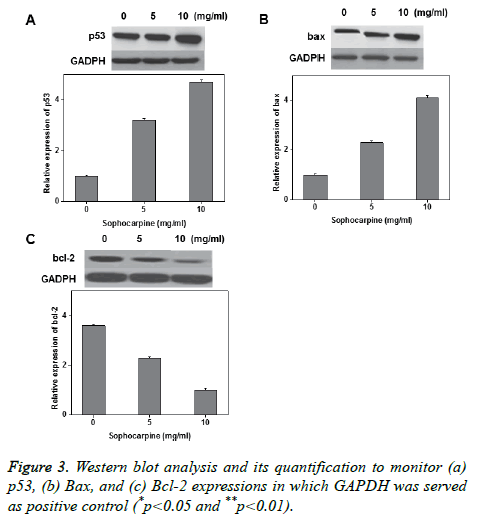
Annexin V-FITC/PI double staining was performed to investigate the sophocarpine mediated apoptosis in prostate cancer cells. The experimental results of flow cytometry suggested that sophocarpine treatment increased cell apoptosis in prostate cancer cells PC-93 (Figures 2A and 2C) and TSUPr1 (Figures 2B and 2D), in a concentration dependent manner. The results indicated that treatment of sophocarpine may help to induce cell apoptosis in human prostate cancer. To determine the possible molecular mechanisms involved in sophocarpine induced apoptosis of human prostate cancer cell lines, the analysis of p53 expressions, Bax and Bcl-2 expressions were performed after treating with various concentrations of sophocarpine. The western blot results showed that p53 and Bcl-2 expressions were reduced whereas increase in Bax expression was monitored, in a concentration dependent manner as shown in Figure 3.
In vivo reduction of cell proliferation
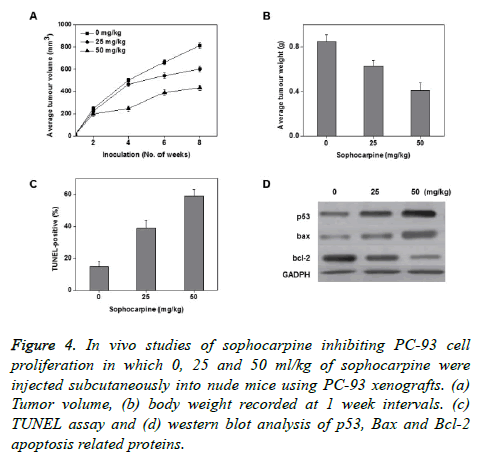
To know the sophocarpine effects on cancer growth in vivo, 3 different sophocarpine dosages were injected intraperitoneally into nude BALB/c mice using PC-93 subcutaneous xenografts. The obtained results showed that upon sophocarpine treatment, the tumour volume and tumour weight of mice markedly reduced in a concentration dependent fashion (Figures 4A and 4B). The concentration dependent increase in the total apoptotic cell counts was measured through TUNEL assay (Figure 4D). These results supports that sophocarpine can influence the reduction of prostate cancer cell development by inducing cell apoptosis, in vivo.
Figure 4. In vivo studies of sophocarpine inhibiting PC-93 cell proliferation in which 0, 25 and 50 ml/kg of sophocarpine were injected subcutaneously into nude mice using PC-93 xenografts. (a) Tumor volume, (b) body weight recorded at 1 week intervals. (c) TUNEL assay and (d) western blot analysis of p53, Bax and Bcl-2 apoptosis related proteins.
Discussion
In the last decade, investigation about biological and pharmacological properties of sophorae alkaloids has been done and these findings remain one of the main focuses of Chinese herbal medicine research [28-30]. Many studies have reported that matrine possesses the ability to inhibit cell proliferation of hepatoma cancer [31,32]. Sophocarpine is structurally related to matrine but no one reported its potential in treatment of prostate cancer so far. We found that sophocarpine treatment can induce cell apoptosis and inhibit cell proliferation in human prostate cancer cells both in vitro and in vivo. MTT assay results showed that treatment of sophocarpine on human prostate cancer cells TSU-Pr1 and PC93 inhibits cell proliferation in concentration and timedependent manner. In vivo tumorigenesis measurements after sophocarpine treatment in xenografts indicated a significant reduction in tumour size and tumour weight in PC-93 subcutaneous xenografts in a concentration dependent manner. The data suggested that sophocarpine treatment helps to inhibit the cell proliferation of prostate cancer both in vitro and in vivo.
