Research Article - Biomedical Research (2016) Volume 27, Issue 4
Anti-neuron apoptosis of Resina Draconis water extracts on rats with traumatic brain injury
Jincheng Zhang1, Kerong Dai2,3*1Department of Orthopaedics, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, PR. China
2Shanghai Key Laboratory of Orthopaedic Implant, Department of Orthopaedics, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, PR. China
3The Key Laboratory of Stem Cell Biology, Institute of Health Sciences, Shanghai Jiao Tong University School of Medicine (SJTUSM) & Shanghai Institutes for Biological Sciences (SIBS), Chinese Academy of Sciences (CAS), PR. China
- *Corresponding Author:
- Kerong Dai
The Key Laboratory of Stem Cell Biology
Institute of Health Sciences
Shanghai Jiao Tong University School of Medicine (SJTUSM)
& Shanghai Institutes for Biological Sciences (SIBS)
Chinese Academy of Sciences (CAS)
639, ZHIZAOJU road, HUANGPU district
Shanghai, 200011 PR. China
Email: zjc850420@163.com / krdai@163.com
Accepted on April 14, 2016
Abstract
Objectives: This study was to observe the effects of Resina Draconis water extracts (RDWE) on traumatic nerve cell apoptosis, oxidative damage and inflammatory reaction of brain in rats.
Methods: 100 healthy Wistar male rats were selected and randomly divided into sham operation group, brain injury group, Resina Draconis water extract 40 or 80 mg/kg group, 25 rats each group. Making traumatic brain injury animal model by improved Feeney's free falling body injury device. The rats were given 1ml of 0.9% sodium chloride and different doses of Resina Draconis (40 and 80 mg kg-1) by intraperitoneal injections for 5 days. Then killed for blood, taken out the whole brain tissue at 2 h, 6 h, 24 h, 1 d, 3 d and 5 d after traumatic brain injury. Serum superoxide dismutase activity (SOD), concentration of MDA (MDA), interleukin 1β, tumor necrosis factor alpha (TNF-α) and IL- 6 (IL-6) concentration were detected. Brain tissue apoptosis of neural cells were detected by in situ terminal transferase labeling technique, and then statistical analysis.
Results: There were no statistically significant differences (p>0.05) that the rat neuronal apoptosis quantity. The SOD activities and the levels of MDA, IL-1, TNF-α and IL-6 of Resina Draconis (80 mg kg-1) group was significantly decreased (p<0.05) compared with the brain injury group at the five times, but shows no statistically significant differences (p>0.05) in 2 h and 6 h after traumatic brain injury.
Conclusions: It is possible to reduce the free radicals and inflammatory responses in vivo, which could inhibit the apoptosis of nerve cells after traumatic brain injury.
Keywords
Traumatic brain injury, Resina draconis, Anti-neuron apoptosis, MDA.
Introduction
As we know, brain free radical reaction and inflammatory reaction usually intensified after traumatic brain injury. The level of MDA, IL- 1 beta, tumor necrosis factor alpha and IL-6 significantly increased and superoxide dismutase (SOD) activity were significantly decreased. The superposition of free radicals, inflammatory factor and multiple damage factors can lead to “intracellular calcium overload”, cause nerve cell apoptosis [1-5]. Resina Draconis is extracted by wood resin of dracaena cochinchinensis which is dracaena plant of containing fat, anti-inflammatory, analgesic and hemostatic effect. This study was to investigate the effect of Resina Draconis on experimental brain injury reaction of nerve cell apoptosis, free radicals and inflammation in rats and make a preliminary discussion on the mechanism of nerve cell apoptosis and effect of Resina Draconis resistance after traumatic brain injury.
Materials and Methods
Materials
Resina Draconis. (Shaanxi Hui Ke plant development Co., Ltd. Batch number: SF20111215), to crush it, Reflux extraction for 7 hours under 100 and then take the suspension after static. Healthy Wistar rats (Shanghai Laboratory Animal Center, Chinese Academy of Medical Sciences), ♂, clean level, experimental animal license No.: 2012-0005 (Shanghai) SCXK; Cell apoptosis detection kit (Zhongshan Beijing Biological Technology Co., Ltd.); Superoxide dismutase activity detection kit and MDA (MDA) ELISA Kit (Nanjing Jiancheng Biological Engineering Institute); ELISA test kit (R&D systems, USA).
