- Biomedical Research (2015) Volume 26, Issue 3
Antioxidant potency of Iranian newly cultivated wild mushrooms of Agaricus and Pleurotus species.
Sharare Rezaeian1, Sara Saadatmand1*, Taher Nejad Sattari1 and Amin Mirshamsi21Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran
2Department of Biotechnology, Faculty of Agriculture, Ferdowsi University of Mashhad, Mashhad, Iran
- *Corresponding Author:
- Sara Saadatmand
Department of Biology
Science and Research Branch
Islamic Azad University
Tehran, Iran
Accepted date: May 27 2015
Abstract
Although Agaricus bisporus and Pleurotus ostreatus are two major cultivated mushrooms in Iran, a range of different other species of Agaricus and Pleurotus mushrooms grow wild in various habitats of the country. However, limited study has been undertaken to investigate antioxidant potency of the Iranian cultivated or wild mushrooms. This study aimed to evaluate antioxidant capacity of methanol-dichloromethane (1:1) extracts of commercially cultivated and newly cultivated wild species of Agaricus and Pleurotus, using chemical and biochemical techniques. In general, the results revealed that all the tested mushroom demonstrated substantial antioxidant activities against various oxidant systems. Specifically, the newly cultivated wild A. devoniensis proved to be a superior antioxidant among the tested mushrooms, due to having high levels of phenols, efficient scavenging activities on 2,2- diphenyl-1-picrylhydrazyl (DPPH) radicals, relatively high chelating abilities, and an apparent high reducing power. In view of total flavonoids and prevention of lipid peroxidation, however, it was a commercial strain of P. ostreatus that ranked the first. The findings of this research might have implications in preventing oxidative stress-related diseases through the diet; thus they may justify efforts to extract natural antioxidant ingredients from the wild mushrooms tested in this study, or to use them directly in the diet.
Keywords
Antioxidant activity, Iranian wild mushrooms, Agaricus devoniensis, Agaricus gennadii, Pleurotus spp.
Introduction
Imbalance between formation and removal of highly reactive micro-molecules including reactive oxygen species (ROS) and reactive nitrogen species (RNS) may cause oxidative stress and cellular damages [1]. Consequently, these cellular damages may lead to cancer, cardiovascular diseases, diabetes, aging, and a vast number of other illnesses and disorders [2]. In living organisms, however, there are several cellular defence systems against damages caused by ROS or RNS, including antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT), or chemical compounds such as α-tocopherol, ascorbic acid, carotenoids, polyphenol compounds and glutathione [3]. In addition to the endogenous defence system, ingestion of exogenous antioxidants through diet and supplements can help prevent oxidative damage [4,5]. Thus far, the most extensively used antioxidant supplements have been propylgallate, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tertbutylhydroquinone.
However, these compounds are suspected of causing liver damage and carcinogenesis [6,7]. Thus, there has been an increasing interest in research on natural antioxidants, namely vegetables, fruits, and mushrooms.
Mushrooms are well known to possess considerable nutritional values due to their high fiber, minerals and protein content, as well as low fat. In addition, they contain useful bioactive metabolites with a broad spectrum of pharmacological benefits [4], including antioxidant activity. In addition to cultivated mushrooms, different wild mushroom species have been demonstrated to have antioxidant activity, many of which have been previously reviewed by Ferreira et al. [8]. The antioxidants found in mushrooms are mainly phenolic compounds (phenolic acids and flavonoids), followed by tocopherols, ascorbic acid and carotenoids, which could be used as antioxidant ingredients. In addition, mushrooms can be used directly into diet and benefit from the synergistic effects of all the bioactive compounds [8].
Mushrooms have a long history of use as a traditional food and medicine in different parts of the world, especially among ethnic groups [4]. However, there is no a clear record on medicinal values of wild mushrooms in Iran, as opposite to medicinal plants that have long been used in the folk medicine of the country. Even today, there is shortage of the public awareness regarding special medicinal values of mushrooms, although a sharp increase in the consumption of mushrooms can be seen. In a previous study, we utilized internal transcribed spacer (ITS) analysis to authenticate several wild isolates from Agaricus and Pleurotus species collected from northeastern Iran. In this study, thus, several commercial strains and ITS-identified wild mushrooms of two genera Agaricus and Pleurotus were subjected to cultivation, followed by assessment of their antioxidant activity through various chemical and biochemical techniques. In particular, it is the first time in Iran that A. devoniensis and A. gennadii have been cultivated and their antioxidant potencies have been studied during this research. In addition, all the commercially cultivated strains utilized in this study are regularly used by the Iranian mushroom market.
Therefore, the findings of this research might be applicable directly to both the mushroom industry and public health.
Materials and Methods
Samples
Totally eight mushroom samples of two genera Agaricus and Pleurotus were analyzed (Table 1). The commercial strains were obtained from our strain collection. The wild strains have previously been collected and authenticated using ITS analysis. Pure cultures of the mushrooms were prepared using a compost extract agar medium. Then, the cultures were subjected to spawn production and mushroom growing based on procedures adapted in our institute. Wild mushrooms A. devoniensis and A. gennadii were grown in the same spawn and compost as those for A. bisporus; however, these mushrooms required a higher temperature (30° C) to grow properly, as compared to the white button mushrooms.