Cell death process, apoptosis is identified by the cellular and molecular processes including cell shrinkage, condensation of chromatids, externalization of phosphatidylserine etc., [33,34]. Cancer formation and progression is associated with uninhibited cell proliferation. Hence, induction of apoptosis furnishes a powerful mechanism for the improvement of anticancer therapies [35,36]. In our current study, treatment of sophocarpine triggered cell apoptosis on prostate cancer in a concentration dependent manner, in vitro. This was further analysed using TUNEL assay and flow cytometry experiments. Apoptosis is associated with different signalling pathways and various molecular markers participating in these signalling pathways have also been determined [37-39]. For instance, in multi-cellular living things, apoptosis and cell cycle maintenance are correlated with p53, concealed by the tumour protein 53 gene [38,39]. During external and internal inducements including infections caused by virus and oxidative stress, p53 can trigger or repress different target genes which are participated in apoptosis including p53, Bax and Bcl-2 [40,41]. Bax participated in a p53 maintenance pathway. p53 assembles in the cytosol and helps to advance Bax expression which helps to permeate mitochondria and assist apoptosis [42,43]. Bcl-2 is involved in avoiding the mitochondrial damage and obstruct the release of cytochrome c from mitochondria [44,45]. The data obtained from the current study revealed that treatment of sophocarpine on prostate cancer cells may end up in an increase in expression of Bax and p53 and reduction of Bcl-2 expression, in a concentration dependent manner. Collectively, the experimental results demonstrated that sophocarpine is a potential drug candidate to regulate the apoptosis related protein expressions in prostate cancer cells PC93, TSU-Pr1 both in vitro and in vivo.
Conclusion
In conclusion, the experimental results obtained revealed that sophocarpine displayed anticancer characteristics in human prostate cancer cells PC93, TSU-Pr1 both in vitro and in vivo. In addition, the observed results proved that anticancer potential of sophocarpine can be correlated to the inhibition of cell proliferation as well as induction of apoptosis. These observations can support a novel access for improvement of prostate cancer, using sophocarpine which is obtained from the genus Sophora.
Acknowledgements
The present study was supported by the Affiliated Zhong Shan Hospital of Dalian University and all the authors would like to thank them for their assistance to complete our work.
References
- Parkin DM, Laara E, Muir CS. Estimates of the worldwide frequency of sixteen major cancers in 1980. Int J Cancer 1988; 41: 184-197.
- Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, CA Cancer. J Clin 1996; 46: 5-27.
- Katahira K, Takahara T, Kwee TC, Oda S, Suzuki Y, Morishita S, Kitani K, Hamada Y, Kitaoka M, Yamashita Y. Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur Radiol 2011; 21: 188-196.
- Gosselaar C, Roobol MJ, Roemeling S, van der Kwast TH, Schröder FH. Screening for prostate cancer at low PSA range: the impact of digital rectal examination on tumor incidence and tumor characteristics. Prostate 2007; 67: 154-161.
- Shi GH, Ye DW, Yao XD, Zhang SL, Dai B, Zhang HL, Shen YJ, Zhu Y, Zhu YP, Xiao WJ, Ma CG. Involvement of microRNA-21 in mediating chemo-resistance to docetaxel in androgen-independent prostate cancer PC3 cells. Acta Pharmacol Sin 2010; 31: 867-873.
- Eisenberger MA, Simon R, O’Dwyer PJ, Friedman HA. A re-evaluation of nonhormonal cytotoxic chemotherapy in the treatment of prostatic carcinoma. J Clin Oncol 1985; 3: 827-841.
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodre C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502-1512.
- Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513-1520.
- Berry DL, Moinpour CM, Jiang CS, Ankerst DP, Petrylak DP, Vinson LV, Lara PN, Jones S, Taplin ME, Burch PA, Hussain MH, Crawford ED. Quality of life and pain in advanced stage prostate cancer: results of a Southwest Oncology Group randomized trial comparing docetaxel and estramustine to mitoxantrone and prednisone. J Clin Oncol 2006; 24: 2828-2835.
