Research Article - Journal of Food Technology and Preservation (2022) Volume 6, Issue 8
Antioxidant and antibacterial activity of plants extracts in food industry and safety.
Sami El Khatib1,2*, Nadine Assaf2
1Department of Biological Sciences, Lebanese International University, Beqaa Valley, Lebanon
2Department of Food Sciences, Lebanese International University, Beqaa Valley, Lebanon
- Corresponding Author:
- Sami El Khatib
Department of Biological Sciences
Lebanese International University
Beqaa Valley, Lebanon
E-mail: sami.khatib@liu.edu.lb
Received: 07-Jul-2022, Manuscript No. AAFTP-22-2730; Editor assigned: 08-Jul-2021, PreQC No. AAFTP-22-2730(PQ); Reviewed: 22-Jul-2021, QC No. AAFTP-22-2730; Revised: 05-Aug-2021, Manuscript No. AAFTP-22-2730(R); Published: 12-Aug-2022, DOI:10.35841/aapcgp-6.8.136
Citation: Khatib SE. Antioxidant and antibacterial activity of plants extracts in food industry and safety. J Food Technol Pres. 2022;6(8):136
Abstract
The use of plants for healing dates to prehistory; plant oils and extracts have been utilized for thousands of years, serving for many purposes, such as food preservatives and medical therapeutic agents. The compounds, that are found in some species and produced by herbs, act as self-defense mechanisms to protect the plant against infectious diseases.
Keywords
Natural compounds, Foods, Food-borne illnesses.
Introduction
Nowadays, little natural compounds are being applied to foods, despite of the high incidence of food-borne illnesses [1]. Those diseases, with the microbial spoilage become a rising problem all around the world, since they cause people to get sick and even die, by infection or intoxication, in addition to the major loss in the food production [2]. So it is important to evaluate the antimicrobial as well as the antioxidant potential of plant extracts, and determine their possible incorporation through the food processing [3].
Therefore, it is worthy to know if these extracts can show efficient antimicrobial activity. Can it be compared to that of standard preservatives or antibiotics used? In which parts of plants, do those bioactive compounds tend to accumulate?
How can they be applied to food products mainly with limited antibiotics lifespan, due to the resistance acquirement? In order to do that, extracts of pants are taken using a solvent. Then, they are assessed for their antimicrobial activity by the Minimal Inhibitory Concentration or the agar diffusion methods, while the antioxidant potential is measured by the DPPH and FRAP assays [4].
In the first section, reason that lead to intensive focus on the potential of having natural antimicrobials are discussed, while in the next part, the techniques used to evaluate the extracts and assess their safety, and their stability in different conditions are mentioned. Then, the last section deliberates the outcomes of the numerous studies conducted in this field, including the responsible compounds and their potential applications. It turned out that most tested extracts exhibited antimicrobial activity, which might be specific against different types of microorganisms.
Microbial spoilage
Thousands of years ago, humans know that food should be processed to be preserved since most of the raw materials are perishable not lasting a few days, which requires proper handling of foods during their preparation, storage and distribution [5]. There are many techniques for food preservation, that were developed with the evolution of humans, consisting of cooking, drying, fermentation (acidification), cooling, freezing, food irradiation and the use of chemical preservatives, sometimes with combination of a high concentration of sugar or salt [6].
Despite that, contamination can occur to various food products by microorganisms, which produce enzymes, causing undesirable reactions, what deteriorate flavour, aroma, and colour, sensory and textural properties of foods [5], contributing to the loss of food quality and safety [7].
Foodborne illnesses
Microbial growth is a major concern [5], causing 25% losses of all food grown for human consumption worldwide [8]. Besides, some microorganisms found in foods may cause foodborne illnesses cases. The most spoilage and pathogenic microorganisms are Listeria monocytogenes, Escherichia coli O157, Salmonella, Staphylococcus aureus, Bacillus cereus, Campylobacter, Clostridium perfringens, Aspergillus niger, and Saccharomyces cerevisiae [9].
Definition & symptoms
According to the World Health organization, a foodborne illnesses, also called food poisoning, is the ingestion of food contaminated by microorganisms (most commonly bacteria, viruses and parasites), or harmful chemicals and toxins. These diseases may cause vomiting, diarrhoea, abdominal pain, fever, chills, and others. These symptoms may vary according to the microorganism of interest and the person case. Infants, elderlies, pregnant women and immune-deficient patients are the most people at risk of foodborne diseases (WHO, 2015).
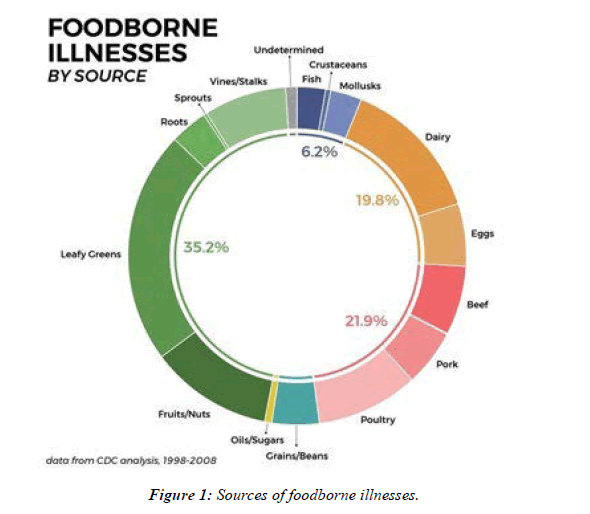
There is a common belief that meats, eggs, chicken and seafood are the ones to blame for causing these diseases. But according to Centers for Disease Control and Prevention (CDC), the Leafy vegetables make up the highest percentage, accounting about 35.2% of many other sources (Figure 1) [10].
Magnitude of food illnesses
Food illnesses are a worldwide growing public health problem; even with the development the food industry sector is achieving [7]. According to the World Health Organization, one out of ten, around the world, becomes ill by foodborne disease each year, and 420000 die as a result. Among them, diarrheal diseases take up more than the half of the global burden of foodborne diseases, causing 550 million people to fall ill and 230000 deaths every year. Almost the third of all deaths occur in children younger than 5 years old, despite that they do not make up more than 9% of the global population [11].
The third highest estimated burden of foodborne diseases per population is occupied by the Eastern Mediterranean Region, after the African and South-East Asia Regions. Every year, more than 100 million people get sick with a foodborne disease and among them 32 million are children under 5 years old (WHO, 2015).