Chemicals
All the reagents used for this study were freshly prepared before use. Butylated hydroxyanisole (BHA), trichloroacetic acid (TCA), and thiobarbituric acid (TBA), methanol (grade 99.9%), dichloromethane (grade 99.9%), ethylenediaminetetraacetic acid (EDTA), sodium dodecyl sulfate (SDS), hydrochloric acid (grade 37%), acetic acid (grade 99.8%), and 1-butanol (grade 99%), were all purchased from Merck (Darmstadt, Germany). Other reagents included gallic acid (Fluka, Spain), 2,2-diphenyl-1- picrylhydrazyl (DPPH) (Sigma-Aldrich, Germany), folinciocaltue (Panreac, Spain), catechin (Fluka, USA), ferrous chloride (Chem-Lab NV, Belgium), ferrozin (Sigma- Aldrich, Germany), and ascorbic acid (Scharlab S.L. Sentmenat, Spain).
Production of mushroom extract
Fresh fruiting bodies were collected, air dried, and powdered. Each 30 g of the mushroom powder was macerated with 300 ml of dichloromethane-methanol (1:1, v/v) mixture at room temperature at 150 rpm for 48 hours. The residue was also re-extracted with additional 100 ml of each solvent. The broths were filtered, combined and concentrated in a rotating vacuum evaporator (RE 300, Heidolph, Germany) at 40° C, and incubated until dryness. The dry extracts were aliquoted (9 g in 10-cm gamma sterile petri dishes) and stored at dark and 4° C until use. The extracts were re-constituted in 100% methanol at 40 mg/ml.
Analysis of total phenolics
Total phenol content (TPC) was quantified based on a modified method of Mayur et al. [9] in 96-well plates. From a stock solution of gallic acid (1.6 g/ml), 10 μL of various dilutions (3.1 up to 1600 μg/ml) was loaded into each well followed by addition of 100 μL of Folin- Ciocalteau 10% (0.2 N). After five minutes, 80 μL of sodium carbonate solution (7.5%, v/w%) was added and the plate was kept in the dark for 30 minutes. The absorbance was then read at 750 nm by a Nanodrop apparatus (Epoch-Biotek, USA). The absorbance for gallic acid generated a standard curve with the following equation: y = 1.3721x + 0.3535 (R2 = 0.9884). The same procedure was carried out with the mushroom extracts at 10 mg/ml. TPC was calculated using the curve equation and expressed as mg of gallic acid equivalents (GAEs) per g of dry weight (dw).
Flavonoid determination
Flavonoids were determined based on modifications of Barros et al. [3]. Shortly, a standard curve was generated using 10 dilutions of catechin (4.9 up to 2500 μg/ml). Then, 62.5 μL of each dilution was mixed with distilled water (312.5 μL) and NaNO2 (5%, 18.7 μL). After five minutes, an AlCl3.H2O solution (10%, 37.5 μL) was added. After six minutes, NaOH (1 M, 125 μL) and distilled water (68.7 μL) were added. Into each well, 125 μL of the mixture was dispensed and the spectrophotometry was done at 510 nm. A standard curve was plotted based on the absorbance of catechin, with the following equation: y = 9×10-5x + 0.0672 (R2 = 0.9966). The same procedure was carried out with each mushroom extract at 10 mg/ml. Flavonoid content of each mushroom sample was measured using the curve equation and expressed as mg of catechin equivalents (CEs) per g of dw.
Scavenging ability on DPPH radicals
Scavenging ability of the mushroom extracts on DPPH radicals was assessed using a micro-titer plate method established by Arbaayah and Kalsom [10] with modifications. Shortly, 50 μL of the mushroom extracts (Twelve 1.5-fold dilutions, starting from 110 μg/ml) and 50 μL of the DPPH solution (0.5 mg/ml in methanol) were added into each well. While methanol replaced the mushroom extracts to make negative controls, BHA served as a positive control. The plates were left to stand in the dark for 30 minutes with shaking. The radical scavenging activity was measured at 517 nm using the following equation: % RSA = (Ac–As/Ac) ×100; where RSA: radical scavenging activity; Ac: the absorbance of the negative control; As: the absorbance of the mushroom samples. The extract concentration providing 50% of radicals scavenging activity (DPPH-EC50) was measured by interpolation using regression analysis.
Reducing power determination
A 96-well plate-based reducing power method was established, modified from Oyaizu [11]. Various concentrations of mushroom methanolic extracts (3.75, 7.5, 15, and 30 mg/ml) at 500 μL were mixed with 500 μL of sodium phosphate buffer (pH 6.6, 200 mM) and 500 μL of potassium ferricyanide, K3Fe(CN)6, (1% w/v). The mixture was incubated at 50º C for 20 min. TCA (10%, 500 μL) was added following by centrifugation at 2500 g for 10 min. The upper layer (500 μL) was mixed with deionised water followed by the addition of 100 μL of ferric chloride (0.1% w/v). The absorbance was measured spectrophotometrically at 700 nm. The extract concentration providing 50% of absorbance (EC50) was calculated from the graph of absorbance. BHA was used as standard.