- Liu M, Liu XY, Cheng JF. Advance in the pharmacological research on matrine. Zhongguo Zhong Yao Za Zhi 2003; 28: 801-804.
- Ma L, Wen S, Zhan Y, He Y, Liu X, Jiang J. Anticancer effects of the Chinese medicine matrine on murine hepatocellular carcinoma cells. Planta Med 2008; 74: 245-251.
- Liu SX, Chiou GC. Effects of Chinese herbal products on mammalian retinal functions. J Ocul Pharmacol Ther 1996; 12: 377-386.
- Jiang H, Hou C, Zhang S, Xie H, Zhou W, Jin Q, Cheng X, Qian R, Zhanget X. Matrine upregulates the cell cycle protein E2F-1 and triggers apoptosis via the mitochondrial pathway in K562 cells. Eur J Pharmacol 2007; 559: 98-108.
- Azzam HS, Goertz C, Fritts M, Jonas WB. Natural products and chronic hepatitis C virus. Liver Int 2007; 27: 17-25.
- Qian H, Shi J, Fan T, Lv J, Chen S, Song C, Zheng Z, Xie W, Chen Y. Sophocarpine attenuates liver fibrosis by inhibiting the TLR4 signaling pathway in rats. World J Gastroenterol 2014; 20: 1822-1832.
- Wang X, Deng H, Jiang B, Yao H. The natural plant product sophocarpine ameliorates dextran sodium sulfate-induced colitis in mice by regulating cytokine balance. Int J Color Dis 2012; 27: 575-581.
- Gao Y, Li G, Li C, Zhu X, Li M, Fu C, Li B. Anti-nociceptive and anti-inflammatory activity of sophocarpine. J. Ethnopharmacol 2009; 125: 324-329.
- Song CY, Zeng X, Chen SW, Hu PF, Zheng ZW, Ning BF, Shi J, Xie WF, Chen YX. Sophocarpine alleviates non-alcoholic steatohepatitis in rats. J Gastroenterol Hepatol 2011; 26: 765-774.
- Chen SX, Xu J, Wang PQ, Zhou BB, Cai MH, Qian FR. Study of sophocarpine in human pharmacokinetic. Shanghai Second Medical Univ J 2005; 25: 1164-1166.
- Guo B, Li C, Deng Z, Chen S, Ji Z, Zhang J, Chen M, Xu F. A new method for measurement of (-)-sophocarpine, a candidate therapeutic for viral myocarditis, in plasma: application to a toxicokinetic study in beagle dogs. Rapid Commun Mass Spectrum 2005; 19: 2840-2848.
- Zhang Y, Wang S, Li Y, Xiao Z, Hu Z, Zhang J. Sophocarpine and matrine inhibit the production of TNF-alpha and IL-6 in murine macrophages and prevent cachexia-related symptoms induced by colon26 adenocarcinoma in mice. Int. Immunopharmacol 2008; 8: 1767-1772.
- Han Y, Zhou Y, Liu Q. Antiendotoxic effects of Sophora alopecuroides L. Zhong Yao Cai 2006; 29: 1066-1069.
- Qavi HB, Wyde PR, Khan MA. In vitro Inhibition of HHV-6 replication by sophocarpines. Phytother Res 2002; 16: 154-156.
- Ding PL, Liao ZX, Huang H, Zhou P, Chen DF. (+)-12alpha-Hydroxysophocarpine, a new quinolizidine alkaloid and related anti-HBV alkaloids from Sophora flavescens. Bioorg Med Chem Lett 2006; 16: 1231-1235.
- Song CY, Zeng X, Chen SW, Hu PF, Zheng ZW, Ning BF., Shi J, Xie WF, Chen YX. Sophocarpine alleviates non-alcoholic steatohepatitis in rats. J Gastroenterol Hepatol 2011; 26: 765-774.
- Zhang S, Ma JH, Zhang PH, Luo AT, Ren ZQ, Kong LH. Sophocarpine attenuates the Na(+)-dependent Ca2(+) overload induced by Anemonia sulcate toxin-increased late sodium current in rabbit ventricularmyocytes. J Cardiovasc Pharmacol 2012; 60: 357-366.
- Song CY, Shi J, Zeng X, Zhang Y, Xie WF, Chen YX. Sophocarpine alleviates hepatocyte steatosis through activating AMPK signaling pathway. Toxicol In Vitro 2013; 27: 1065-1071.