Animals and groups
100 healthy Wistar rats of quality equivalent (250~300 g), ♂were selected and randomly divided into sham operation group, brain injury group, Resina Draconis. Water extract 40 mg/kg group and Resina Draconis. water extract 80 mg/kg group, 25 rats each group, and then divided into five subgroups according to five time points of 2 h, 6 h, 1 d, 3 d and 5 d, 5 rats in each subgroup. Rats were received intraperitoneal anesthesia by sodium pentobarbital. 50 mg/kg, cut the scalp at 1 mm after the right side of the coronary artery, 2 mm next to the middle of the midline. Drill a bone hole of 5 cm in diameter by improved Feeney's free falling body injury device [6,7], with hammer of 40 g from 25 cm free fall impact rod, then sutured scalp. Rats in sham operation group were cut scalp and suture the scalp without striking hammer. Rats in sham operation group and brain injury group were treated with 1 mL sodium chloride injection immediately after the completion of the operation or brain injury model. Infection for five successive days. Intraperitoneal injection of doses of 40 and 80 mg/kg after brain injury with Resina Draconis. water extract group rats, medication five successive days. The rats were anesthetized by sodium pentobarbital intraperitoneal injection (80 mg/kg) at 2 h, 6 h, 1 d, 3 d and 5 d after the formation of traumatic brain injury model. The heads of rats were killed and the whole brains were got out quickly.
Apoptotic cell detection
Detection of apoptosis nerve cells in brain tissue was finished by in situ terminal transferase labeling technique. Nuclear staining was brown or brownish red, which was defined as positive staining of cells. In the Olympus-CH optical microscope to calculate the 10 high-power mirror field of vision, each field of view count 100 cells, a total of 1000 cells, calculate the average positive rate (%).
Serological marker detection
The activity of superoxide dismutase in serum was detected by xanthine oxidase; Determination of MDA in serum by using the method of the determination of serum MDA; The determination of serum IL-1 and tumor necrosis factorαand IL-6 concentrations by using the ELISA method.
Statistical methods
Using SPSS/11.0 software for statistical analysis, experimental
data is expressed with 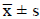 . The analysis of variance was used
to compare between groups, LSD method was used to compare
between the two groups.
. The analysis of variance was used
to compare between groups, LSD method was used to compare
between the two groups.
Results
Traumatic brain injury model on Wistar rats
The activity of serum superoxide dismutase (SOD) was significantly decreased, the concentrations of the serum MDA (MDA), IL-1β, tumor necrosis factor α and IL-6 was significantly increased, the amounts of apoptosis of brain nerve cells increased significantly.
The effect of RDWE on serum MDA levels in rats with traumatic brain injury [8,9]
The concentration of serum MDA in rats has not statistically significant difference (p>0.05) between RDWE (40 mg/kg) group and brain injury group at 2 h, 6 h, 3 d and 5 d after traumatic brain injury, and it was significantly decreased (p<0.05) in Resina Draconis. (80 mg kg-1) group compared with the brain injury group in 24 h, 3 d and 5 d after traumatic brain injury, but no statistical significance (p>0.05) in 2 h and 6 h after traumatic brain injury (Table 1).
| Group | MDA /nmol·mL-1 | ||||
|---|---|---|---|---|---|
| 2h | 6h | 1d | 3d | 5d | |
| Sham operation group | 7.0 ± 0.3 | 7.0 ± 0.4 | 7.0 ± 0.5 | 7.0 ± 0.6 | 7.0 ± 0.5 |
| Traumatic brain injury group | 9.0 ± 0.7 | 10.2 ± 0.8 | 15.5 ± 1.0 | 13.1 ± 1.2 | 11.5 ± 0.9 |
| RDWE 40mg·kg-1 | 8.8 ± 0.6 | 9.7 ± 0.9 | 14.9 ± 1.4 | 12.8 ± 1.2 | 10.6 ± 1.0 |
| RDWE 80mg·kg-1 | 8.7 ± 0.7 | 9.2 ± 1.1 | 13.8 ± 1.2# | 11.5±1.1# | 9.3 ± 0.9# |
| Note: # indicated p<0.05, compared with brain trauma group. | |||||
Table 1. Effects of RDWE on serum MDA levels in the rats with
traumatic brain injury (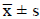 ± s, n=5).