Chelating abilities against ferrous ions
Chelating ability assay was adapted from a method of Yeh et al. [6]. To 400 μL of each mushroom dilution (the same as those utilized for the DPPH assay), 50 μL of ferrous chloride (2 mM) was added and shaken vigorously. The reaction was initiated by the addition of 50 μL of ferrozine (5 mM). Then, 100 μL/well of the mixture was dispensed and allowed to stand at room temperature for 10 minutes, after which the absorbance was measured at 562 nm. Methanol served as the negative control, while EDTA (at the same doses as the mushrooms) was taken as the reference standard. The chelating ability percentage was determined using the following equation: % CA = (Ac–As/Ac) ×100; where CA: chelating ability; Ac: the absorbance of the negative control; As: the absorbance of the mushroom samples.
Inhibition of lipid peroxidation
The inhibition effect of the mushrooms on lipid peroxidation was evaluated based on a modified TBARS (Thiobarbituric acid reactive substances) assay [12], using egg yolk homogenate as lipid rich media. Briefly, fresh egg homogenate (10%, in ice-cold 1.15% KCl, w/v) was centrifuged at 2500 g for 15 minutes. Then, 500 μL of the supernatant was mixed with 100 μL of the mushroom samples (at 40 and 20 mg/ml), prepared immediately before use, and made up to 1.0 mL with sterile distilled water. FeSO4 (50 μL, 10 μM) and ascorbic acid (50 μL, 100 μM) were added to induce lipid peroxidation, followed by incubation at 37° C for 30 minutes. The following controls were also taken: BHA (as the reference control) and sterile distilled water (as the fully oxidized control). The reaction was stopped by addition of 50 μL of TCA (20%, pH 3.5), 1.5 ml of TBA (0.8%, w/v, in 1.1% SDS, w/v) and 1.5 ml of acetic acid (20%, w/v, pH 3.5), followed by heating at 100° C for 60 minutes. After cooling, 5.0 ml of 1-butanol was added to each tube to stabilize the colour, followed by centrifugation at 2500g for 15 minutes. The absorbance of the organic upper layer was measured at 532 nm. Inhibition rate was determined through the following formula: Inhibition ratio (%) = (A-Al)/A × 100; where A was the absorbance value of the fully oxidized and A1 was the absorbance value of the sample.
Statistical analysis
All the treatments were applied in triplicate, and each experiment was independently repeated at least three times. GraphPad Prism version 6 was utilized to conduct statistical analyses and ANOVA tests. Means were compared using Tukey method with a significance level (p value) of 0.05.
Results and Discussion
DPPH radical scavenging activity
