Research Article - Biomedical Research (2018) Volume 29, Issue 13
Antioxidants, antimicrobial and cytotoxic potential of Swertia chirata
Muhammad Atif Khan1, Muhammad Zia2, Muhammad Arfan3, Abdul Nazir4, Nighat Fatima1, Muhammad Naseer5, Shujaat Ali Khan1, Tariq Ismail1, Yasser MSA Alkahraman1, Ghulam Murtaza6*, and Abdul Mannan1*
1Department of Pharmacy, COMSATS University Islamabad (Abbottabad Campus 22060), Pakistan
2Department of Biotechnology, Quaid-i-Azam University, Islamabad, Pakistan
3Department of Chemistry, School of Natural Sciences, National University of Science and Technology, Islamabad, Pakistan
4Department of Environmental Science, COMSATS Institute of Information Technology, Abbottabad, Pakistan
5Department of Environmental Science, International Islamic University, Islamabad, Pakistan
6Department of Pharmacy, COMSATS University Islamabad (Lahore Campus), Pakistan
- *Corresponding Author:
- Abdul Mannan
Department of Pharmacy
COMSATS University
Abbottabad Campus, Pakistan
Accepted on June 5, 2018
DOI: 10.4066/biomedicalresearch.29-18-685
Visit for more related articles at Biomedical ResearchAbstract
The current study was designed to evaluate the properties of various fractions of methanolic extract prepared from aerial parts of Swertia chirata. Five fractions, RA-78, RA-91, RA-112, RA-14 and RA-15 showed significantly high DPPH scavenging capacity. In finding out antimicrobial activities, different fractions have shown mild activities against Bordetella bronchiseptica and Micrococcus luteus, while all fractions showed significant results against Aspergillus fumigatus followed by Mucor species and five fractions were active against Aspergillus niger. In measurement of brine shrimp cytotoxic potential of different fractions, three fractions RA-12, RA-34 and RA-78 had shown the best cytotoxic results with zero LD50. In determining antileishmanial properties of different fractions, the best results were exhibited by fraction RA-78 and RA-112 with zero percent survival of Leishmania parasite. Various fractions of methanolic extract prepared from aerial parts of Swertia chirata promisingly possess antioxidant, antimicrobial, brine shrimp cytotoxic, and antileishmanial activities.
Keywords
Swertia chirata, Phytochemical evaluation, DPPH free radical scavenging activity, Antimicrobial properties, Cytotoxicity assay, Antileishmanial activity.
Introduction
Medicinal plants are as assumed as major source for active constituents which are used for development of drugs. There are about half million plants around the world, and most of their medicinal activities have not explored yet [1]. According to WHO, about 80% of world population relies on plant based conventional medicines especially for their primary health care need [2]. It was a common perception on the basis of tradition, that there is no harm in the uses of natural medicinal plants [3].
The medicinal plant Swertia chirata (Roxb.) Buch-Ham. ex C. B. Clarke belongs to the family gentianaceae. Its folk use names are Chirata, Chareta, and chirayta. It is being used in different remedies as antipyretic and anthelmintic [4] and in arthritis [5]. It is also used for gastrointestinal and urogenital tract disorders [4]. This plant is also being used traditionally for treatment of cancer [6].
The objectives of present study were to prepare crude methanolic extract and its different fractionations obtained from the aerial parts of Swertia chirata and evaluate their phytochemical, antioxidant, antimicrobial, cytotoxic, and antileishmanial potential.
Materials and Methods
Extract preparation and its fractionation
Swertia chirata plant material was collected from Galyat region of Khyber Pakhtunkhwa, Pakistan and identified by the taxonomist and verified by comparing with available literature and herbarium specimens. The specimen was deposited in the herbarium and given the voucher number “PAC1009”.
The aerial parts of the plant material were shade dried, crushed into fine powder. The powdered plant material (4.3 kg) was macerated in methanol (10 L) for 10 d. Methanolic extract was filtered through muslin cloth and filter paper and dried under reduced pressure for further analysis.
