Research Article - Biomedical Research (2017) Volume 28, Issue 3
Anti-osteosarcoma effect of novel pyran-annulated heterocycles derivatives
Qing Bi1#, Wei-Wei Ruan2#, Li Cao3 and You-Jia Xu4*1Department of Orthopedic Surgery, Soochow University, Soochow, Jiangsu, China
2Department of Orthopedic Surgery, Wenzhou Medical University, Wenzhou, Zhejiang, China
3Department of Orthopaedics, Zhejiang Provincial People's Hospital, Hangzhou, Zhejiang, China
4Department of Orthopaedics, the Second Affiliated Hospital of Soochow University, Soochow, Jiangsu, China
#These authors contributed equally to this paper
- *Corresponding Author:
- You-Jia Xu
Department of Orthopaedics
The Second Affiliated Hospital of Soochow University
Jiangsu, China
Accepted date: August 05, 2016
Abstract
The synthesis and characterization of four novel pyran derivatives (1-4) were realized by means of IR, 1H NMR, HRMS, and single crystal X-ray crystallography. The anti-osteosarcoma activities of the four compounds were investigated against three human osteoma sarcomatosum cells (HuO9, 143B and MG-63) by MTT assay. It has been found that compounds 3 and 4 exerted much more potent activities against the three cell lines than the other two compounds.
Keywords
Pyran, Crystal, Anti-osteosarcom.
Introduction
According to the statistics, nearly 7 million lives die from different kinds of cancer every year in the world, the high fatality rate of cancer makes people turn pale at the mention of it [1]. Moreover, the evidence showed that the treatment of metastatic cancer cannot achieve the desired effect only by surgery or radiation, or even combining surgery with radiation. As a result, much more attention has been paid on conventional chemotherapy to cure cancer [2], while this type of treatment could cause severe side effects for it usually fails to differentiate between normal cells and tumor cells. In the last decade, the adoption of molecular targeted therapies plays an important role in blocking the growth of tumor cell by interfering with specific receptors and signaling pathways, which is a new generation of selective cancer drugs that has made treatments more tumor-specific [3,4].
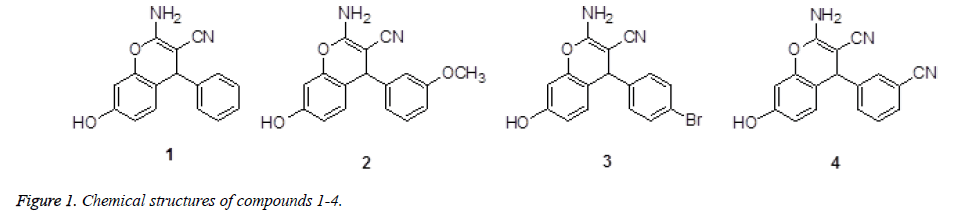
Several derivatives of the pyran or of fused pyran ring systems are endowed with different types of biological activities. It has been reported that pyran derivatives exhibit antimicrobial activity, growth stimulating effects, antifungal and plant growth regulation effects, antitumor activity, Central Nervous System (CNS) activity and hypotensive effect [5,6]. Moreover pyran derivatives are well known for antihistaminic activity, platelet antiaggregating activity and local anaesthetic activity, antiallergenic effect, antidepressant effect and as antiproliferation agents [7]. As mentioned above, we are here to report the synthesis of four new dihydropyran derivatives (Figure 1) with their anticancer activity.
Experimental
Apparatus and materials
A Brucker Equinox-55 spectrophotometer IR was used to capture IR spectra (400-4000 cm-1). 1H NMR spectra and Mass spectra were captured by using a Varian Inova-400 spectrometer (at 400 MHz) and a micrOTOF-Q II mass spectrometer respectively. A XT-4 micro melting apparatus was made use of measuring the melting points, and the thermometer remained uncorrected.
Synthesis and characterization of compounds 1-4
The synthesis of compounds 1-4 were on the basis of a reported procedure [8]. Refluxing the mixture of mdihydroxybenzene (10 mmol), benzaldehyde (3- methoxybenzaldehyde, 4-bromobenzaldehyde and 3- cyanobenzaldehyde) (10 mmol), malononitrile (10 mmol) and 4-(dimethylamino)pyridine (DMAP) (1 mmol) in ethanol (100 mL) for 2-3 h and then cooling to room temperature. Through filtering the precipitates, they were sequentially washed with ice-cooled water and ethanol and then dried under a vacuum.
2-Amino-7-hydroxy-4-phenyl-4H-chromene-3-carbonitrile (1): Yield: 65%. 189-190°C. IR (KBr pellet cm-1): 3640, 2038, 1816, 1110, 1032, 954, 906 cm-1. 1H NMR (DMSO-d6, δ, ppm): 9.724 (s, 1H), 7.288-7.325 (t, 2H), 7.157-7.222 (q, 3H), 6.900 (s, 2H), 6.793-6.814 (d, 1H), 6.470-6.494 (q, 1H), 6.408 (s, 1H), 4.619 (s, 1H). HRMS (ESI+): m/z: calcd for C16H12N2O2: 287.0791 [M+Na+]; found: 287.0766.