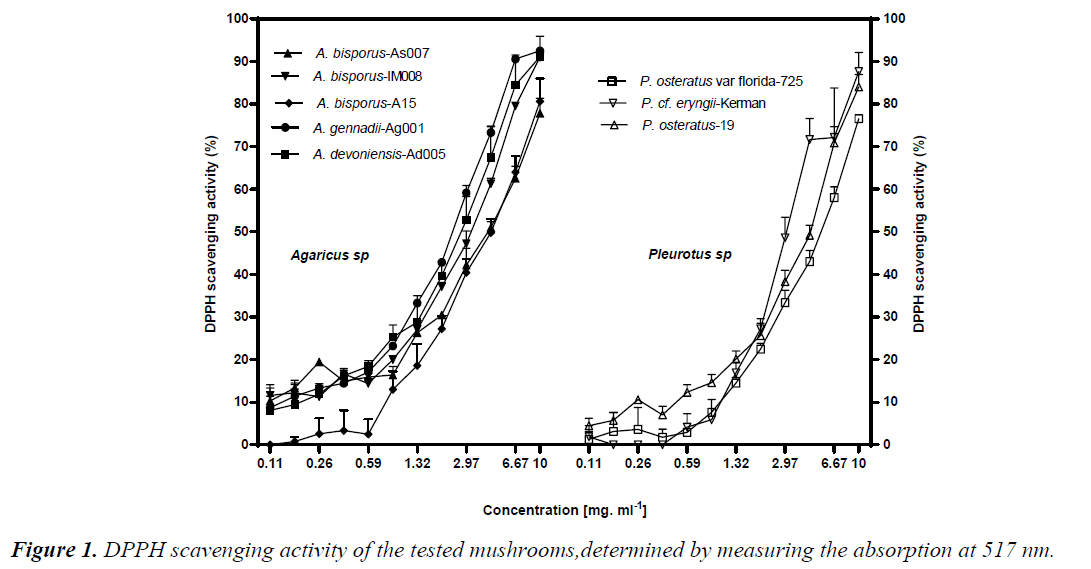
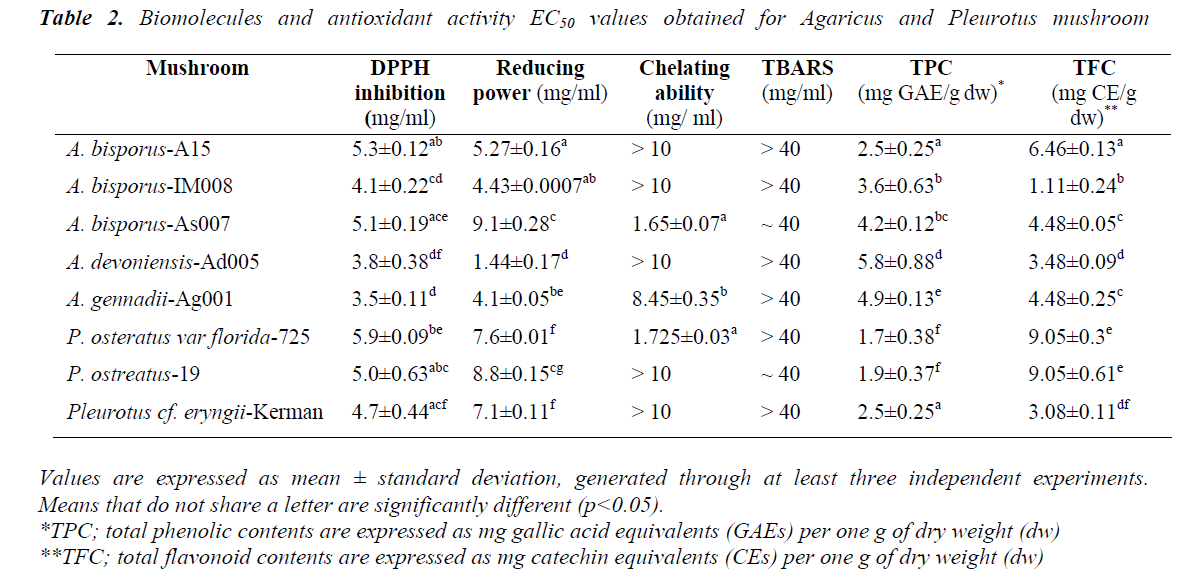
DPPH radical scavenging activity was performed through measuring decrease in DPPH radical absorption after exposure to the mushroom extracts. It is assumed that putative antioxidants present in the mushrooms react with DPPH radicals, resulting in discolouration of DPPH radicals from purple to yellow. Overall, all the mushrooms exhibited levels of DPPH inhibition in a dose-dependent manner. Except A. bisporus- A15, dose-dependent DPPH scavenging active to the Pleurotus ones (p<0.05) (Fig. 1). A.bisporus-IM008, A. devoniensis-Ad005, and A. gennadii-Ag001 showed the most remarkable DPPH scavenging activities (higher than 90%) at 10 mg/ml, as compared to the rest (p<0.05). The maximal DPPH inhibition at 10 mg/ml in Pleurotus mushrooms were 87% and 84% for P. cf. eryngii-Kerman and P. ostreatus-19, respectively (p<0.05). Compatible with these findings, the DPPHEC50 values of the afore-mentioned Agaricus and Pleurotus mushrooms were significantly lower than the rest (p<0.05) (Table 2).
According to our findings, two mushrooms A. devoniensis- Ad005 and A. gennadii-Ag001 could, thus, be considered the most powerful DPPH scavengers amongst the tested mushrooms. To the best of our knowledge, this is the first report on assessment of antioxidant potential of wild A. gennadii and A. devoniensis. Regarding the A. bisporus strains, the DPPH-EC50 values represent higher free radical inhibition than methanolic extracts of Turkish [13] and Portuguese [14] wild A. bisporus mushrooms, where they showed EC50 values of 19.51 and 9.61 mg/ml, respectively. In contrast, another study revealed that a Turkish isolate of A. bisporus showed a considerable DPPH inhibition of 77.5% at only 180 μg/ml [15]. Also, an ethanolic extract of Chinese A. bisporus [16] showed over 90% radical inhibition with an EC50 of only 0.38 mg/ml.
The maximal DPPH inhibition obtained with P. ostreatus-19 at 10 mg/ml (84%) was much higher than the methanolic extract of an Indian P. ostreatus isolate (44%) [17] and higher than ethanolic extract of a Romanian P. ostreatus isolate (78%) [18]. In line with these findings, the DPPHEC50 of P. ostreatus-19 (5 mg/ml) was much lower than that of Turkish [13] and Malaysian [10] isolates of P. ostreatus. In contrast, another Turkish isolate of P. ostreatus showed a considerable DPPH inhibition of 77.5% (at only 180 μg/ml) [15]. In our study, P.cf. eryngii-Kerman showed a great DPPH inhibition (87%, EC50 of 4.7 mg/ml), which might be comparable to a Korean P. eryngii (15%) [19].