Based on data from Centers for Disease Control and Prevention, the annual foodborne illnesses, in the United States, were estimated to cost up to 77 billion dollar, for medical bills, lost work productivity. Despite this number is high, it does not include yet the costs to the food industry, such as reduced consumer confidence and recall losses [12] (Figure 2).
Use of preservatives
Food additives are substances not usually consumed by them; they do not constitute a particular characteristic of the food. They are usually added to achieve a functional purpose. They can be classified into six categories: preservatives, nutritional, colouring, flavouring, texturizing, and miscellaneous compounds [13].
Preservatives, added to maintain the quality of food products, are divided into two types: antimicrobials and antioxidants agents [13]. Antimicrobials inhibit, delay or prevent the growth and proliferation of bacteria, yeast and mold, and prevent any alterations in the taste, the aroma or the appearance due to microorganism growth [1]. Antioxidants prevent or delay lipid oxidation, which is favoured with the presence of oxygen, light, heat, moisture and transition metals, can induce a rancid flavour and off- odours [13]. Besides some products of lipid oxidation, such as malondialdehyde and cholesterol oxidation products, can be cytotoxic, genotoxic and may promote the occurrence of cardiovascular diseases, atherosclerosis and cancers [14].
Regulation of additives
Before using any of the additives mentioned in the earlier section, they are subjected to safety assessment and authorization procedure. After toxicological study, a maximum level of a permitted material is developed, leading to the determination of the acceptable daily intake, which refers to the amount of the component in the food product that can be ingested on a daily basis over a lifetime without an appreciable health risk (European Food Safety Authority, 2016).
Current preservatives & mode of action
To determine whether a compound can be used as preservative in a food product, it should be effective at low concentrations against the microorganisms of the product, be non-toxic and compatible with other constituents added in its processing, and be stable for the shelf life of the product. Table 1 shows the preservatives approved by the Food and Drug Administration, the type of microorganisms they are effective on and their applications in the food industry. Preservatives are known to interfere with the cell wall of the microorganisms, or inhibit their enzyme systems. Nitrite for instance, when added to meat, converts into nitric oxide, and combines with myoglobin to form nitrosyl- myoglobin, a heat stable pigment. Nitrite curing inhibits growth of microbes such as Clostridium and Steptococcus and lowers the temperature required to kill C. botulinum and thus helps in decreasing the chances of botulism. In bacteria, nitric oxide reacts with ferredoxin enzyme and destroys the cells as they not are able to synthesize ATP and die due to lack of energy [6].
| Compound(s) | Microbial target | Primary food applications |
|---|---|---|
| Acetic acid, Acetates, Diacetates, Dehydroacetic acid | Yeasts, bacteria | Baked goods, condiments, confections, dairy products, fats/oils, meats, sauces. |
| Benzoic acid, Benzoates | Yeasts, Molds | Beverages, fruit products, margarine |
| Dimethyl bicarbonate | Yeasts | Beverages |
| Lactic acid, Lactates | Bacteria | Meats, fermented foods |
| Lactoferrin | Bacteria | Meats |
| Natamycin | Molds | Cheese |
| Nisin | Clostridium botulinum and other bacteria |
Cheese, other products |
| Nitrite, Nitrate | Clostridium botulinum | Cured meats |
| Parabens (alkyl esters propyl, methyl, heptyl) of p-hydroxybenzoic acid) |
Yeasts, Molds, Bacteria (Gram-positive) | Beverages, baked foods, syrups |
| Propionic acid, Propionates |
Molds | Bakery products, dairy products |
| Sorbic acid, Sorbates | Yeasts , molds, bacteria | Most foods, beverages, wines |
| Sulphites | Yeasts, molds | Fruits, fruit products, potato products, wines |
Table 1. Food preservatives approved by the Food and Drug Administration.
Antioxidants, based on their activity, are classified into enzymatic and non-enzymatic antioxidants. Enzymatic antioxidants break down and remove free radicals by donating hydrogen, while the others interrupt free radicals chain of reactions, such as vitamin C and vitamin E [15]. There are natural antioxidants, like ascorbic acid, carotenoids, phenolic compound and tocopherols, used in food [13], as well as synthetic, such as butylated hydroxyl toluene (BHT), butylated hydroxyl anisole (BHA), and propyl gallate (PG) [14].
Side effects
Despite mentioned benefits, there has been a public concern about the adverse effects these preservatives might have. For instance, there was an increase in the risk of the toxic residues in the products treated by benzimidazoles, aromatic hydrocarbons and sterol biosynthesis inhibitors in the postharvest stage. Also, sulphiting agents, which are used in various fruits, may induce asthma identified by shortness of breath, wheezing and coughing, headache and even cancer. Nitrates and nitrites used in meat products may cause stomach cancer [7]. Also, synthetic antioxidants, mentioned earlier, may promote cancer [15]. Therefore, there is a need to reassess their effects or even to invent new alternatives [16].
Antimicrobial resistance
Besides the adverse effects of synthetic preservatives, antimicrobial resistance is one of the most concerning problems the health sector is facing all across the world. It happens when antimicrobial agents would not inhibit the growth of microorganisms anymore. Microbes require this resistance gene due to spontaneous mutations, or due to the natural evolution of microorganisms. The fact that they can transfer these genes to other microorganisms sensitive to that antimicrobial agent makes the problem of antimicrobial resistance more serious. Antibacterial resistance is more likely to occur than antiviral resistance [17]. It caused in 2012, 25000 deaths in the European Union, more than 38000 deaths in Thailand, and more than 23000 deaths in the United States of America [18].
Consumer awareness
Consumers, nowadays, are more aware about health issues, they increasingly demand for chemical free and more natural food products. This was really obvious by the increasing consumption of fresh fruits and vegetables, by more than 30%, during the past few decades in the United States [19]. It is evident that eating fresh crops is essential for good health [20], and it has been proved that the regular intake of these products reduce the rates of heart diseases, cancers, aging, other degenerative diseases [21], and chronic conditions such as cataracts, asthma, and bronchitis. This protection against diseases is attributed to the presence of bioactive compounds with anti-oxidant activity, such as phenolic compounds, carotenoids, and vitamins [20]. Even though, these compounds, when compared to their synthetic counterparts, have a low potency, they apparently provide positive longterm health effects, when regularly being consumed [21]. Nowadays, there is an increasing choice of fresh-cut fruits, a more convenient product, especially when family members are incorporated in the labour market [20].