2-Amino-7-hydroxy-4-(3-methoxy-phenyl)-4H-chromene-3- carbonitrile (2): Yield: 60%. 178-179°C. IR (KBr pellet cm-1): 3436, 2330, 1672, 1523, 1310, 1071, 761 cm-1. 1H NMR (DMSO-d6, δ, ppm): 9.717 (s, 1H), 7.204-7.243 (t, 1H), 6.884 (s, 2H), 6.827-6.848 (d, 1H), 6.771-6.794 (t, 1H), 6.715-6.735 (d, 2H), 6.475-6.502 (q, 1H), 6.402-6.407 (d, 1H), 4.509 (s, 1H), 3.721 (s, 3H). HRMS (ESI+): m/z: calcd for C17H14N2O3: 317.0897 [M+Na+]; found: 317.0877.
2-Amino-4-(4-bromo-phenyl)-7-hydroxy-4H-chromene-3- carbonitrile (3): Yield: 55%. 169-170°C. IR (KBr pellet cm-1): 3081, 1671, 1600, 1570, 1435, 1325, 1085, 757 cm-1. 1H NMR (DMSO-d6, δ, ppm): 9.752 (s, 1H), 7.497-7.518 (d, 2H), 7.124-7.145 (d, 2H), 6.943 (s, 2H), 6.775-6.796 (d, 1H), 6.479-6.505 (q, 1H), 6.405-6.410 (d, 1H), 4.656 (s, 1H). HRMS (ESI+): m/z: calcd for C16H11BrN2O2: 364.9896 [M +Na+]; found: 364.0788.
2-Amino-4-(3-cyano-phenyl)-7-hydroxy-4H-chromene-3- carbonitrile (4): Yield: 60%. 170-171°C. IR (KBr pellet cm-1): 3432, 2210, 1662, 1513, 1210, 1051, 760 cm-1. 1H NMR (DMSO-d6, δ, ppm): 9.792 (s, 1H), 7.705-7.723 (d, 1H), 7.671 (s, 1H), 7.495-7.568 (m, 2H), 7.019 (s, 2H), 6.806-6.827 (d, 1H), 6.491-6.517 (q, 1H), 6.423-6.427 (d, 1H), 4.779 (s, 1H). HRMS (ESI+): m/z: calcd for C17H11N3O2: 312.0743 [M+Na+]; found: 312.0754.
Crystal structure determination
According to the evaporation of chloroform solution, suitable single crystals of compound 3 came to hand. The collection of the diffraction data were on a Bruker Smart Apex CCD area detector using a graphite monochromated Mo Kα radiation (λ=0.71073 Å) at room temperature. The structure was solved by using the program SHELXL-97 [9] and Fourier difference techniques, and refined by full-matrix least-squares method on F2. All hydrogen atoms were added academically. The followed Table 1 lists the Crystallographic data for compound 3.
| Formula | C16H11BrN2O2 |
| Mr | 343.17 |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a/Å | 16.2042 (7) |
| b/Å | 5.9590 (3) |
| c/Å | 19.6663 (8) |
| α/° | 90 |
| β/° | 98.699 (1) |
| γ/° | 90 |
| V/Å3 | 1877.15 (15) |
| Z | 4 |
| Dcalc/g·cm-3 | 1.214 |
| μ(Mo Kα)/mm-1 | 2.194 |
| θ range/° | 3.21 to 25.00 |
| Reflections collected | 22935 |
| No. unique data [R(int)] | 3296 |
| No. data with I ≥ 2σ(I) | 2386 |
| R1 | 0.0671 |
| ωR2(all data) | 0.2087 |
| CCDC | 1497253 |
Table 1. Crystal data, data collection and structure refinement of compound 3.
MTT Assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was chosen to use in the anticancer activity of compounds 1-4 and doxorubicin. First 1 × 104 cells per well were seeded in Dulbecco's modified Eagle's medium (DMEM, 100 μL) supplemented with 10% fetal bovine serum in each well of 96-well microculture plates and incubated for 24 h at 37°C in a CO2 incubator. Compounds were deliquated to the planned concentrations in culture medium and added to the wells with respective vehicle control. After 48 h of incubation, MTT (10 μL, 5 mg•mL-1) was replenished each well and the plates continued to be incubated for another 4 h. Then the supernatant from each well was carefully removed, compounds were dissolved in DMSO (100 μL) and absorbance at 570 nm was recorded.
Results and Discussion
Molecular structure
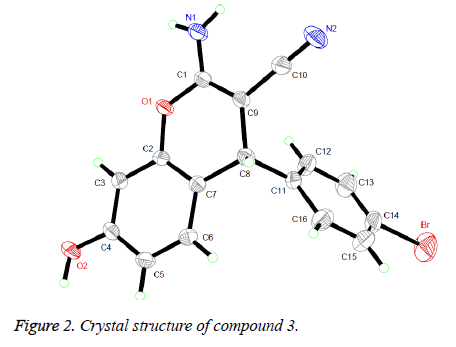
The 1H NMR, IR and MS for the products are in good accordance with the title compounds. In the 1H NMR spectra of compounds 1-4, single peaks at δ 4.619, δ 4.509, δ 5.656 and δ 4.779 ppm characteristic of the CH group respectively. Single crystals of compound 3 were cultured for X-ray diffraction analysis for the purpose of sustaining the configuration of the product.