- Ma L,Wen S, Zhan Y, He Y, Liu X, Jiang J. Anticancer effects of the Chinese medicine matrine on murine hepatocellular carcinoma cells. Planta Med 2008; 74: 245-251.
- Azzam HS, Goertz C, Fritts M, Jonas WB. Natural products and chronic hepatitis C virus. Liver Int 2007; 27: 17-25.
- Liu JY, Hu JH, Zhu QG, Li FQ, Wang J, Sun HJ. Effect of matrine on the expression of substance P receptor and inflammatory cytokines production in human skin keratinocytes and fibroblasts. Int Immunopharmacol 2007; 7: 816-823.
- Zhang JQ, Li YM, Liu T, He WT, Chen YT, Chen XH. Antitumor effect of matrine in human hepatoma G2 cells by inducing apoptosis and autophagy. World J Gastroenterol 2010; 16: 4281-4290.
- Wang Y, Yao R, Gao S, Wen W, Du Y, Szabo E. Chemopreventive effect of a mixture of Chinese herbs (antitumor B) on chemically induced oral carcinogenesis. Mol Carcinog 2013; 52: 49-56.
- Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, Cohen GM. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ 2009; 16: 1030-1039.
- Silva MT. Secondary necrosis: the natural outcome of the complete apoptotic program. FEBS Lett 2010; 584: 4491-4499.
- Frew AJ, Johnstone RW, Bolden JE. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett 2009; 280: 125-133.
- Hou J, Wang D, Zhang R, Wang H. Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res 2008; 14: 5519-5530.
- Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov 2008; 7: 979-987.
- Hu W, Ge Y, Ojcius DM, Sun D, Dong H, Yang XF, Yan J. p53 signaling controls cell cycle arrest and caspase-independent apoptosis in macrophages infected with pathogenic Leptospira species. Cell Microbiol 2013; 15: 1642-1659.
- Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y, Giorgetti-Peraldi S, Cormont M, Bertolotto C, Deckert M, Auberger P, Tanti JF, Bost F. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res 2010; 70: 2465-2475.
- Fernandez-Gomez FJ, Llecha N, Comella JX, Prehn JH, Jordan J. 6-Hydroxydopamine activates the mitochondrial apoptosis pathway through p38 MAPK-mediated, p53-independent activation of Bax and PUMA. J Neurochem 2008; 104: 1599-1612.
- Ramaiah MJ, Pushpavalli SN, Lavanya A, Bhadra K, Haritha V, Patel N, Tamboli JR, Kamal A, Bhadra U, Pal-Bhadra M. Novel anthranilamide-pyrazolo[1,5-a]pyrimidine conjugates modulate the expression of p53-MYCN associated micro RNAs in neuroblastoma cells and cause cell cycle arrest and apoptosis. Bioorg Med Chem Lett 2013; 23: 5699-5706.
- Deng Y, Wu X. Peg3/Pw1 promotes p53-mediated apoptosis by inducing Bax translocation from cytosol to mitochondria. Proc Natl Acad Sci USA 2000; 97: 12050-12055.
- Gogada R, Prabhu V, Amadori M, Scott R, Hashmi S, Chandra D. Resveratrol induces p53-independent, X-linked inhibitor of apoptosis protein (XIAP)-mediated Bax protein oligomerization on mitochondria to initiate cytochrome C release and caspase activation. J Biol Chem 2011; 286: 28749-28760.
- Bishayee K, Chakraborty D, Ghosh S, Boujedaini N, Khuda-Bukhsh AR. Lycopodine triggers apoptosis by modulating 5-lipoxygenase, and depolarizing mitochondrial membrane potential in androgen sensitive and refractory prostate cancer cells without modulating p53 activity: signaling cascade and drug-DNA interaction. Eur J Pharmacol 2013; 698: 110-121.
- Liu Y, Yang Y, Ye YC, Shi QF, Chai K, Tashiro S, Onodera S, Ikejima T. Activation of ERK-p53 and ERK-mediated phosphorylation of Bcl-2 are involved in autophagic cell death induced by the c-Met inhibitor SU11274 in human lung cancer A549 cells. J Pharmacol Sci 2012; 118: 423-432.