Fractionation of methanolic extract (RA-16) was carried out by using gravity column chromatography. For column packing, slurry of silica gel (400 g) prepared in chloroform was poured into it, chloroform was further added with continuous tapping to develop the column and methanolic extract was applied slowly on the top. The solvent systems of 5% CH3OH/ AHCl3-10%, 15%, and 20% CH3OH/AHCl3 were run and different fractions were collected, labeled as RA-12, RA34, RA-56, RA-78, RA-91, RA-112, RA-13, RA-14, and RA-15.
Total phenolic and flavonoid contents
The procedure used to determine the total phenolic contents, using Folin-Ciocalteu reagent [7]. The absorbance of reaction mixture was recorded at 715 nm. Gallic acid was used as standard and total phenolic contents were express as gallic acid equivalent. In addition, the method utilized for evaluation of total flavonoid contents was aluminium chloride colorimetric method [8]. The absorbance of the reaction mixture was taken at 415 nm. Quercetin was used as a standard and flavonoid contents were expressed as quercetin equivalent. The experiments were repeated in triplicate.
DPPH radical-scavenging activity
The antioxidant activities of crude methanolic extract and its fractions were determined by using DPPH free radical scavenging assay, as described previously [9]. The absorbance was taken at 517 nm by micro plate reader and scavenging percentage was calculated. The experiment was performed in triplicate. Ascorbic acid was used as a positive control, whereas methanol was used as a negative control.
Antimicrobial assay
The antibacterial activity of each fraction was determined by using disc diffusion method [10] using three strains of bacteria. One strain Micrococcus luteus was gram-positive while two strains Bordetella bronchiseptica and Salmonella typhi were gram-negative. The inoculum of each bacterium was spread smoothly on surface of sterilized solidified nutrient agar media present in the petri plate. Then 5 μl of each fraction was loaded on autoclaved filtered paper discs and placed on the respective places in the petri plates. Each plate contained two disc of positive and one disc of negative control. The plates were incubated at 37°C for about 24 h. The zone of inhibition (mm) was measured and assay was performed in duplicate.
The antifungal activity of each plant fraction was evaluated by using disc diffusion method [11]. Three different fungal strains were used, which were Mucor specie, Aspergillus fumigates, and Aspergillus niger. After solidification of sterilized sabouraud dextrose agar media in the petri plate, inoculum of each fungus was swabbed on its surface to produce the lawn. Then 5 μl of each fraction was loaded on filter paper disc and each disc was placed on its respective place in labeled plate. Each plate contains two disc of positive and one disc of negative control. The plates were then incubated at 28°C for 24 h. After incubation, zones of inhibition were measured and experiment was performed in triplicate.
Brine shrimp lethality assay
The cytotoxic effects of all fractions were measured by using brine shrimp lethality bioassay [8]. Three concentrations of each fraction (1000 μg/ml, 100 μg/ml, and 10 μg/ml) and three concentrations of standard drug were prepared (10 μg/ml, 1 μg/ml, and 0.1 μg/ml). 96 plate was used for this assay and 250 μl of sea water was added to the well so that the sample was dissolved in the sea water. Then 10 shrimps (Artemisa salina) were transferred to each well. The volume of the wells was raised up to 300 μl to attain desired concentration of each well. The plates were incubated for 24 h at room temperature. Then shrimps were taken from each well and counted the survivors under magnifying glass. The lethal dose of crude fractions against brine shrimps was calculated by using Finny software.
Antileishmanial assay
Pre-established Leishmania tropica KWH23 strain was incubated at 24 ± 1°C for 6-7 d in 199 medium containing 10% fetal bovine serum. The in vitro antileishmanial assay was performed according to the protocol previously described previously [12] with a slight modification. Stock solution of each fraction (4,000 ppm) was prepared by dissolving 4 mg in 1000 μL of distilled water. The stock solutions were serially diluted in 96 well plates. Positive and negative control was Amphotericin B and distilled water, respectively. The microtiter plates were incubated at 24°C for 72 h. The experiment was performed in triplicate. After 72 h, about 15 μl of test culture were then transferred to improved neubauer counting chamber and live promastigotes were counted under light microscope. For LC50 calculation, probit regression analysis of SPSS Ver. 21 was used.