New alternatives
Research is being pushed towards developing natural food additives that may replace the synthetic ones [3]. This could be related to several reasons including the adverse effects of currently used food additives [7], the antibiotic resistance of food borne pathogens, the increasing regulatory restrictions on food additives [1], the raise of health consciousness among consumers and their increasing demand for the least processed food products or chemical free products [22].
These reasons fostered research on the screening of active compounds from plant origin, whether their extracts, juices, seeds, pastes, powders, peels, essential oils, leaves, stems or roots [22]. Special interest is focused on screening agricultural waste for identifying new compounds and testing their antimicrobial and antioxidant activity [3]. Worldwide, the agro-food industry produces several million tons of plants waste annually, around 25 to 30% of nonedible products such as skins and seeds [20]; their disposal could cause environmental problems such as water pollution, unpleasant odours, vegetation damage and greenhouse gas emission. These wastes could be sources of natural antioxidants, since they have a high concentration of phenolic compounds especially found in peels, skins and seeds. These compounds have been associated to a wide range of physiochemical properties such as anti-allergic, anti- inflammatory, antimicrobial and antioxidants [23].
So the purpose of this seminar is to screen plant components or extracts from different fruits, vegetables, legumes and plants for their chemical composition and to evaluate their antioxidant and antimicrobial activities against specific food borne pathogens or spoilage microorganisms, and their potential uses in the food industry.
Compounds
The bioactive compounds that turned out to have antimicrobial properties belong to the family of phytoestrogens; they include phenols, terpenoids, alkaloids, lectins, polypeptides, polyamines, glucosinolates and glucosides [24]. Polyphenols are the most important group of compounds to be found effective [13]. These compounds can be classified into subtypes flavonoids and phenolic acids.
The most important flavonoids are:
• Quercetins, present in glycosylated forms.
• Catechin and epicatechin and their oligomers.
Some of the most phenolic acids are:
• Caffeic acid, present in esterified form with quinic acid
• P-coumaric acid, present in esterified form with quinic acid (Markowshi, 2006).
This phenolic composition exists in apples, so other compounds, such as tannins, stilbene and lignans, may be characteristic of other plant extracts [14].
Applications
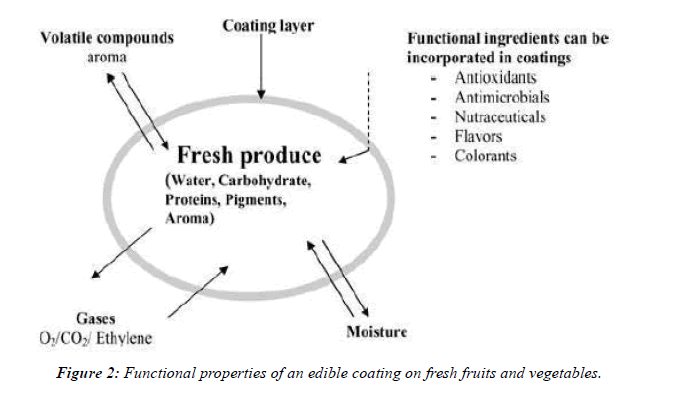
There are different ways of applying the active agents into the fresh-cut of fruits and vegetables, which are dipping, spraying, impregnation and coating. The latter, so far, was studied the most [5]. Fresh cut of fruits and vegetables are so delicate, since they still living tissues, but the fact that they are wounded make them more subjected to microbial spoilage. So the concept of using edible coatings to increase the shelf life is very effective, since it protects them from harmful environmental effects, and minimizes the exchange of moisture and air because of the semi-permeable membrane formed on the surface [25] (Table 2).
| Types of based | Composition | Food Additives | Applications & Results |
|---|---|---|---|
| Polysaccharides | Chitosan, distilled water, Tween 80, palm stearin | Chitosan (Anti-microbial agent) |
Chitosan-stearin edible coating on star fruits (Averrhoa carambola L.) are able to extend the shelf life at low temperature and maintaining their firmness and appearance |
| Cassava starch, copaiba oil, distilled water | Copaiba oil (Anti-microbial agent) | Coating with cassava starch and copaiba oil on organic strawberry at low temperature shows the lower counts of mesophilic and psychotropic microorganism, yeast and mold | |
| Chitosan, glycerol, Tween 80, distilled water | Chitosan (Anti- microbial agent) | Coating based on chitosan- glycerol to delay “Berangan” banana ripening process at ambient air is an effective method. | |
| Polysaccharide & Protein |
Whey protein, soy protein, alginate, carrageenan, glycerol, distilled water | Alginate & sunflower oil (Anti-oxidation agent) | Effect of using different based of edible coating on fresh cut apples to extend their shelf life. The whey protein and soy protein is the most effective properties for coating and additional of sunflower oil helps to improve the fruits quality. |
| Protein | Soy protein, lauric acid, propylene glycol, distilled water | - | Using soy protein based is improving the shelf life and overall quality of minimal process jujubes |
| Gum acacia, garlic, cinnamon | Garlic, cinnamon (Anti-microbial agent & Anti- oxidation agent) | Gum acacia edible coating incorporated with garlic and cinnamon as natural preservative for meat and fish shows garlic and cinnamon can be used as the antimicrobial and antioxidant agent. The shelf life is extended until 3 weeks and the microbial present decrease week wise. |
Table 2. Summary of edible coatings materials.
Therefore, what are the active compounds that can show antimicrobial properties? In which parts of the plants are they concentrated the most or even present? At which microorganisms are they effective the most? Do they offer other health benefits? Are they safe for human consumption? Can fresh-cut fruits be preserved by their own by-products? Can malolactic fermentation in wine making be controlled by phenolic extracts instead of sulphur dioxide (SO2)? Do they offer opportunities to develop value added products?
In the next section, we will be discussing how these compounds can be extracted.
Evaluation Methods
Sample preparation & extraction
Seeds: A study was carried out on the seeds of Millettia ferruginea [26], and Vicia faba, to evaluate their phytochemical constituents, to investigate their nutritional values [21], and their antioxidant and antimicrobial potential. They are dried and grounded with mortar and pestle [26], then kept in closed container to be further assessed as nutritional value. The powder (50 g) of Vicia faba is subjected to methanol (200 mL) extraction, left for 24 hours, and then filtered. Vacuum is used to evaporate the solvent, and the extract is kept at 4°C [21]. The powder (10 g) of Millettia ferruginea is subjected to chloroform, methanol and water extraction, left for 2 hours with 100 mL of each solvent, and then filtered. Rotary evaporator is used to remove the methanol and chloroform solvents, while lyophiliser is used to freeze-dry the aqueous extract [26].