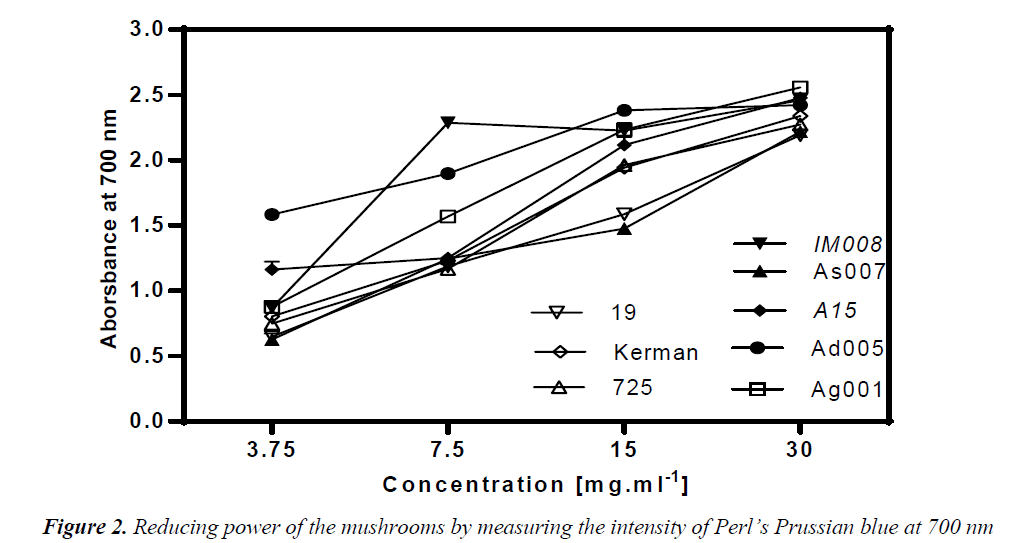
Reducing power
Reducing capacity of the mushroom samples was assessed using their ability to convert Fe3+ to Fe2+. Intensity of Perl’s Prussian blue caused by this reduction was measured at 700 nm; a higher absorbance indicated higher reducing power. Thus, reducing ability of a compound can be contributed to its antioxidant properties. As depicted in Fig. 2, the amounts of reducing power of the mushroom extracts were functions of their concentrations. At 30 mg/ml, all the mushroom ext racts exhibited an outstanding reducing power of over 2. The amount of reducing ability remained over 2 at the concentration 15 mg/ml in the Agaricus spp, except A. bisporus- As007. In particular, A. devoniensis-Ad005 and A. bisporus- A15 exhibited a reducing power of over 1 even at the concentration 3.75 mg/ml. The data showed that Agaricus mushrooms had lower EC50 as compared to Pleurotus strains (p<0.05), except A. bisporus-As007 with an EC50 of 9.1 mg/ml (Table 2). The lowest EC50 (1.44 mg/ml) was found with A. devoniensis-Ad005 that significantly differed from the rest (p<0.01). In addition, Pearson’s correlation test showed that the reducing power-EC50 values considerably correlated with those of the DPPH-EC50 (r= + 0.72).
Similar studies from Portugal reported strains of A. bisporus having had EC50-reducing power values of 3.63 (mg/ml) [3], and 1.80 (mg/ml) [20], which were lower than Agaricus mushrooms (except A. devoniensis) tested in the present study. In addition, other studies from China [16] and France [21] reported substantial reducing power values for an ethanolic extract (EC50 = 0.37 mg/ml) and a methanolic extract (EC50 = 1.26 mg/ml) of A. bisporus, respectively. However, no data was found in the literature as to reducing power of A. devoniensis, which showed a distinguished reducing power among the mushrooms tested in our study.
With regard to oyster mushrooms, the ethanolic extract of a Romanian P. osreatus at 10 mg/ml showed a reducing power similar to our findings [18]. However, P. osreatus strains reported from India [17] and Malaysia [10] at 10 mg/ml had reducing power values of 1 and 0.397, respectively; which were weaker than those of our findings. In contrast, the methanolic extract of a Turkish isolate of P. ostreatus showed significant amounts of reducing power at very low concentrations (600 μg/ml) [15].
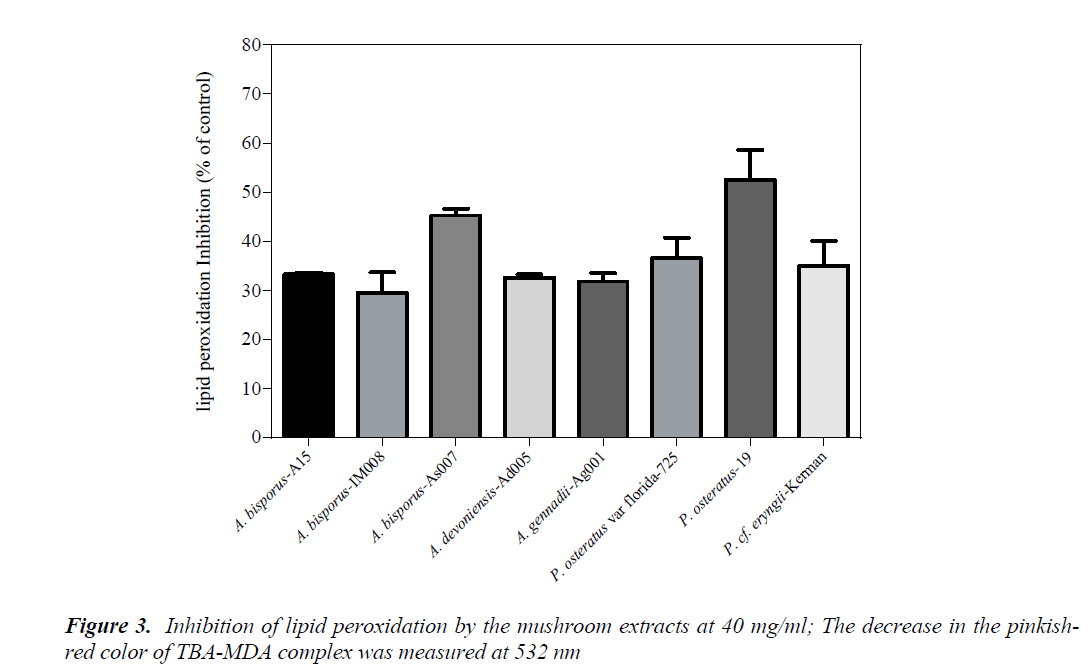
Inhibition of lipid peroxidation
Effect of the mushroom extracts on lipid peroxidation was measured by a modified TBARS method using egg yolk homogenates as lipid rich media. Amounts of decrease in the pinkish-red color developed by TBA-MDA complex were attributed to inhibitory effects of the mushrooms on lipid peroxidation. The results showed that no considerable color change was occurred at concentrations below 40 mg/ml (data not shown). Therefore, the lipid peroxidation assay was performed using the mushroom extracts at 40 mg/ml. Amongst the mushroom extracts, only P. ostreatus-19 exhibited over 50% inhibitory potency, followed by A. bisporus-As007 with about 45% inhibition (Fig. 3) While, there was no significant difference in lipid peroxidation inhibition between P. ostreatus-19 and A. bisporus-As007 (p>0.05), these two mushrooms significantly differed from the rest (p<0.05). Consistent with our findings, the methanolic extract of a Romanian P. ostreatus had significant lipid peroxidation prevention; 70% (at 10 mg/ml) [18].
As opposed to the data obtained with DPPH and reducing power, the tested mushrooms were found to exert moderate or slight inhibition of lipid peroxidation. These findings are in agreement with those of Barros et al. [3], where they reported that the antioxidant activities generated by chemical assays were greater than those of biochemical assays such as lipid peroxidation. Accordingly, TBAR-EC50 for A. bisporus was found to be higher than 50 mg/ml.
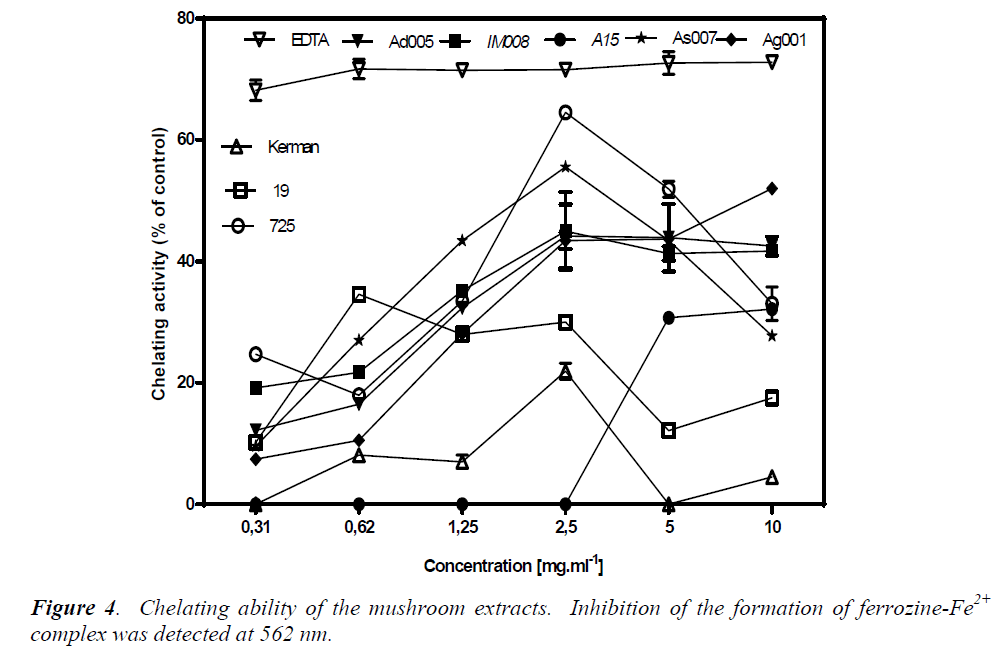
Chelating ability
Chelating effects of the test mushrooms on Fe2+ were determined by the formation of ferrozine-Fe2+ complexes. The ability of an agent to chelate Fe2+ is based on the assumption that it can capture Fe2+ before ferrozine, thus preventing the formation of ferrozine-Fe2+ complex. This reaction can be spectrophotometrically detected through absorbance at 562 nm; less absorbance indicates higher chelating ability. Firstly, the chelating ability of EDTA was assessed as a reference. EDTA at all the tested concentrations (0.31-10 mg/ml) showed a plateau of about 70% chelating ability (Fig. 4). This amount of chelating ability remained stable down to the concentration 0.11 mg/ml, after which it fell to 40% (data not shown).
Generally, the chelating effects of all the mushroom extracts increased as their concentrations increased from 0.31 mg/ml to 2.5 mg/ml. Among the mushroom extracts, A. devoniensis-Ad005 and A. gennadii-Ag001 showed an obvious dose-dependent increase in chelating ability as the concentrations increased up to 10 mg/ml, where they exhibited 42% and 52% chelating activity, respectively. However, the other mushrooms showed fluctuations in the chelating activity at the concentrations above 2.5 mg/ml. At 2.5 mg/ml, P. osteratus var florida-725, A. bisporus-As007, and A. bisporus-IM008 showed notable chelating effects of 64%, 55%, and 45%, respectively, which were significantly different from each other and the rest of mushrooms as well (p<0.05). A. bisporus-A15, P. osteratus-19, and P. cf. eryngii-Kerman did not show considerable chelating activities at any tested concentrations (Fig. 4). Therefore, the order of chelating effects of the mushrooms at 2.5 mg/ml on ferrous ion was P. osteratus var florida-725 (64%)> A. bisporus-As007 (55%)> A. bisporus-IM008 (45%)> A. devoniensis-Ad005 (44%)>A. gennadii-Ag001 (43%)> P. osteratus-19 (30%)> P. cf. eryngii-Kerman (22%)> A. bisporus-A15 (0%).
Other studies showed higher levels of iron chelating for Agaricus and Pleurotus mushrooms as compared to our findings; the methanolic extract of a Turkish A. bisporus [15] with 58% inhibition at only 100 μg/ml, P. osteratus reported from India [17], and Romania [18] with 90% inhibition, and P. osteratus reported from Turkey with 62.5% inhibition (at 10 mg/ml) [15]. The present study revealed that P. osteratus var florida-725, wild A. bisporus- As007, A. devoniensis-Ad005 and A. gennadii- Ag001, could be considered good ferrous chelators. Basically, the metal chelating ability is considered important, as it may lower the level of transition metals that involve in generating the first few radicals to initiate the radical-mediated oxidative chain reactions in both biological and food systems [6]. Therefore, the aforementioned mushrooms may serve as chelating agents to protect against oxidative stress.
Estimation of total phenolic contents (TPC)
Phenolic content of the mushrooms was quantitatively measured using Folin–Ciocalteu assay method adapted in 96-well plate. Thus, TPC of each sample was measured and expressed as mg gallic acid equivalent per one g dry weight (Table 2). Overall, Agaricus sp mushrooms were found to possess higher amounts of TPC in comparison to Pleurotus sp mushrooms (p<0.05), except A. bisporus- A15 that exhibited the same TPC as P. cf. eryngii-Kerman (2.5 mg GAEs/g dw). While A. devoniensis-Ad005 showed the highest amount of TPC with 5.8 mg GAEs/g dw, the lowest amount was seen in P. osteratus var florida- 725 with only 1.7 GAEs/g dw. The TPC values of both A. devoniensis-Ad005 and A. gennadii-Ag001 were significantly greater than the rest of mushrooms (p<0.05); however there was no significant difference between A. devoniensis-Ad005 and A. gennadii-Ag001 (p>0.05).
The maximal TPC observed in wild A. devoniensis is lower than that of wild A. bisporus isolated reported from Turkey (13 GAEs.g-1) [15] and China (6.8 GAEs.g-1) [16]. This amount of TPC, on the other hand, is higher than extracts of wild A. bisporus from another Turkish study (4.02 GAEs.g-1) [13] and from Portugal (4.49 GAEs.g-1) [14], respectively. Total phenols observed in the Pleurotus strains tested in our study were almost twice as high as those of Pleurotus sp reported from India [17]. Malay sian wild and commercial strains of P. ostreatus showed around 6 and 5 GAEs.g-1, respectively [22].
Some authors have expressed TPC as mg GAEs per extract or fresh weight of mushroom. Thus, in addition to dry weight, we also calculated the TPC values to be expressed as mg GAEs per extract (data not shown) in order to make them more comparable with similar reports. Accordingly, TPC values in P. osteratus var florida-725, P. cf. eryngii-Kerman and P. osteratus-19 were 9.5, 14, and 10.5 GAEs.g-1 extract, respectively. These amounts are much less than those reported with P. ostreatus reported from Romania [18] and Malaysia [10].
Phenols are major antioxidant component in mushrooms and their measurement has been well known to be associated to antioxidant activity, including free radical scavenging and chelating ability. Accordingly, a strong negative Pearson’s correlation (r = - 0.80) was observed between DPPH-EC50 values and total phenols. Similarly, total phenols significantly correlated with reducing power-EC50s (r= - 0.69).
Estimation of total flavonoid contents (TFC)
Flavonoid contents were quantitatively measured through an AlCl3 method adapted in 96-well plate, expressed as mg catechin equivalent per one g dry weight. Contrary to the findings obtained with phenolic contents, two Pleurotus strains P. osteratus-19 and P. osteratus var florida- 725 exhibited considerable amount of flavonoids (9.05 CEs/g dw) that was significantly greater (p<0.01) than those of Agaricus mushrooms (Table 2). Significant amounts of flavonoid in Pleurotus mushrooms tested in the present study are in agreement with other studies, displaying a significant amount of flavonoids(18). Despite having a low TPC, A. bisporus-A15 showed a noticeable amount of flavonoids (6.46 CEs/g dw) that was significantly higher than the rest of Agaricus mushrooms. The lowest TFC was seen in A. bisporus-IM008 with just over 1 mg CEs/g dw (Table 2).
The TFC values of A. bisporus were higher than ones reported for cultivated strains of A. bisporus from Portugal [3] and Malaysia [5], while flavonoids reported from methanolic extracts of white and brown button mushrooms in Tuskegee, Alabama (USA) were significantly higher than ours [23].
TFC values in Pleurotus strains tested in present study were much greater than those reported from India [17], and Malaysia [10,22]. Expressed as mg CEs/g extract, TFC values for P. osteratus var florida-725, P. cf. eryngii- Kerman and P. osteratus-19 were 88.78, 134.44, and 48.85, respectively. A P. ostreatus isolate from Romania was shown to possess TFC as much as 212 and 118 (mg quercetin equivalent per g extract) for ethanolic and methanolic extracts, respectively [18].
Conclusion
This is the first report on antioxidant potency of wellauthenticated wild mushrooms in Iran. Our findings revealed that the wild and commercial stains of Pleurotus and Agaricus had considerable in vitro antioxidant activities against various oxidant systems. Specifically, wild A. devoniensis might be regarded as a superior natural antioxidant, as compared to other tested mushrooms, due to having high levels of phenols, efficient DPPH scavenging and chelating abilities, and an outstanding reducing power. To the best of our knowledge, this is the first report on antioxidant activity of this mushroom in the literature. In addition, its cultivation process has been standardized in this study, which may make it more applicable and reliable source of natural antioxidants. However, in light of total flavonoids and prevention of lipid peroxidation, commercial strain of P. ostreatus-19 ranked the first. At the moment, the importance of protein content of mushrooms has been taken into its place in Iran; however, other medicinal properties, including antioxidant activity, of mushrooms is overlooked. Thus, the findings presented here might have implications in the population diet and disease prevention through diet. Fractionation and profiling of main antioxidant components present in these mushrooms would merit further studies.
Acknowledgements
This research was carried out as part of the PhD thesis of S. Rezaeian. The experiments were performed in the laboratory of Industrial Fungi Biotechnology Research Department, Iranian Academic Centre for Education, Research and Culture (ACECR)-Mashhad Branch.
References
- Vijay P, Vimukta S. The role of natural antioxidants in oxidative stress induced diabetes mellitus. Res J Pharm Sci 2014; 3: 1-6.
- Bras MLE, Clement MV, Pervaiz S, Brenner C. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol Histopathol 2005; 20: 205-219.
- Barros L, Falcao S, Bartista P, Freire C, Vilas-Boas M, Ferreira LCFR. Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem 2008; 111: 61-66.
- Alvarez-Parrilla E, Dela Rosa LA, Martinez NR, Gonzalez Aguilar GA. Total phenols and antioxidant activity of mushrooms from Chihuaua, Mexico. Chenciay Tecnologia Alimentaria 2007; 5: 329-334.
- Gan CH, Nurul B, Asmah AR. Antioxidant analysis of different types of edible mushrooms (Agaricus bisporous and Agaricus brasiliensis). Int Food Res J 2013; 20: 1095-1102.
- Yeh JY, Hsieh LH, Wu KT, Tsal CF. Antioxidant properties and antioxidant compounds of various extracts from the edible basidiomycete Grifola frondosa (Maitake). Molecules 2011; 16: 3197-3211.
- Singh P, Singh A, D’Souza L M, Roy U, Singh SM. Chemical constituents and antioxidant activity of the Arctic mushroom Lycoperdon molle Pers. Polar Res 2012; 31: 17329.
- Ferreira LCFR, Barros L, Abreu RMV. Antioxidants in wild mushrooms. Curr Med Chem 2009; 16: 1543-1560.
- Bafna MR, Sanchetis S, Seo SY. Antioxidant and α- glucosidase inhibitory properties of Carpesium abrotanoides L. J Med Plants Res 2010; 4: 1547-1553.
- Arbaayah HH, Umikalsom Y. Antioxidant properties in the oyster mushrooms (Pleurotus spp.) and split gill mushroom (Schizophyllum commune) ethanolic extracts. Mycosphere 2013; 4: 661-673.
- Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet 1986; 44: 307-315.
- Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem 2000; 69: 167-174.
- Keles A, Koca I, Gencelep H. Antioxidant properties ofwild edible mushrooms. J Food Process Technol 2001; 2: 130.
- Barros L, Duenas M, Ferreira IC, Baptista P, Santos- Buelga C. Phenolic acids determination by HPLC-DADESI/ MS in sixteen different Portuguese wild mushrooms species. Food Chem Toxicol 2009; 47: 1076-1079.
- Elmastas M, Isildaka O, Turkekulb I, Temur N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J Food Compos Anal. 207; 20: 337- 345.
- Liu J, Jia L, Kan J, Jin CH. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem Toxicol 2013; 51: 310-316.
- Jeena GS, Punetha H, Prakash O, Chandra M, Kushwaha KPS. Study on in vitro antioxidant potential of some cultivated Pleurotus species (Oyster mushroom). Indian J Nat Prod Resour 2014; 5: 56-61.
- Vamanu E. Antioxidant properties and chemical compositions of various extracts of the edible commercial mushroom, Pleurotus Ostreatus, in Romanian Markets. Revista de Chimie 2013; 64: 49-54.
- Kim MY, Seguin P, Ahn JK, Kim JJ, Chun SC, Kim EH, Seo SH, Kang EY, KIM SL, Park YJ, Ro HM, Chung IM. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J Agric Food Chem 2008; 27: 7265-7270.
- Reis FS, Martins A, Barros L, Ferreira ICFR. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: a comparative study between in vivo and in vitro samples. Food Chem Toxicol 2012; 50: 1201-1207.
- Savoie JM, Minvielle N, Largeteau ML. Radical scavenging properties of extracts from the white button mushroom, Agaricus bisporus. J Sci Food Agric 2008; 88: 970-975.
- Yim H S, Chye FY, Ho SK, Ho CW. Phenolic profiles of selected edible wild mushrooms as affected by extraction solvent, time and temperature. Asian J Food Agro-Ind 2009; 2: 392-401.
- Abugria DA, Mcelhenney WH. Extraction of total phenolic and flavonoids from edible wild and cultivated medicinal mushrooms as affected by different solvents. J Nat Prod Plant Resour 2013; 3: 37-42