Results and Discussion
Total phenolic and flavonoid contents
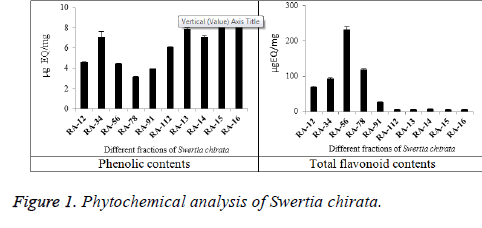
According to results of fractions and crude methanolic extract of Swertia chirata, high total phenolic contents were found in four fractions, RA-15, RA-13, RA-34 and RA-14 (8.61 ± 0.08, 7.92 ± 0.07, 7.16 ± 0.51 and 7.16 ± 0.06 μg EQ/mg respectively) (Figure 1). Similar range of total phenolic contents was found in Polygonatum species [13]. Moreover, these concentrations may depend on variation in collection time and site [14]. High total flavonoid contents (Figure 1) were found in three fractions, RA-56, RA-78 and RA-34 (232.5 ± 7.4, 118.855 ± 3.5 and 93.185 ± 3.2 μg EQ/mg, respectively). The highest total flavonoid contents were found in the fraction RA-56 followed by RA-78 and RA-34. These concentrations of total flavonoid contents present in the plant were higher than that present in Torilis leptophylla [15].
DPPH radical-scavenging activity
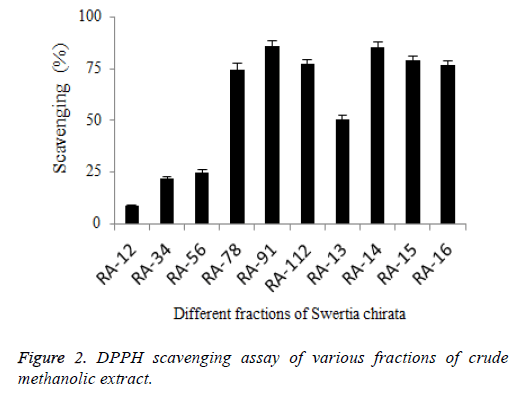
All fractions showed statistically significant (P<0.05) DPPH scavenging capacities (%). DPPH scavenging activity (Figure 2) of five fractions RA-78, RA-91, RA-112, RA-14, and RA-15 had shown significantly (P<0.05) high scavenging potential (74.3 ± 3.4 %, 85.77 ± 2.49 %, 77.17 ± 2.1 %, 85.29 ± 2.61 % and 78.81 ± 2.1 %, respectively). RA-91 fraction showed 10.4% more potential with respect to extract, showing significantly (P<0.05) higher antioxidant potential. As compared to DPPH results of Thymus serpyllum, it was found that methanolic extract of plant showed better antioxidant results then the aqueous and n-hexane plant extract [16].
Antimicrobial assay
All fractions did not show any activity (Table 1) against Salmonella typhimurium. However, RA-112, RA-13, RA-14, RA-15 and RA-16 were active against Micrococcus luteus. The encouraging activities were shown by all fractions against Bordetella bronchiseptica. These results are contrary to the results in which methanolic extract prepared from bark, fruits and leaves of Ficus microcarpa was used and high antibacterial activity was reported [17].