Roots: Roots extracts of Carica papaya L. [27], Thespesia populnea Linn [28] are tested to determine their chemical composition, and to investigate their antimicrobial activity against some infectious bacteria. After washing the fresh roots with tap water and rinsing them with sterile distilled water, they are divided into two portions. Electric blender is used to blend the fresh portion, while hot air oven is used to dry the second for 3 days at 40°C, then they are milled with mortar and pestle, to be kept protected from sunlight. The two portions (20 g of powder) are subjected to cold and hot extraction with water, methanol and acetone (100 mL of solvent), dilution is done with 50% of dimethylsulphoxide for organic extracts and sterile distilled water for aqueous ones [27].
The roots of Thespesia populnea Linn are dried in the shade and pulverised to be water and ethanol extracted. The powder (100 g) is added to 500 mL of distilled water and boiled for 1.5 hours. The mixture is filtered with clean white cloth after being cooled for 6 hours. Rotatory evaporator is used to concentrate the filtrate under vacuum before being stored at 4°C till their next usage. As it concerns the ethanolic extract, the powder (200 g) is mixed with ethanol, left at room temperature for 72 hours and then filtered. Rotatory evaporator is also used to concentrate the filtrate with reduced pressure [28,29].
Agricultural wastes
By-products of food industries are also screened for their antimicrobial and antioxidants potentials, such as olive pomace, peanut skins, pomegranate peels [16], and grape pomace, peanut skins, orange peels. They are first washed, rinsed with distilled water and dried in a hot air oven at 50°C for 8 hours [14], or in an air draft drying oven at 40°C, until no more than 12% moisture content [16], and then milled. The powder (10 g) is mixed with 100 mL of ethanol 70%, methanol 80%, and acetone 80%, left being agitated overnight at room temperature, to be filtered. The residues are re-subjected to extraction. Rotatory evaporator is used to remove the organic solvents below 40°C [14]. The samples are protected from light degradation by being wrapped with aluminium foil while being subjected to extraction [16].
The hulls (100 g) of legumes Vigna radiate (mung bean), Cicer arietinum (chickpea), and Cajanus cajan (pigeon pea), are mixed with 1 L of distilled water, refluxed for 1 hour before being cooled and filtered with a cheesecloth. Centrifugation for 20 minutes is applied to the extract, and lyophilisation is achieved to pulverise the extract [3].
The same procedure applies on legumes Faidherbia albida [30], Red clover (Trifolium pratense) plant [31,32], but may differ in the solvent type, the concentration of powder and solvent, and the drying process. Essential oil is recovered from Crassocephalum rubens leaves, by hydro distilling them for 3 hours, using a Clevenger apparatus, and then dried over anhydrous sodium sulphate [7].
Identification & quantification of compounds
Gas chromatography was used to analyse the essential oil of Crassocephalum rubens with flame ionization detector and a column DB5. It is followed by GC quadruple mass spectrometry in order to identify the compounds [7]. It was also used in the case of faba seeds, with helium as a carrier gas. To identify the detected compounds, it was compared to the libraries already standardized according to the family of compo nd ch a ma pectr m li rary a richa.
Phenolic compounds
Folin-Ciocalteu method is used to determine the concentration of total phenolic compounds, expressed as mg of gallic acid per gram of extract because gallic acid is used as reference. Its basic mechanism is the oxidation of the hydroxyl groups of phenols in alkaline medium, leading to blue colour, measured by spectrophotometer, where the absorption is related to the concentration of phenolic compounds [31].
High Pressure Liquid Chromatography HPLC is used to identify the phenols according to their retention times and to quantify each according to peak area [32,33]. Flavonoids are first identified by the development of red colour after adding a few drops of concentrated hydrochloric acid to the sample [30]. Their content is determined by Aluminium chloride colorimetric method [3], expressed as 1g of Catechin (as standard) equivalents per mg of weight of extracts. The absorbance is measured at 430 nm 10 minutes after mixing 0.5 mL of sample extract with 1.0 ml of 2% methanolic AlCl3.6H2O [14]. In another study, the sample extract is measured at 765 nm against DMSO blank [21].
Proximate analysis
Besides the phenolic compounds and the phytoestrogens molecules of interest, proximate analysis is done for perennial legumes. Kjeldahl method is used to determine the crude protein content with a conversion factor of 6.25, Soxhlet extraction with hexane for crude fat content, acid/alkaline hydrolysis for crude fiber content, and incineration at 550°C for crude ash content. Concentrations of soluble sugars, glucose, starch and minerals such as potassium, sodium, calcium, magnesium, zinc and iron. Flame atomic absorption spectroscopy is used to determine minerals concentration after digestion with nitric acid and hydrogen peroxide, expressed as g of macro-element and mg of micro-element per 100 g on a dry matter basis [34].
Antimicrobial Activity
Tested microorganisms
Plant samples are tested for their antibacterial, antifungal activities [35]. Microorganisms are either isolated from commonly consumed food products that might be considered as potential source of illnesses, such as meat soup and cooked maize flour, or standard strains [7]. In the case of testing extracts in the aim of controlling the malolactic fermentation in wine, Lactic Acid Bacterias (LAB) is isolated from red wines [31]. Clinical isolates of numerous bacteria were used in the case of papaya roots extract, including Streptococcus pneumonia, Streptococcus pyogenase, Salmonella typhi and Shigella flexneri [27]
Tested bacteria are classified between Gram+ and Gram-, pathogenic and spoilage bacteria. Gram+ non spore-forming bacteria include Staphylococcus aureus, Staphylococcus epidermidis, and Enterococcus faecalis. Gram+ spore-forming bacteria include Bacillus cereus and Bacillus subtilis. While the Gram- bacteria tested are Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa and Proteus vulgaris. The fungi tested are Candila albicans [35,37], Cryptococcus neoformans [35], and Aspergillus niger [36,37].
Growth conditions
Strains of microorganisms are inoculated by a loop into the Nutrient broth medium, and then incubated at 37°C on a rotary shaker overnight. Muller-Hinton agar medium is mixed with the culture, and poured into sterilized plates after 45°C temperature is reached. The plates are placed at room temperature under laminar flow to solidify [29].
While in other study, the bacterial strains are cultivated in Trypticase Soy Agar, at 37°C for 18 to 20 hours, and the fungus Candila albicans is cultivated in Sabouraud Dextrose Agar at 25°C for 48 hours. 0.9% sodium chloride solution is used to wash the cultured microorganisms from the agar surface and then 0.5 McFarland standards are used to standardize the suspensions [34].