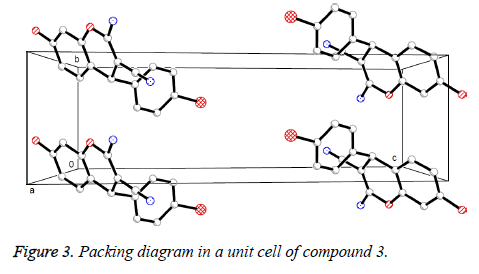
Presented in Figure 2 is the crystal structure of compound 3. The pyran ring and the benzene ring are virtually vertical and is nearly coplanar with the mean plane of the adjacent phenyl ring. Besides, for the compound, the molecules are gathered to form a three-dimensional structure via π-π stacking together with no intermolecular hydrogen bonding interactions, which has been depicted in Figure 3.
In vitro anticancer screening
The evaluation of the four newly synthesized compounds for their in vitro cytotoxic activity against three human osteosarcoma cell lines (HuO9, 143B and MG-63) selected doxorubicin as the reference drug that is based on the method described as reported by Vichai and Kirtikara [10]. The assessment of cytotoxicity was at concentrations of 0, 0.01, 0.1, 10 and 100 μg/mL. The relation between remaining fraction and drug concentration was plotted to obtain survival curves of the three tumor cell lines after accretion of the specified compounds. IC50 is chose as the parameter here for it is in line with the concentration required for 50% inhibition of cell viability. The comparison of IC50 values between the synthesized compounds and the reference drug are listed in Table 2. According to data we obtained, it could be found that among the tested compounds, the compounds 3 and 4 with an IC50 value of 7-10 μM has the most powerful cytotoxic effect against the three human osteosarcoma cell lines than other two compounds. From the antitumor screening results the against three human osteosarcoma cell lines (HuO9, 143B and MG-63), some structure activity relationships can be suggested. The strong electron-withdrawing group (Br, CN) in the phenyl ring in compounds 3 and 4 favors the activity; the strong electron-donating group (OCH3) in the phenyl ring in compound 2 inactive the activity.
| Compounds | Half maximum inhibitory concentration in μM (IC50) | ||
|---|---|---|---|
| HuO9 | 143B | MG-63 | |
| 1 | 30 | 25 | 28 |
| 2 | 28 | 30 | 30 |
| 3 | 7 | 10 | 8 |
| 4 | 8 | 7 | 9 |
| Doxorubicin | 5 | 6 | 6 |
Table 2. Results of in vitro cytotoxic activity of the synthesized compounds and doxorubicin on three human osteosarcoma cell lines.
Conclusion
In summary, four new pyran derivatives were synthesized and their cytotoxic activity against the three human osteosarcoma cell lines was then investigated. Among the tested compounds with IC50 value from 7 to 10 μM, the activity of compounds 3 and 4 was the highest. What is more, it could also come to a conclusion from the antitumor screening that the strong electron-withdrawing group in the phenyl ring favors the activity.
Acknowledgement
This work was supported by grants from the scientific and technological project in Zhejiang province (2016C37123) and medical and health platform research program in Zhejiang province (2012ZDA003).
References
- Bruijnincx PC, Sadler PJ. New trends for metal complexes with anticancer activity. Curr Opin Chem Biol 2008; 12: 197-206.
- Smith B, Land H. Anticancer Activity of the Cholesterol Exporter ABCA1 Gene. Cell Rep 2012; 2: 580-590.
- Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem 2005; 280: 25284-25290.
- Bonsignore L, Loy G, Secci D, Calignano A. Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivatives. Eur J Med Chem 1993; 28: 517-520.
- Khan AT, Lal M, Ali S, Khan MM. One-pot three-component reaction for the synthesis of pyran annulated heterocyclic compounds using DMAP as a catalyst. Tetra Lett 2011; 52: 5327-5332.
- Mochida S, Shimizu M, Hirano K, Satoh T, Miura M. Synthesis of naphtho[1,8-bc]pyran derivatives and related compounds through hydroxyl group directed C-H bond cleavage under rhodium catalysis. Chem-An Asian J 2010; 5: 847-851.
- Mulwad VV, Langi BP, Chaskar AC. Synthesis of novel biologically active heterocyclic compounds from 2-oxo-2H-benzopyran-6-ylimidazolidine. Acta Pol Pharm 2011; 68: 39-47.
- Eshghi H, Zohuri GH, Sandaroos R, Damavandi S. Synthesis of novel benzo[f]chromene compounds catalyzed by ionic liquid. Heterocycl Commun 2012; 18: 67-70.
- Sheldrick GM. SHELXS 97, Program for the Solution of Crystal Structure. University of Göttingen, Germany 1997.
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 2006; 1: 1112-1116.