| Sample code | Zone of inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| S. typhi | M. luteus | B. septica | A. niger | Mucor Sp. | A. fumigatus | |
| RA-12 | 0 | 0 | 6.5 | 0 | 14 ± 1.41 | 13 ± 1.41 |
| RA-34 | 0 | 0 | 6 | 0 | 10.5 ± 0.71 | 10 ± 2.83 |
| RA-56 | 0 | 0 | 6 | 0 | 16 ± 1.41 | 11 ± 1.41 |
| RA-78 | 0 | 0 | 6 | 7 ± 0.15 | 13 ± 1.41 | 7 ± 0 |
| RA-91 | 0 | 0 | 6 | 7 ± 0.17 | 14.25 ± 3.18 | 10 ± 0 |
| RA-112 | 0 | 6 | 6 | 7 ± 0.19 | 9 ± 1.41 | 6 ± 0 |
| RA-13 | 0 | 6 | 6 | 7 ± 0.21 | 15 ± 4.24 | 9 ± 1.41 |
| RA-14 | 0 | 6 | 6 | 7 ± 0.21 | 15.5 ± 0 | 21 ± 1.41 |
| RA-15 | 0 | 7 | 6 | 0 | 10 ± 0 | 8 ± 2.83 |
| RA-16 | 0 | 6 | 6 | 0 | 10.5 ± 4.95 | 14.5 ± 0.71 |
| Control (Cefotaxime/terbinafine) | 23 | 21 | 17 | 33 ± 1.52 | 30 ± 1.0 | 30 ± 1.52 |
Table 1. Antibacterial and antifungal activity of crude extract and its fractions.
Five fractions (RA-78, RA-91, RA-112, RA-13, and RA-14) were slight active against Aspergillus niger (Table 1). However, all fractions have shown significantly (P<0.05) results against Mucor species where RA-56 showed highest inhibition followed by RA-91. Comparative fungal growth inhibition cause by all fractions against Aspergillus fumigatus was highest. Fraction RA-14 showed highest growth inhibition in the present antifungal study. Our results are better than those published in another study performed previously [16].
Brine shrimp lethality assay
In brine shrimp lethality assay, LD50 value lower than 1000 μg/ml is considered cytotoxic [18]. Three fractions RA-12, RA-34 and RA-78 showed the best cytotoxic results with zero LD50. The other significant results were obtained by the fraction RA-13 with 16.54 μg/ml LD50 and by fraction RA-112 with 17.37 μg/ml LD50 (Table 2). On correlation of our work with brine shrimp lethality of Artemisia dubia extract, our all fractions showed encouraging cytotoxic activities [8].
| Sample code | Brine shrimp lethality assay (percentage death) | |||
|---|---|---|---|---|
| 1000 µg/ml | 500 µg/ml | 250 µg/ml | LD50 µg/ml | |
| RA-12 | 43 | 35 | 23 | 0 |
| RA-34 | 23 | 20 | 10 | 0 |
| RA-56 | 53 | 33 | 13 | 901.2 |
| RA-78 | 33 | 25 | 15 | 0 |
| RA-91 | 60 | 56 | 53 | 92.91 |
| RA-112 | 96 | 83 | 73 | 17.37 |
| RA-13 | 96 | 86 | 77 | 16.54 |
| RA-14 | 86 | 80 | 75 | 69.5 |
| RA-15 | 100 | 70 | 56 | 144.94 |
| RA-16 | 100 | 73 | 50 | 249.52 |
| Doxorubicin | 100 | 85 | 70 | 1.98 |
Table 2. Results of brine shrimp lethality assay. Sample code Brine shrimp lethality assay (percentage death) 1000 μg/ml 500 μg/ml 250 μg/ml LD50 μg/ml RA
Antileishmanial activity
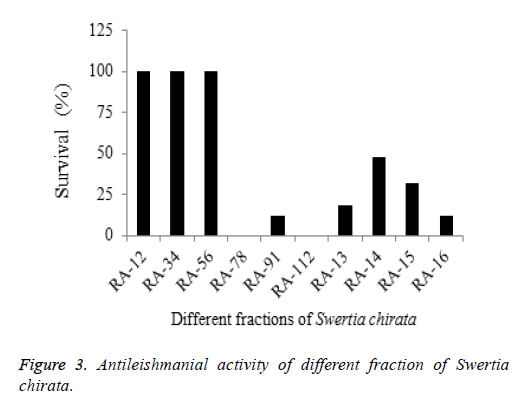
All fractions were also subjected to antileishmanial activities and found positive against leishmaniasis disease (Figure 3). The best results were shown by fraction RA-78 and RA-112 with 0% survival of Leishmania parasite. The second best results were shown by fractions RA-16 and RA-91 by 12% survival rate. Amphotericin-B used as a standard drug against Leishmania protozoa. The results of current study were quite better than the previous work done by in vitro assessment of anticutaneous leishmaniasis activity of Sudanese plants [19,20].
Conclusion
It can be concluded that various fractions of methanolic extract prepared from the aerial parts of Swertia chirata promisingly possess various chemical and biological properties including antioxidant, antimicrobial, brine shrimp cytotoxic, and antileishmanial activities.
References
- Bassam ARH. Medicinal plants (Importance and uses). Pharm Anal Acta 2012; 3: 4172.
- Boon E, Ahenkan A. Impact of deforestation on medicinal plants in Ghana 2008.
- Haq I safety of medicinal plants. Pak J Med Res 2004; 43: 1-8.
- Tabassum S. An overview of medicinal importance of Swertia chiraytia. Int J Appl Sci Technol 2012; 2: 298-304.
- Kacker S, Chirata S, Sanctum O. Rheumatoid arthritis, phenylbutazone, dexamethasone. A study on the activity of Swertia chirata and Scimum Sanctum in animal model of arthritis. J Evol Med Dent Sci 2013: 1311-1317.
- Verma KV, Khomendra KS, Kumar A, Zaman K. Comparison of hepatoprotective activity of Swertia chirayita and andrographis paniculata plant of North-East India against CCl4 induced hepatotoxic rats. J Pharm Res 2013; 7: 647-653.
- Clarke G, Ting K, Wiart C, Fry J. High Correlation of 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all three assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants 2013; 2: 1-10.
- Haq I. Antibacterial activity and Brine shrimp toxicity of Artemisia dubia extract. Paki J Botany 2012; 44: 1487-1490.
- Lin YW. Free radical scavenging activity and antiproliferative potential of Polygnonum cuspidatum root extracts. J Nat Med 2010; 64: 146-152.
- Zaidi MA, Sidney AC. Biologically active traditional medicinal herbs from Balochistan, Pakistan. J Ethnopharmacol 2005; 96: 331-334.
- Manavathu EK. A comparative study of the post-antifungal effect (PAFE) of amphotericin B, triazoles and echinocandins on Aspergillus fumigatus and Candida albicans. J. Antimicrob Chemother 2004; 53: 386-389.
- Ma Y. Activity of dihydroartemisinin against Leishmania donovani both in vitro and vivo. Chin Med J 2004; 117: 1271-1273.
- Huang WY. Comparative analysis of bioactivities of four Polygonum species. Planta Medica 2008; 74: 43-49.
- Djeridane A. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem 2006; 97: 654-660.
- Saeed N, Muhammad RK, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med 2012; 12: 221.
- Rehman A. Biological evaluation of wild Thyme (Thymus serpyllum). Pharm Biol 2009; 47: 628-633.
- Ao C. Evaluation of antioxidant and antibacterial activities of Ficus Microcarpa L. Fil. extract. Food Control 2008; 19: 940-948.
- Krishnaraju AV, Tayi VNR, Dodda S, Mulabagal V. Biological screening of medicinal plants collected from Eastern ghats of India using Artemia salina ( Brine shrimp test ). Int J Appl Sci Eng 2006; 4: 115-125.
- MacLaughin JL, Chnag CJ, Smith DL. Bench-top bioassays for the discovery of bioactive natural product. Atta Ur-Rahman. Elsevier Science Publisher B.V. Amsterdam 1991; 10-103.
- Fatima KA, Nazar AM, Husam M, Abdalla MT, Mubark M, Magzoub, Siddig MA. In vitro Assessment of anti-cutaneous leishmaniasis activity of some Sudanese plants. Acta Parasitol Turc 2005; 29: 3-6.