Minimal inhibitory concentration
The minimal inhibitory concentration (MIC) method consists of determining the lowest concentration of the antimicrobial compound, which would inhibit the visible growth of microorganisms after overnight incubation. Other similar method is the minimal bactericidal concentration (MBC) which is the minimal concentration that would prevent the growth of any microorganism after subculture on an antibiotic free media. The test organism, already diluted to 0,5 McFarland turbidity, is introduced by a loop, into the test tube containing 0,5 mL of varying concentration of extracts with 2 mL of Nutrient Broth. A positive control was used by adding standard antibiotics (ciprofloxacin and streptomycin) to the tube and excluding the extracts. And the negative control consists of adding the organism to the broth only. The tubes are incubated at 37°C for 24 hours, and then examined for microbial growth by observing turbidity [27]. Another study is carried out to measure the effect of the extract on the viable count of two microorganisms Escherichia coli and Staphylococcus aureus. Bacterial suspensions containing of 107 CFU/mL of the two microorganisms each alone, are inoculated to the Muller Hinton Broth (MHB) with the MIC of the essential oil in test, and kept at 37°C. Samples are taken out at 0, 20, 40, 60, 80, 100 and 120 minutes. Buffer peptone is used to dilute 0, 1 mL sample of each treatment, and then spread on MHB. The controls are made by inoculating samples of treatment without the essential oil, with the same procedural conditions. The plates are incubated at 37°C for 24 hours, and they are counted for colonies [7].
While to determine the MBCs, the previous tubes with no visible microbial growth are collected, a loop of broth is inoculated into sterile Nutrient agar by streaking. Controls are carried out by streaking the respective test organism into the agar only, and then they are all incubated at 37°C for 24 hours. The MBC is considered the concentration at which no visible growth was seen [27].
Agar diffusion
The agar diffusion method is commonly used to determine the MIC in solid media. It involves the application of antibiotic solutions of different concentrations to cups [27], wells [35] or paper discs [36], placed on the surface of agar plates seeded with the test bacterial strain. Clear zones are formed when the antimicrobial material diffuses into the agar and inhibits the growth of microorganisms [38]. Bacterial suspensions containing of 107 CFU/mL are inoculated into MHA plates before solidification, and after a sterile borer is used to make wells in the agar plates. About 100 L solutions containing 1 mg of each extract is dispended in the wells. Positive controls are carried out by dispending one of the following standard drugs penicillin G, streptomycin and gentamicin, while negative ones are made by adding nothing to the wells. The antimicrobial activity is measured after 24 hours of incubation at 37°C, and it is expressed as the diameter of the inhibition zone produced around each well [35]. In the paper diffusion method, 5 mm sterilized filter paper disc are impregnated with 25L of the test extract samples, allowed to dry and placed into inoculated plates for incubation. Ciprofloxacin and fluconazole are used as positive controls, while negative ones consist of disc impregnated with 10L of distilled water. The diameter of the inhibition zones is measured in millimetres [29].
Effect of pH and temperature
Only one study considered the effect the temperature and the pH might have on the antimicrobial activity of the natural extracts. The same procedure is applied as previously mentioned in the section II.3.3, after some test tubes were treated at 4°C in the refrigerator, at 60°C and at 100°C in water bath for 30 minutes. As concerning the pH, 1 N HCl and 1 N NaOH is used to make the pH of the test tubes 2.5, 5 and 10 respectively. The antimicrobial activity of the extracts being neutralized with the same compounds was tested after 30 minutes of treatment [27].
Antioxidant activity
The antioxidant activity of the extracts is measured by different methods, almost all of them based on absorbance measurement after a redox reaction. The mainly used include the following.
DPPH
DPPH (2, 2-diphenyl-1-picryl-hydrazyl-hydrate) free radical method consists of an oxidized free radical stable at room temperature with a violet colour. When the antioxidant is added, it reduces the free radical, leading to colourless solution, which is measured by a spectrophotometer at 517 nm. The mixture of 1 mL of sample extract with 500L of DPPH in ethanol is shaken vigorously and left for 20 minutes incubated in the dark. The absorbance is measured [3].
FRAP assay
Ferric Reducing Antioxidant Activity consists of redox reaction with a change in colour. The reduction of a ferric complex (colourless) to ferrous (blue) is examined by measuring absorbance. The mixture of 900 L of freshly prepared FRAP reagent with 100 L of extract solution containing 0.1 mg extracts, is allowed to stand at 37°C for 4 minutes. The absorbance is measured at 593 nm against blank, and as calibration standard BHT is used. The results are expressed as mg of BHT equivalents/g of extract [4].
Evaluation of DNA damage
Iso-flavonoids (barbigerone, calopogonium isoflavone-A and durmillone) isolated from the seeds of Millettia ferruginea, are tested for their DNA fragmentation activity against human Peripheral Blood Mononuclear Cells (PBMC). The blood samples were collected from healthy and non-smoking in-house donors. Comet assay was carried out, dimethyl sulfoxide (DMSO) is used to dissolve these compounds at concentration of 10 mmol/L, this solution is used to dilute the PBMC suspension, so the final concentrations are 1mol/L and 1 mmol/L, and they were incubated for one hour at 37°C. A negative control was made by treating the PBMCs with 10 L of SMSO taken in 1 mL of nuclease-free deionized water, and what served as positive control is adding H2O2 to the cells. All samples were subjected to other procedures before the percentage of DNA is measured [35].
Statistical analysis
All experiments, mentioned earlier, were carried out in triplicate, and the average values were reported. ANOVA, the one way analysis of variance, is used to test the significant difference of all the data recorded in the study, considering P values <0, 05 as significant [30]. It is followed by D ncan‟ te t to test for simple main differences among treatments [34].
Sensory evaluation
When some of the extracts are added to a food product, sensory evaluation is needed to evaluate the organoleptic characteristics of the new formulated product. For instance, the biscuits fortified with 12% faba seeds flour using wheat as the main flour, are baked according to the normal baking procedures and then stored in airtight containers. The sensory characteristics are evaluated by a 9 point hedonic scale, rating their features such as colour, texture and crunchiness. The nutritional quality is evaluated by analysing the moisture, ash, protein, crude fat, and crude fiber contents [21].