± s, n=5).
The serum SOD activity in the rats has not statistically significant difference (p>0.05) between RDWE (40 mg/kg) group and brain injury group at 2 h, 6 h, 24 h, 1 d, 3 d and 5 d after traumatic brain injury. And it was significantly increased (p<0.05) in Resina Draconis. (80 mg kg-1) group compared with the brain injury group in 1 d, 3 d and 5 d after traumatic brain injury, but no statistical significance (p>0.05) in 2 h and 6 h after traumatic brain injury, (Table 2).
| Group | SOD activity / U·L-1 | ||||
|---|---|---|---|---|---|
| 2h | 6h | 1d | 3d | 5d | |
| Sham operation group | 245.1 ± 5.1 | 243.2 ± 4.2 | 241.5 ± 5.7 | 242.7 ± 5.2 | 240.1 ± 5.3 |
| Traumatic brain injury group | 215.4 ± 11.5 | 193.1 ± 12.7 | 146.1 ± 9.6 | 165.2 ± 13.0 | 179.2 ± 13.7 |
| RDW 40 mg·kg-1 | 210.0 ± 7.3 | 190.4 ± 11.5 | 163.2 ± 10.5 | 174.5 ± 12.1 | 188.2 ± 11.5 |
| RDW 80 mg·kg-1 | 221.5 ± 8.5 | 213.7 ± 9.2# | 194.8 ± 15.1# | 217.2 ± 14.6# | 228.5 ± 13.3# |
| Note: # indicated p<0.05, compared with brain trauma group. | |||||
Table 2. Effects of RDWE on serum SOD activity in the rats with traumatic brain injury (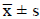 ± s, n=5).
± s, n=5).
Effects of RDWE on serum IL-6 in the rats with traumatic brain injury [10]
The serum IL-6 in the rats has not statistically significant difference (p>0.05) between RDWE (40 mg/kg) group and brain injury group at 2 h, 6 h, 1 d, 3 d and 5 d after traumatic brain injury. And it was significantly decreased (p<0.05) in Resina Draconis (80 mg kg-1) group compared with the brain injury group in 24 h, 1 d, 3 d and 5 d after traumatic brain injury, but no statistical significance (p>0.05) in 2 h and 6 h after traumatic brain injury (Table 3).
| Group | IL-6/pg·mL-1 | ||||
|---|---|---|---|---|---|
| 2h | 6h | 1d | 3d | 5d | |
| Sham operation group | 9.7 ± 1.1 | 9.7 ± 1.2 | 9.6 ± 0.9 | 9.9 ± 1.1 | 9.8 ± 1.3 |
| Traumatic brain injury group | 14.7 ± 2.5 | 15.9 ± 2.5 | 25.4 ± 3.3 | 22.3 ± 3.5 | 20.1 ± 3.5 |
| RDWE 40mg·kg-1 | 14.6 ± 0.8 | 15.1 ± 2.2 | 23.2 ± 1.8 | 19.5 ± 1.5 | 19.1 ± 1.2 |
| RDWE 80mg·kg-1 | 12.5 ± 2.3 | 14.1 ± 0.9 | 19.1 ± 1.3# | 15.3±1.8# | 15.5±1.6# |
| Note: #indicated p <0.05, compared with brain trauma group. | |||||
Table 3. Effects of RDWE on serum IL-6 in the rats with traumatic brain injury (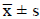 ± s, n=5).
± s, n=5).
Effects of RDWE on serum IL-1β in the rats with traumatic brain injury [11,12]
The serum IL-1β in the rats has not statistically significant difference (p>0.05) between RDWE (40 mg/kg) group and brain injury group at 2 h, 6 h, 1 d, 3 d and 5 d after traumatic brain injury. And it was significantly decreased (p<0.05) in Resina Draconis. (80 mg kg-1) group compared with the brain injury group in 24 h, 1 d, 3 d and 5 d after traumatic brain injury, but no statistical significance (p>0.05) in 2 h and 6 h after traumatic brain injury, seen from Table 4.
| Group | serum IL-1β/ pg·mL-1 | ||||
|---|---|---|---|---|---|
| 2h | 6h | 1d | 3d | 5d | |
| Sham operation group | 279.5 ± 28.1 | 291.8 ± 35.6 | 308.2 ± 31.1 | 294.0 ± 25.3 | 296.6 ± 32.9 |
| Traumatic brain injury group | 441.5 ± 42.3 | 518.5 ± 64.2 | 870.1 ± 68.5 | 713.2 ± 92.3 | 573.2 ± 65.1 |
| RDWE 40 mg·kg-1 | 445.2 ± 33.1 | 542.1 ± 58.3 | 831.2 ± 88.5 | 691.6 ± 56.3 | 551.9 ± 65.2 |
| RDWE 80 mg·kg-1 | 346.5 ± 28.7 | 461.8 ± 39.5 | 702.5 ± 61.4# | 545.2 ± 92.3# | 477.1 ± 93.7# |
| Note: # indicated p<0.05, compared with brain trauma group. | |||||
Table 4. Effects of RDWE on serum IL-1β in the rats with traumatic brain injury (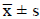 ± s, n=5).
± s, n=5).
Effects of RDWE on serum TNF-α in the rats with traumatic brain injury [13]
The on serum TNF-α in the rats has not statistically significant difference (p>0.05) between RDWE (40 mg/kg) group and brain injury group at 2h6h24 h1d3d and 5 d after traumatic brain injury. And it was significantly decreased (p<0.05) in Resina Draconis. (80 mg kg-1) group compared with the brain injury group in 24 h, 1 d, 3 d and 5 d after traumatic brain injury, but no statistical significance (p>0.05) in 2 h and 6 h after traumatic brain injury (Table 5).
| Group | serum TNF-α/ pg·mL-1 | ||||
|---|---|---|---|---|---|
| 2h | 6h | 1d | 3d | 5d | |
| Sham operation group | 825.6 ± 43.2 | 831.5 ± 44.6 | 841.2 ± 56.3 | 835.7 ± 52.7 | 843.9 ± 67.1 |
| Traumatic brain injury group | 1185.2 ± 126.4 | 1526.7 ± 141.5 | 2479.3 ± 223.6 | 1874.2 ± 236.1 | 1671.3 ± 137.9 |
| RDWE 40 mg·kg-1 | 1059.3 ± 115.8 | 1531.1 ± 128.5 | 2147.3 ± 219.1 | 1826.4 ± 148.2 | 1381.5 ± 138.9 |
| RDWE 80 mg·kg-1 | 1183.6 ± 167.3 | 1412.1 ± 1512.7 | 1672.5 ± 208.4# | 1147.2 ± 139.4# | 1365.1 ± 117.6# |
| Note: # indicated p<0.05, compared with brain trauma group. | |||||
Table 5. Effects of RDWE on serum TNF-α in the rats with traumatic brain injury (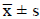 ± s, n=5).
± s, n=5).
Effects of RDWE on the amount of neuronal cell apoptosis in the rats with traumatic brain injury [14]
The amount of neuronal cell apoptosis in the rats has not statistically significant difference (p>0.05) between RDWE (40 mg/kg) group and brain injury group at 2 h, 6h, 1d, 3d and 5 d after traumatic brain injury. And it was significantly decreased (p<0.05) in Resina Draconis. (80 mg kg-1) group compared with the brain injury group in 1 d, 3 d and 5 d after traumatic brain injury, but no statistical significance (p>0.05) in 2 h and 6 h after traumatic brain injury (Table 6).
| Group | Neuronal cell apoptosis /% | ||||
|---|---|---|---|---|---|
| 2h | 6h | 1d | 3d | 5d | |
| Sham operation group | 9.2±0.5 | 9.1±0.6 | 9.1±0.4 | 9.2±0.5 | 9.3±0.7 |
| Traumatic brain injury group | 19.5±2.2 | 23.4±2.5 | 41.3±3.6 | 35.8±2.9 | 28.4±1.7 |
| RDWE 40mg·kg-1 | 18.1±3.4 | 22.5±1.7 | 37.2±3.6 | 32.3±3.1 | 25.9±3.8 |
| RDWE 80mg·kg-1 | 17.6±2.7 | 20.4±1.5 | 27.1±2.9# | 24.8±2.2# | 21.1±2.4# |
| Note: # indicated p<0.05, compared with brain trauma group. | |||||
Table 6. Effects of RDWE on the amount of neuronal cell apoptosis in the rats with traumatic brain injury (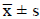 ± s, n=5).
± s, n=5).
Discussion
The pathological and physiological mechanism of brain injury is complex, free radical reaction, inflammatory reaction and apoptosis are important components of secondary brain injury after traumatic brain injury. After traumatic brain injury, Free radical production, inflammatory reaction increased, leading to neuronal apoptosis, which further aggravate the traumatic brain injury, affecting the prognosis. The current study gradually found that peripheral superoxide dismutase, MDA (MDA), IL-1β, serum TNF-α and IL-6 and nerve cell apoptosis has a direct relationship, and the changes in their levels can be significant influences on nerve cell apoptosis process. The study of the anti-lipid peroxidation effect of Resina Draconis phenol on tissues erythrocytes of rats also obtained the same conclusion. The dragon Resina Draconis phenols have scavenged effects on oxygen free radicals, and can inhibit the damage to the body. In addition, it can increase the level of cAMP in plasma, and reduce the level of cGMP in plasma. Resina Draconis in the treatment of hemorrhagic diseases has bidirectional regulation effect, and has strong antiinflammatory and analgesic, can promote the repair of skin and enhance the role of immunity of human body. At the same time, plant defensins have inhibitory effect to many bacteria, and it can promote the body metabolism, and then achieve hemostasis, pain, swelling, and ultimately cure hemorrhoids objective.
This study found serum superoxide dismutase activity significantly decreased, serum MDA, IL-1β and TNF-α and IL-6 concentration increased and the number of apoptotic cells was significantly increased in brain tissue after traumatic brain injury. It showed that free radical production, inflammatory response increased, apoptosis of neurons increased. Further research found that high dose (80 mg/kg) intraperitoneal injection of Resina Draconis. Water extract can obviously reduce apoptosis, inflammation and free radical reaction in rats with traumatic brain injury. Previous studies have found that the ethanol extract from Resina Draconis can significantly inhibit the level of NOPGE2IL-1β and TNF-α stimulated by the lipopolysaccharide (LPS). And iNOS and COX-2 expression was down regulated by the P65 gene of the NF-κB pathway, thus inhibiting the release of NO and PGE2. NO is involved in cellular factors, especially the cell associated with inflammation. In the inflammatory response, the regulation of the child has cytotoxicity and anti inflammation, double action, monocyte macrophages, and vascular smooth muscle cells. LPS, TNF- alpha, IL-1 and other functions, can produce a large number of NO. In addition, the ethanol extract of Resina Draconis. has obvious antioxidation effect and can induced overexpression of macrophage heme enzyme-1.
At the same time, the total phenolic of Resina Draconis. pills can significantly reduce brain infarct volume, improve brain function. It showed that the drug can increase cerebral blood flow, so as to enhance the brain tissue oxygen supply and the level of energy metabolism. This effect may relate to the effect of anti-thrombosis, inhibiting platelet aggregation, reducing the level of fibrinogen of the Resina Draconis [15]. To alleviate the pain and urgent to animals, avoid animals subjected to unnecessary pain, so as not to affect the experimental results, the rats were given deep anesthesia by 80 mg/kg sodium pentobarbital intraperitoneal. We calculated that the water extracts productivity was 5% (5 g extracts from 1 kg crude medicine). There was no pain on rats, which demonstrated during the operation process, thus ensuring the stability of test data. Therefore, Resina Draconis. water extracts could significantly reduce the inflammation and free radical reaction in rats with traumatic brain injury, thus it could inhibit apoptosis of nerve cells and showed a significant neuroprotection activity.
Acknowledgement
This work was supported by grant from the Chinese Academy of Sciences (No.XDA01030502) and a grant from the National Natural Science Foundation of China (No. 81190133)
References
- Meng XE, Zhang Y, Li N, Fan DF, Yang C, Li H, Guo DZ, Pan SY. Effects of hyperbaric oxygen on the Nrf2 signaling pathway in secondary injury following traumatic brain injury. Genet Mol Res 2016.
- Tian R, Hou Z, Hao S, Wu W, Mao X, Tao X, Lu T, Liu B. Hydrogen-rich water attenuates brain damage and inflammation after traumatic brain injury in rats.Brain Res 2016.
- Meng XE, Zhang Y, Li N, Fan DF, Yang C, Li H, Guo DZ, Pan SY. Hyperbaric Oxygen Alleviates Secondary Brain Injury After Trauma Through Inhibition of TLR4/NF-κBSignaling Pathway. Med SciMonit2016;22:284-288.
- Wang B, Armstrong JS, Reyes M. White matter apoptosis is increased by delayed hypothermia and rewarming in a neonatal piglet model of hypoxic ischemic encephalopathy. Neuroscience 2016; 316:296-310.
- Kim JY, Choi K, Shaker MR. Promotion of Cortical Neurogenesis from the Neural Stem Cells in the Adult Mouse Subcallosal Zone. Stem Cells 2015.
- Tsai W, Hsieh H, Chen C.Characterization of the antiplatelet effects of (2S)-5-methoxy-6-methylflavan-7-ol from DraconisResina. Eur J Pharmacol1998;346: 103-110.
- Ren W, Jing G, Shen Q. Occludin and connexin 43 expression contribute to the pathogenesis of traumatic brain edema. Neural Regen Res 2013; 8: 2703-2712.
- Orhan N, UgurYilmaz C, Ekizoglu O. Effects of beta-hydroxybutyrate on brain vascular permeability in rats with traumatic brain injury. Brain Res 2016; 1631:113-26.
- Deryugina AV, Krylov VN, Shumilova AV. Using Mexicor To Correct The Functional Parameters Of Red Blood Cells In Rats With Traumatic Brain Injury ModelEkspKlinFarmakol 2015; 78:14-17.
- Meng XE, Zhang Y, Li N. Hyperbaric Oxygen Alleviates Secondary Brain Injury After Trauma Through Inhibition of TLR4/NF-κBSignaling Pathway. Med SciMonit2016;22:284-288.
- Dash PK, Zhao J, Kobori N. Activation of Alpha 7 Cholinergic Nicotinic Receptors Reduce Blood-Brain Barrier Permeability following Experimental Traumatic Brain Injury. J Neurosci 2016; 36:2809-2818.
- Heo SK, Yi H, Yun H. Ethylacetate extract from DraconisResina inhibits LPS-induced inflammatory responses in vascular smooth muscle cells and macrophages via suppression of ROS production. Food and Chemical Toxicology 2010; 48: 1129-1136.
- Feng Y, Cui Y, Gao JL. Resveratrol attenuates neuronal autophagy and inflammatory injury by inhibiting the TLR4/NF-κBsignaling pathway in experimental traumatic brain injury. Int J Mol Med 2016.
- Dash PK, Zhao J, Kobori N. Activation of Alpha 7 Cholinergic Nicotinic Receptors Reduce Blood-Brain Barrier Permeability following Experimental Traumatic Brain Injury. J Neurosci 2016; 36:2809-2818.
- Shu L, Wang C, Wang J. The neuroprotection of hypoxic preconditioning on rat brain against traumatic brain injury by up-regulated transcription factor Nrf2 and HO-1 expression. NeurosciLett 2016; 611:74-80.