Beef burgers formulated with extracts from grape pomace, olive pomace and peanut skins are evaluated for their sensory characteristics too. The control burgers contain 60% meat, 7.1% fat, 5% water, 12% rehydrated texturized soy, 5.5% fresh egg, 5% fresh onion, 1.4% ground bread crust, 1.5% salt and 1.5% spices. While others are formulated with 200 ppm of the synthetic antioxidant butylated hydroxyl toluene (BHT), while the natural extracts are added at concentrations between 400 and 800 ppm. They are all aerobically packed and stored at -18°C for three months. Twenty panellists evaluate the cooked burgers at zero time and each month of storage, for their colour, appearance, taste, tenderness, juiciness and overall acceptability [14].
Effect of Natural Compounds
Chemical composition of extracts
The extracts, tested with Gas Chromatography or High Pressure Liquid Chromatography for the identification of compounds, showed the presence of carbohydrates, glycosides, steroids, tannins, polyphenols, alkaloids, triterpenoids, flavonoids, anthocyanins and proteins [36]. Their presence is dependent on the type of extract, aqueous, methanolic, ethanoic or any other organic solvent used in the extraction, as shown in table 3 [36].
| Sample | Methanol extract | Aqueous extract |
|---|---|---|
| Test for terpenes | + + + | + + |
| Test for flavonoids | - | - |
| Test for saponins | - | + + + |
| Test for steroids | + + + | - |
| Test for cardiac glycosides | + | + |
| Test for proteins | + | - |
| Test for carbohydrates• Monosaccharide • Reducing sugars • Carbohydrates | ||
| + + + | + + | |
| - | - | |
| + | + | |
| Test for tannins and phenolic compounds | - | + |
| Test for alkaloids | - | + |
+ + +, High concentration; + +, moderate concentration; +, low concentration; -, absence
Table 3. Results of phytochemical screening of methanol and aqueous extracts.
It is interesting to mention that the isoflavonoids concentration increased 7 fold when the tissues were collected 24 hours after cutting, as shown in table 4 [32]. For the first time, they compared the effect of three isoflavonoids, namely barbigerone, calopogonium isoflavone-A and durmillone each one by itself and not as total phenols fraction, on the antimicrobial activity. They were used at different concentrations after been isolated from the mature seeds of Millettia ferruginea, and the positive control used was the antibiotic streptomycin, [35].
| Sample | Formononetin, 1mol/gdw | Biochanin A, 1mol/gdw |
|---|---|---|
| Plot 1, 0 h | 1.2 ± 0.22 | 0.51 ± 0.1 |
| Plot 1, 24 h | 7.8 ± 0.5 | 5.1 ± 0.15 |
| Plot 2, 0 h | 1.3 ± 0.012 | 0.92 ± 0.028 |
| Plot 2, 24 h | 9.7 ± 1.12 | 6 ± 0.57 |
Table 4. Formononetin and Biochanin A (mmol/ g dry weight) in extracts of red clover harvested zero and 24 hours after cutting plants.
Abundance of bioactive compounds
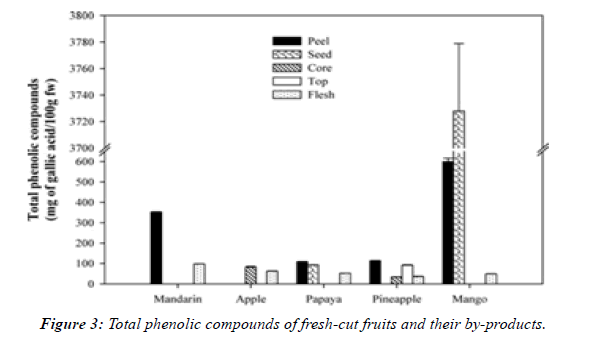
Non-edible part of fruits and vegetables accounts for 25 to 30%, and the production of fresh-cut fruits to be packed, produces even more by-products, such as in diced papayas where 47.1% is lost, in pineapple where 48.08% is recovered from the whole fruit, and in mangos where 42.44% is lost. It has been proposed that waste materials may contain considerable amounts of useful compounds. A study was carried out to test that proposal by comparing the concentration of phenolic compounds and flavonoids in different parts of each of mandarin, apple, papaya, pineapple and mango (Figure 3). It was reported that peel and seed contain high amounts of phenolic compounds with antioxidant and antimicrobial activities, being more pronounced in mango peels and seeds [20].
Other studies conducted in this extend showed that the peels of lemons, oranges and grapefruits were 15% higher than that of the pulp of these fruits, peels from apples, peaches, pears, yellow and white flesh nectarines contain twice the amount of total phenolic compounds as that contained in the fruit pulp. The edible pulp of banana is found to contain phenolic compounds about 25% of that present in the peel, and this percentage is reduced to 9.78% when comparing the pomegranate pulp and peels. It is also in the case of tomato pulp versus tomato seeds and peels with significantly higher levels of total phenolic compounds, total flavonoids, lycopene, ascorbic acid and antioxidant activity [20].
Antimicrobial activity
First, an extract with MIC values less than 100 mg/mL is classed as strong inhibitor, at 100-500 mg/mL as moderate inhibitor, at 500-1000 mg/mL as weak inhibitor and at more than 1000 mg/mL as inactive inhibitor [29]. Most tested extracts exhibited antimicrobial activity varying in its intensity according to the plant extracted from, and higher sensitivity for Gram positive than Gram negative bacteria [35]. This was explained by the fact that the outer membrane in Gram- bacteria acts as permeability barrier [29]. The results were comparable to antibiotics [35]. However, Gram- showed higher susceptibility to other extracts, such as the ones of roots of papaya. This result disagrees with the earlier report indicating that plant extracts are more active against Gramnegative bacteria [27]. Other extracts such as of perennial legumes showed potency to inhibit both Gram+ and Grambacteria, among them multi-resistant microorganisms, but had no effect on the growth of yeast Candila albicans. This inefficacy of the extracts can be explained by the complex yeast cell wall, preventing the extract from contacting the cell structures [34].
As for the source of the used strain, it had no significant implication on the MIC values. While the MIC determined for Escherichia coli ATCC (2.18 mg/mL) is the double of the isolated one from meat soup (1.09 mg/mL) (Table 5), it is the opposite in the case of Staphylococcus aureus isolated from cooked maize flour compared to the homologue strain [7].
| Germs tested | MIC (mg/mL) |
|---|---|
| Escherichia coli | 1.09 |
| Escherichia coli ATCC | 2.18 |
| Staphylococcus aureus | 1.09 |
| Staphylococcus aureus ATCC | 0.54 |
| Salmonella typhi | 2.19 |
| Streptococcus faecalis β-hemolysante | 1.05 |
| Candida albicans | 4.38 |
Table 5. MIC values for essential oil of leaves of Crassocephalum rubens.
The type of extract, related to the used solvent, also affects the antimicrobial activity. For instance, in the case of the roots of tested papaya, the methanol extract was the most effective, followed by the acetone extract; the hot water extract and the cold water extract (Table 6). The latter one did not show any activity against the test organism. This was due to the chemical composition of each extract; while the methanol extract contain saponins, alkaloids, tannins and phenols, the hot water extract contain saponins and glycosides, and the cold extract contain only glycosides. This composition is related to the better solubility of the active components in organic solvents. The latter one did not show any activity against the test organism [27].
| Organism | MIC (mg/ml) | MBC mg/ml) | ||||
|---|---|---|---|---|---|---|
| WE | AE | ME | WE | AE | ME | |
| S. aureus | +++ | 100 | 100 | +++ | 200 | 200 |
| S. pyogenase | +++ | 200 | 100 | +++ | +++ | +++ |
| S. pneumoniae | +++ | 150 | 100 | +++ | 200 | 200 |
| B. cereus | +++ | 150 | 200 | +++ | 200 | 200 |
| E. coli | +++ | 150 | 100 | +++ | 150 | 150 |
| P. aeruginosa | +++ | 150 | 100 | +++ | 150 | 200 |
| P. mirabilis | +++ | 150 | 100 | +++ | 200 | 200 |
| S. typhi | +++ | 50 | 50 | +++ | 200 | 200 |
| S. flexneri | +++ | 100 | 100 | +++ | 200 | 200 |
+++ = profuse growth; WE = water extract; AE = acetone extract; ME = methanol extract
Table 6. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of extracts of C. papaya.
Mode of Action
The antibacterial activity of the extracts can be explained in different ways. The bioactive compounds are considered able to disturb the cell membrane, disrupt the proton motive force electron flow, alter the active transport and coagulate the cell contents. In the case of tested essential oil, the lipophilic compounds could accumulate in the lipid bilayer and distort the lipid-protein interaction [7].
Effect of pH and temperature
The effect of temperature and pH was studied in the case of C. papaya roots extract. Concerning the temperature, the activity of extracts increased with an increase in temperature, as shown in table 7. This indicates that the bioactive compounds are heat stable, and can withstand hard processing stages [27].
| Test organism | Temperature (°C)/ Zone of inhibition (nm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NT (30ºC) WE | 4ºC | 60ºC | 100ºC | |||||||||
| AE ME | WE AE ME | WE AE ME | WE AE ME | |||||||||
| S. aureus | 0 | 8 | 8 | 0 | 8 | 8 | 0 | 10 | 12 | 0 | 10 | 12 |
| S. pyogenase | 0 | 10 | 10 | 0 | 10 | 10 | 0 | 12 | 14 | 0 | 12 | 14 |
| S. pneumoniae | 0 | 8 | 10 | 0 | 10 | 10 | 0 | 10 | 14 | 0 | 10 | 14 |
| B. cereus | 0 | 6 | 8 | 0 | 6 | 8 | 0 | 8 | 10 | 0 | 8 | 12 |
| E. coli | 0 | 6 | 8 | 0 | 6 | 8 | 0 | 8 | 10 | 0 | 10 | 12 |
| P. aeruginosa | 0 | 8 | 14 | 0 | 10 | 14 | 0 | 10 | 16 | 0 | 10 | 16 |
| P. mirabilis | 0 | 10 | 12 | 0 | 10 | 12 | 0 | 12 | 14 | 0 | 12 | 14 |
| S. typhi | 0 | 4 | 14 | 0 | 4 | 14 | 0 | 6 | 18 | 0 | 6 | 18 |
| S. flexneri | 0 | 6 | 8 | 0 | 6 | 8 | 0 | 8 | 10 | 0 | 8 | 10 |
NT = non-treated extract; WE = water extract; AE = acetone extract; ME = methanol extract
Table 7. Effect of temperature on the antibacterial activity of root extracts of C. papaya on the test organisms.
As for the pH, the activity of the extracts decreased at alkaline conditions (Table 8). This acid stability indicates that the extracts when refined can be favorable for oral administration, since the stomach contains acidic secretions. These plant extracts used have the potential to be used in the production of novel drugs for the treatment of gastroenteritis, urethritis, otitis media, and typhoid fever and wound infections, since they showed antibacterial activities against the bacteria causing these diseases [27].
| Test organism | pH/ Zone of inhibition (nm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NT (4.3) | 2.5 | 5 | 10 | |||||||||
| WE AE ME | WE AE ME | WE AE ME | WE AE ME | |||||||||
| S. aureus | 0 | 8 | 8 | 0 | 10 | 10 | 0 | 10 | 14 | 0 | 4 | 6 |
| S. pyogenase | 0 | 10 | 10 | 0 | 10 | 12 | 0 | 12 | 14 | 0 | 4 | 8 |
| S. pneumoniae | 0 | 8 | 10 | 0 | 10 | 12 | 0 | 10 | 14 | 0 | 4 | 6 |
| B. cereus | 0 | 6 | 8 | 0 | 6 | 8 | 0 | 8 | 12 | 0 | 4 | 6 |
| E. coli | 0 | 6 | 8 | 0 | 6 | 10 | 0 | 8 | 10 | 0 | 4 | 6 |
| P. aeruginosa | 0 | 8 | 14 | 0 | 10 | 14 | 0 | 10 | 16 | 0 | 4 | 8 |
| P. mirabilis | 0 | 10 | 12 | 0 | 10 | 12 | 0 | 12 | 14 | 0 | 4 | 8 |
| S. typhi | 0 | 4 | 14 | 0 | 6 | 14 | 0 | 6 | 14 | 0 | 4 | 8 |
| S. flexneri | 0 | 6 | 8 | 0 | 8 | 10 | 0 | 8 | 10 | 0 | 4 | 6 |
NT = non-treated extract; WE = water extract; AE = acetone extract; ME = methanol extract
Table 8. Effect of pH on the antimicrobial activity of root extracts of C. papaya on the test organisms.
Antioxidant activity
Phenolic compounds in plants can donate oxygen or electrons and form stable radical intermediate that ‟why they are considered to e power l in vitro antioxidant and flavonoids to have the highest antioxidant potential. In one study, they tested the antioxidant activity of aqueous hull (seed coat which has the highest concentration of phenolic compounds) extracts of these legumes mung bean, chickpea and pigeon pea, consumed in India. As shown in figure 4, there was a gradual increasing antioxidant activity with increasing concentration of extracts. The pigeon pea and the chickpea exhibit
References
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(suppl 1):5-16.
- Ayala‐Zavala JF, Rosas‐Domínguez C, Vega‐Vega V. Antioxidant enrichment and antimicrobial protection of fresh‐cut fruits using their own byproducts: Looking for integral exploitation. J Food Sci. 2010;75(8):R175-81.
- Barth M, Hankinson TR, Zhuang H. Microbiological spoilage of fruits and vegetables. InCompendium of the microbiological spoilage of foods and beverages. 2009:135-183.
- Bonev B, Hooper J, Parisot J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J Antimicrob Chemother. 2008;61(6):1295-1301.
- Butkutė B, Padarauskas A, Cesevicienė J. Perennial legumes as a source of ingredients for healthy food: proximate, mineral and phytoestrogen composition and antibacterial activity. J Food Sci Technol. 2017;54(9):2661-9.
- Chodosh S. Don't worry about eggs-these other foods are way more likely to give you Salmonella. Popular Science2017.
- Choudhury M, Shiferaw Y, Sur K. Antimicrobial activity and DNA-fragmentation effect of isoflavonoids isolated from seeds of Millettia ferruginea, an endemic legume tree in Ethiopia. J Coast Life Med. 2016;4(7);556-563.
- Doughari JH, Elmahmood AM, Manzara S. Studies on the antibacterial activity of root extracts of Carica papaya L. Afr J Microbiol Res. 2007;1(3):37-41.
- El-Shourbagy G, El-Zahar K. Oxidative stability of ghee as affected by natural antioxidants extracted from food processing wastes. Ann Agric Sci. 2014;59(2):213-220.
- European Food Safety Authority. Call for food additives usage level and/or concentration data in food and beverages intended for human consumption. EFSA J. 2010;8(1):1457.
- Flythe M, Kagan I. Antimicrobial effect of Red Clover (Trifolium pratense) phenolic extract on the ruminal hyper ammonia-producing bacterium, Clostridium sticklandii. Curr Microbiol. 2010;61(2):125-31.
- Garcia E, Oldoni T, Alencar S. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz Dent J. 2012;23(1):22-27.
- García-Ruiz A, Cueva C, González-Rompinelli E. Antimicrobial phenolic extracts able to inhibit lactic acid bacteria growth and wine malolactic fermentation. Food Control.2012;28(2):212-219.
- Garg N, Kumar P. Food antimicrobials: challenges and prospects. 2015.
- Havelaar A, Zwietering M. The global burden of foodborne disease; overview and implications. Int Ass Food Prot. 2016.
- Hintz T, Matthews K, Di R. The use of plant antimicrobial compounds for food preservation.
Biomed Res Int. 2015;2015:246264. - Ibrahium M, Osheba A, Nageib A. Using of some natural antioxidants extracted from food processing wastes for improvement quality attributes of beef burger products. Middle East J Appl Sci.2015;5(4):929-939.
- Ismail M, Mohamed E, Marghany M. Preliminary phytochemical screening, plant growth inhibition and antimicrobial activity studies of Faidherbia albida legume extracts. J Saudi Soc Agric Sci. 2014;15(4):112-117.
- Kanatt SKA, Sharma A. Antioxidant and antimicrobial activity of legume hulls. Food Res Int. 44(2011);3182–3187.
- Lin D, Zhao Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr Rev Food Sci Food Saf. 2007;6(3):60-75.
- Lucera A, Costa C, Conte A. Food applications of natural antimicrobial compounds. Front Microbiol. 2012;8(3):287.
- Mane BG, Khurana SK, Choudhary S. Effect of natural antimicrobials on foodborne pathogens and shelf life: a review. J Biosci Bioeng. 2014;1:22-31.
- Markowski J, Płocharski W. Determination of phenolic compounds in apples and processed apple products. J Fruit Ornam Plant Res. 2006;14(2):133-141.
- Nimse S, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. R Soc Chem. 2015;5(35):27986-28006.
- Pasricha V, Satpathy G, Gupta R. Phytochemical & Antioxidant activity of underutilized legume Vicia faba seeds and formulation of its fortified biscuits. J Pharmacogn Phytochem. 2014;3(2):75-80.
- Petruzzi L, Corbo M, Sinigaglia M. The microbial quality of food. Wood head Publishing Series in Food Science, Technology and Nutrition. 2017:1-21.
- Preethi R, Devanathan V, Loganathan M. Antimicrobial and antioxidant efficacy of some medicinal plants against food borne pathogens. Adv Biol Res 2010;4(2):122-125.
- Santos-Sanchez NF, Salas-Coronado R, Valadez-Blanco R. Natural antioxidant extracts as food preservatives. Acta Sci Pol Technol Aliment. 2017;16(4):1-10.
- Scharff, RL. Economic burden from health losses due to foodborne illness in the United States. J Food Prot. 2012;75(1):123-31.
- Senthil-Rajan D, Rajkumar M, Srinivasan R. Investigation on antimicrobial activity of root extracts of Thespesia populnea Linn. Trop Biomed. 2013;30(4):570–578.
- Sharif ZIM, Mustapha FA, Jai J. Review on methods for preservation and natural preservatives for extending the food longevity. Chem Eng Res Bull. 2017;19:145-153.
- Utt E, Wells C. The global response to the threat of antimicrobial resistance and the important role of vaccines. Pharm Policy Law. 2016;18(1-4):179-197.
- Yasmeen F, Rauf T, Babar M. Cedrus deodara (Deodar) and Zanthoxylum armatum (Timur) evaluated as antimicrobial and antioxidant agents. Int J Pharm Pharm Sci. 2015;2(2):110-118.
- Udakis L. Antimicrobial resistance. MicroSoc. 2011.
- Voon HC, Bhat R, Rusul G. Flower Extracts and Their Essential Oils as Potential Antimicrobial Agents for Food Uses and Pharmaceutical Applications. Compr Rev Food Sci Food Saf. 2012;11(1):34-55.
- World Health Organisation. Food safety. 2017.
- Yehouenou B, Wotto V, Bankole H. Chemical study and antimicrobial activities of volatile extracts from fresh leaves of crassocephalum rubens (juss & jack) s. Moore against food-borne pathogens. Scientific Study & Research: Chemistry & Chemical Engineering, Biotechnology, Food Industry. 2010;11(3):341-9.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref