Research Article - Biomedical Research (2018) Volume 29, Issue 12
Antimicrobial sensitivity pattern of pathogenic bacteria isolated from older women with asymptomatic bacteriuria
Ahmed Abduljabbar Jaloob Aljanaby1* and Israa Abduljabbar Jaloob Aljanaby2
1Department of Biology, Faculty of Science, University of Kufa, Iraq
2College of Pharmacy, University of Kufa, Iraq
- *Corresponding Author:
- Ahmed Abduljabbar Jaloob Aljanaby
Department of Biology
Faculty of Science
University of Kufa, Iraq
Accepted date: May 15, 2018
DOI: 10.4066/biomedicalresearch.29-18-601
Visit for more related articles at Biomedical ResearchAbstract
Objectives: Asymptomatic bacteriuria is one of the most important silent diseases frequently infect women in worldwide.
Aims: To determine the antimicrobial susceptibility pattern of pathogenic bacteria isolated from older women with asymptomatic bacteriuria.
Materials and Methods: A total of 263 culture positive bacterial isolates from 862 urine samples were collected from older women (60-70 years old) admitted to Al-Najaf hospital in Al-Najaf Governorate, Iraq during January 2016 to December 2017. All bacterial isolates were identified according to standard microbiological tests. Antimicrobial susceptibility test was done according to method by Kirby-Bauer.
Results: Out of 263 culture positive urine samples, Escherichia coli was the most dominant bacterial isolates 109 (41.44%) followed by Klebsiella pneumoniae 85 (32.32%), Acinetobacter baumannii 32 (12.16%), Pseudomonas aeruginosa 18 (6.84%), Serratia marcescens 11 (4.18%) and Staphylococcus saprophyticus 8 (3.06%). Among all antimicrobials, Imipenem 10 μg showed the highest activity (100%) against E. coli, S. marcescens and S. saprophyticus isolates While, Amikacin 30 μg showed the highest activity (100%) against S. marcescens only. Most bacterial isolates 240 (91.3%) were multi-drug resistance (MDR), 16 isolates (6%) and 7 isolates (2.7%) were extensive drug-resistance (XDR) and pandrug- resistance (PDR), respectively.
Conclusions: There were many older women infected with asymptomatic bacteriuria caused by bacteria with high resistant to different antimicrobials; therefore, the antimicrobial sensitivity test must be done periodically.
Keywords
Asymptomatic bacteriuria, Older women, Antimicrobials, Pathogenic bacteria
Introduction
Urinary tract infection (UTI) is one of the most important common infections in worldwide [1]. Older women and children are the most infected with this infection [2,3]. Urinary tract infection is broadly define as an infection of the urinary system and may involve the lower urinary tract or the upper urinary tract or both [4,5]. Multi-drug resistance pathogenic bacteria such as E. coli and K. pneumoniae are the main pathogens cause urinary tract infection [6,7]. The presence of pathogenic bacteria in the urine of an individual without symptoms of urinary tract infections is defined as a symptomatic bacteriuria [8]. Asymptomatic bacteriuria is generally not recommended in all times only during in some cases such as in pregnancy and at preoperative of men before urological procedure [9,10]. Overuse of different antimicrobials in asymptomatic bacteriuria treatment in older women infected with recurrent urinary tract infections is common, in whom antibiotics are routinely administered, therefore, most pathogenic bacteria cause asymptomatic bacteriuria characterized by highly resistant against many antimicrobials [11,12]. In Iraq, there are no data focuses on the prevalence of asymptomatic bacteriuria, which is among the facilitative factors urinary tract infections and no data on the relationship between asymptomatic bacteriuria treatment and the risk of higher antibiotic resistance. Therefore, the aim of this study was to investigations of the prevalence of asymptomatic bacteriuria in older women and the antimicrobials resistance pattern of pathogenic bacteria isolated from this infection during two years in Al-Najaf Governorate, Iraq.
Materials and Methods
Study design and women selection
This is a cross-sectional study carried out in Al-Najaf hospital in laboratory of microbiology in Al-Najaf Governorate, Iraq during January 2016 to December 2017. A total of 862 older women (60-70 years old) were included in this study. All women were not suffering from any signs and symptoms of urinary tract infections, not received any antibiotic treatments for any reason within the last one week and accepted being a part in the study.
Samples collection, bacterial identification and antimicrobials susceptibility test
Eight hundred and sixty two midstream urine samples were collected from women (two samples from each women for two days) in sterile disposable containers (Hi-media, India), immediately the urine samples were streaked by loop (Himedia, India) onto blood agar (Oxoid, UK) surface and MacConkey agar (Oxoid,UK) surface and incubated under aerobic conditions at 37°C for 24 h. If the single bacterial isolate were in titer ≤ 105 colony forming units were considered as a positive growth. All bacterial isolates were identified according to standard microbiological methods [13,14]. Antimicrobials susceptibility testing was determined by a disc diffusion test method (Kirby-Bauer) [15]. Antimicrobial susceptibility and resistance was determined according to CLSI guidelines (2017) [16] according to strain growth zone diameter.
Statistical analysis
Statistical analysis was preforming according to (SPSS) version 12.0 for windows software to compare (in percentages) between the prevalence of pathogenic bacteria isolated from women and between sensitive and resistance of pathogenic bacteria to antimicrobials.
Results
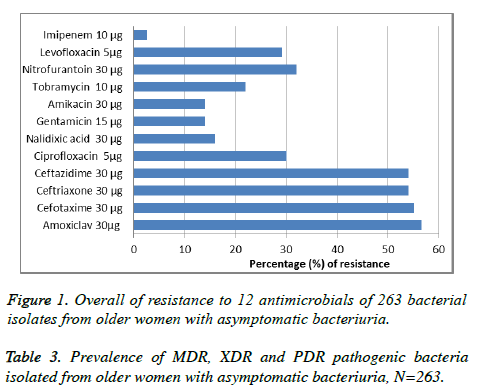
Of the 862 urine samples were collected from women, 263 were positive for bacterial growth (30.5% of women were infected with asymptomatic bacteriuria). The most frequently isolated bacteria included E. coli (41.44%), K. pneumoniae (32.32%), A. baumannii (12.16%), P. aeruginosa (6.84%), S. marcescens (4.18%) and S. saprophyticus (3.06%) (Table 1). Among the antimicrobials used for susceptibility testing, Imipenem 10 μg showed the full activity (100%) against S. marcescens and S. saprophyticus, While, Amikacin 30 μg showed the highest activity (100%) against S. saprophyticus only. Most isolates were resistance to Amoxiclav 30μg, Cefotaxime 30 μg, Ceftriaxone 30 μg and Ceftazidime 30 μg with highly percentages (<50%). The antimicrobials susceptibility pattern of all isolates is shown in Table 2 and Figure 1. Among all 263 isolates, 240 (91.3%) were MDR, 16 (6%) XDR and 7 (2.7%) PDR, The resistance type of all isolates is shown in Table 3.
| E. coli | 109 (41.44) |
| K. pneumoniae | 85 (32.32) |
| A. baumannii | 32 (12.16) |
| P. aeruginosa | 18 (6.84) |
| S. marcescens | 11 (4.18) |
| S. saprophyticus | 8 (3.06) |
| Total | 263 (100) |
Table 1. Numbers and percentages of pathogenic bacteria isolated from older women with asymptomatic bacteriuria.
| Antimicrobials | E. coli (N=109) | K. pneumoniae (N=85) | A. baumannii (N=32) | P. aeruginosa (N=18) | S. marcescens (N=11) | S. saprophyticus (N=8) |
|---|---|---|---|---|---|---|
| Amoxiclav 30 µg | 45 (41.2) | 40 (47) | 14 (43.7) | 8 (44.4) | 6 (45.5) | 1 (12.5) |
| Cefotaxime 30 µg | 40 (36.6) | 42 (49.4) | 20 (62.5) | 7 (38.8) | 7 (63.6) | 2 (25) |
| Ceftriaxone 30 µg | 43 (39.4) | 45 (52.9) | 17 (53.1) | 7 (38.8) | 8 (72.7) | 2 (25) |
| Ceftazidime 30 µg | 43 (39.4) | 48 (56.4) | 15 (46.8) | 6 (33.3) | 7 (63.6) | 2 (25) |
| Ciprofloxacin 5 μg | 88 (80.7) | 55 (64.7) | 20 (62.5) | 10 (55.5) | 8 (72.7) | 3 (37.5) |
| Nalidixic acid 30 µg | 97 (88.9) | 74 (87) | 28 (87.5) | 8 (44.4) | 8 (72.7) | 6 (75) |
| Gentamicin 15 µg | 90 (82.5) | 77 (90.5) | 28 (87.5) | 16 (88.8) | 9 (81.8) | 6 (75) |
| Amikacin 30 µg | 91 (83.4) | 74 (87) | 28 (87.5) | 15 (83.3) | 11 (100) | 7 (87.5) |
| Tobramycin 10 µg | 89 (81.6) | 70 (82.3) | 20 (62.5) | 11 (61.1) | 8 (72.7) | 7 (87.5) |
| Nitrofurantoin 30 µg | 78 (71.5) | 65 (76.4) | 18 (56.2) | 9 (50) | 6 (45.5) | 3 (37.5) |
| Levofloxacin 5 μg | 85 (77.9) | 65 (76.4) | 19 (59.3) | 8 (44.4) | 5 (45.4) | 4 (50) |
| Imipenem 10 µg | 109 (100) | 80 (94.1) | 31 (96.8) | 17 (94.4) | 11 (100) | 8 (100) |
Table 2. Numbers and percentages of pathogenic bacteria isolated from older women with asymptomatic bacteriuria that were sensitive to antimicrobials. N=263.
| MDR | XDR | PDR | |
|---|---|---|---|
| E. coli (N=109) | 106 (97.2) | 3 (2.7) | 0 (0.0) |
| K. pneumoniae (N=85) | 71 (83.5) | 9 (10.5) | 5 (5.8) |
| A. baumannii (N=32) | 28 (87.5) | 3 (9.3) | 1 (3.1) |
| P. aeruginosa (N=18) | 16 (88.9) | 1 (5.5) | 1 (5.5) |
| S. marcescens (N=11) | 11 (100) | 0 (0.0) | 0 (0.0) |
| S. saprophyticus (N=8) | 8 (100) | 0 (0.0) | 0 (0.0) |
| Total 263 (100) | 240 (91.3) | 16 (6) | 7 (2.7) |
Table 3. Prevalence of MDR, XDR and PDR pathogenic bacteria isolated from older women with asymptomatic bacteriuria, N=263.
Discussion
This is the first study in Iraq (to the best of our knowledge) aimed to investigate the prevalence of older women infected with asymptomatic bacteriuria caused by pathogenic bacteria and antimicrobial susceptibility pattern of these pathogens during two years. According to our data, of the 862 older women, 263 (30.5%) were infected with asymptomatic bacteriuria, this result is similar to the previous reports [17-20]. Mostly, asymptomatic bacteriuria is common in the older women over than 60 years old; many factors may be lead to asymptomatic bacteriuria such as; age related in urologic function, multiple comorbid chronic disease and neurogenic bladder caused by various pathologies [21]. In this study as shown in table 1, of the 263 positive culture, E. coli 109 (41.44%) and K. pneumoniae 85 (32.32%) were the most predominant bacteria were responsible of asymptomatic bacteriuria, while S. saprophyticus was the lowest predominant bacteria 8 (30.06%). These results are in agreement with previous studies [22,23]. Many members of enterobacteriaceae such as E. coli, K. pneumoniae, P. aeruginosa and A. baumannii are able to cause recurrent urinary tract infections lead to asymptomatic bacteriuria [24,25]. Mainly, recurrent urinary tract infections are caused by gram-negative bacteria as well as by gram-positive bacteria such as uropathogenic E. coli, K. pneumoniae, S. saprophyticus and P. aeruginosa [26-28]. These uropathogens have the ability to bind to the bladder epithelium and form biofilms-like intracellular bacterial communities that are responsible for colonization and persistence and protect their members from antibiotics and neutrophils in urinary tract and lead to cause infection [29,30]. In this study, most bacterial isolates were sensitive to Imipenem 10 μg, Amikacin 30 μg, Gentamicin 15 μg and Nalidixic acid 30 μg, respectively as shown in Table 3 and Figure 1. Klebsiella pneumoniae, A. baumannii and P. aeruginosa were the most pathogens resistant to most antimicrobials. Amoxiclav 30 μg, Cefotaxime 30 μg, Ceftriaxone 30 μg and Ceftazidime 30 μg were with low activity against all isolates. These results are in agreement with many previous studies [31-34]. The recurrent urinary tract infection is one of the most serious problems particularly in older individuals who are living in developing countries [35]. The overuse of the same antimicrobials such as 3rd and 4th generation cephalosporins against same bacteria without using of antimicrobial sensitivity test lead to cause asymptomatic bacteriuria and emerging of new bacterial strains with highly resistance to different antimicrobials [36-38]. Our finding in the current study showed that among 263 bacterial isolates, 240 (91.3%) were MDR (bacteria resist three different classes of antimicrobials), E. coli 3 (2.7%), K. pneumoniae 9 (10.5%), A. baumannii 3 (9.3%) and P. aeruginosa 1 (5.5%) were sensitive to only one or two types of antimicrobials (XDR isolates), while K. pneumoniae 5 (5.8%), A. baumannii 1 (3.1%) and P. aeruginosa 1 (5.5%) were resistance to all types of antimicrobial classes (PDR isolates) (Table 3). These results are similar with other studies in different countries [39-41]. Multi-drug resistance gram negative and gram positive bacteria became the most problem numerous pathogens cause serious infections worldwide such as burns and urinary tract infections [42,43]. Recently, extensive and pan- drug resistance bacteria such as K. pneumoniae , A. baumannii and P. aeruginosa were considered are the most problem pathogens resist about 98% of antimicrobials [44]. Many reasons lead to increase the prevalence of MDR, XDR and PDR uropathogenic bacteria include, the incorrect administration of antimicrobials and increase in the production of beat-lactamases enzymes by pathogenic bacteria and lack of good controlling mechanisms in hospitals [45].
Conclusion
In Iraq, asymptomatic bacteriuria is a common neglected disease among older women over than 60 years old. E. coli is the most commonly bacterium were isolated. Klebsiella pneumoniae, A. baumannii and P. aeruginosa are a highly dangerous drug resistant pathogens cause this silent infection. In spite of imipenem 10 μg is expensive, but is still one of the best antimicrobials against different pathogenic bacteria cause asymptomatic bacteriuria.
Limitations
The definition of asymptomatic bacteriuria relies on a single urine sample. This is not entirely in accordance with the Infectious Diseases Society of America guidelines for the diagnosis of asymptomatic bacteriuria in adults that requires two consecutive urine specimens with isolation of the same bacterial strain in women.
References
- Rezaee MA, Abdinia B. Etiology and antimicrobial susceptibility pattern of pathogenic bacteria in children subjected to UTI: A referral hospital-based study in Northwest of Iran. Medicine 2015.
- Ma JF, Shortliffe LM. Urinary tract infection in children: etiology and epidemiology. Urol Clin North Am 2004; 31: 517-526.
- Zorc JJ, Kiddoo DA, Shaw KN. Diagnosis and management of pediatric urinary tract infections. Clin Microbiol Rev 2005; 18: 417-422.
- Dulczak S, Kirk J. Overview of the evaluation, diagnosis and management of urinary tract infections in infants and children. Urol Nurs 2005; 25: 185-191.
- Sobel JD, Kaye D. Urinary tract infections. In: Mandell G, Bennett JC, Dolin R (Eds). Mandell, Douglas, and Bennett’s: Principles and Practice of Infectious Disease. 7. Philadelphia, PA: Elsevier 2010.
- Merino-Bohorquez V, Docobo-Pérez F, Sojo J, Morales I, Lupión C, Martín D, Cameán M, Hope W, Pascual Á, Rodríguez-Baño J. Population pharmacokinetics and pharmacodynamics of fosfomycin in non-critically ill patients with bacteremic urinary infection caused by multidrug-resistant Escherichia coli. Clin Microbiol Infect 2018; 18: 30153-30158.
- Aljanaby AAJ, Alhasnawi HMRJ. Phenotypic and molecular characterization of multidrug resistant Klebsiella pneumoniae isolated from different clinical sources in Al-Najaf province-Iraq. Pak J Biol Sci 2017; 20: 217-232.
- Cai T, Nesi G, Mazzoli S, Meacci F, Lanzafame P, Caciagli P, Mereu L, Tateo S, Malossini G, Selli C, Bartoletti R. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infections. Clin Infect Dis 2015; 61: 1655-1661.
- Nicolle LE. Asymptomatic bacteriuria: review and discussion of the IDSA guidelines. Int J Antimicrob Agents 2006; 28: S42-S48.
- Dalal S, Nicolle L, Marrs CF, Zhang L, Harding G, Foxman B. Longterm Escherichia coli asymptomatic bacteriuria among women with diabetes mellitus. Clin Infect Dis 2009; 49: 491-497.
- Cai T, Mazzoli S, Mondaini N, Meacci F, Nesi G, D'Elia C, Malossini G, Boddi V, Bartoletti R. The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: to treat or not to treat? Clin Infect Dis 2012; 55: 771-777.
- Raz R. Asymptomatic bacteriuria: clinical significance and management. Int J Antimicrob Agents 2003; 22: 45-47.
- MacFaddin JF. Biochemical tests for identification of medical bacteria, 3rd (Edn), Williams and Wilkins, Philadelphia 2000.
- Mahon CR, Lehman DC, Manuselis G. Textbook of diagnostic microbiology. 3rd (Edn). Philadelphia: W.B. Saunders Company 2007.
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by standard single disc method. Am J Clin Pathol 1966; 45: 493-496.
- Performance standards for antimicrobial susceptibility testing; twenty-third informational supplement. USA: Clinical and Laboratory Standards Institute (CLSI), 2017.
- Lin Y, Chen L, Lin M, Hwang S. Asymptomatic Bacteriuria among the institutionalized elderly. J Chin Med Assoc 2006; 69: 213-217.
- Rodhe N, Englund L, Mölstad S, Samuelsson E. Bacteriuria is associated with urge urinary incontinence in older women. Scand J Prim Health Care 2008; 26: 35-39.
- Mody L, Juthani-Mehta M. Urinary tract infections in older women: A clinical review. JAMA 2014; 311: 844-854.
- Lorenzo Gómez MF, Collazos Robles RE, Virseda Rodríguez ÁJ, García Cenador MB, Mirón Canelo JA, Padilla Fernández B. Urinary tract infections in women with stress urinary incontinence treated with transobturator suburethral tape and benefit gained from the sublingual polibacterial vaccine. Ther Adv Urol 2015; 7: 180-185.
- O’Donnell JA, Hofmann MT. How to manage nursing home patients with or without chronic catheterization. Geriatrics 2002; 57: 45-58.
- Tabasi M, Karam MR, Habibi M, Mostafavi E, Bouzari S. Genotypic Characterization of virulence factors in Escherichia coli isolated from patients with acute cystitis, pyelonephritis and asymptomatic bacteriuria. J Clin Diagn Res 2016; 10: DC01-DC07.
- Ekwedigwe KC, Sunday-Adeoye I, Eliboh MO, Isikhuemen ME, Uro-Chukwu H, Ezeonu P, Daniyan ABC, Yakubu EN. Prevalence and antimicrobial susceptibility of asymptomatic bacteriuria among women with pelvic organ prolapse in Abakaliki, South-East Nigeria. BMC Womens Health 2018; 18: 53.
- Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am 2014; 28: 1-13.
- Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015; 13: 269-284.
- Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med 2002; 113: 14S-19S.
- Nielubowicz GR, Mobley HL. Host-pathogen interactions in urinary tract infection. Nature Rev Urol 2010; 7: 430-441.
- Ayelign B, Abebe B, Shibeshi A, Meshesha S, Shibabaw T, Addis Z, Gelaw A, Dagnew M. Bacterial isolates and their antimicrobial susceptibility patterns among pediatric patients with urinary tract infections. Turk J Urol 2018; 44: 62-69.
- Kalas V, Hibbing ME, Maddirala AR, Chugani R, Pinkner JS, Mydock-McGrane LK, Conover MS, Janetka JW, Hultgren SJ. Structure-based discovery of glycomimetic FmlH ligands as inhibitors of bacterial adhesion during urinary tract infection. Proc Natl Acad Sci U S A 2018; 115: E2819-E2828.
- Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 2003; 301: 105-107.
- Khan IU, Mirza IA, Ikram A, Afzal A, Ali S, Hussain A, Fayyaz M, Ghafoor T. Antimicrobial susceptibility pattern of bacteria isolated from patients with urinary tract infection. J Coll Physicians Surg Pak 2014; 24: 840-844.
- Aljanaby AAJ, Aljanaby IAJ. Profile of antimicrobial resistance of aerobic pathogenic bacteria isolated from different clinical infections in Al-Kufa Central Hospital-Iraq during period from 2015 to 2017. Res J Pharm Tech 2017; 10: 3264-3270.
- Galindo-Méndez M. Molecular characterization and antimicrobial susceptibility pattern of extended-spectrum β-lactamase-producing Escherichia coli as cause of community acquired urinary tract infection. Rev Chilena Infectol 2018; 35: 29-35.
- Rizwan M, Akhtar M, Najmi AK, Singh K. Escherichia coli and Klebsiella pneumoniae sensitivity/resistance pattern towards antimicrobial agents in primary and simple urinary tract infection patients visiting University Hospital of Jamia Hamdard New Delhi. Drug Res 2018.
- Aljanaby AA, Gafil FA. Effect of different antibiotics on aerobic pathogenic bacteria and urinary tract infection in Al-Manathera City, Iraq: a comparative study. Res Chem Intermed 2013; 39: 3679-3687.
- Alemu A, Moges F, Shiferaw Y, Tafess K, Kassu A, Anagaw B, Agegn A. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at University of Gondar Teaching Hospital, Northwest Ethiopia. BMC Res Notes 2012; 5: 197.
- Spellberg B. The future of antibiotics. Crit Care 2014; 18: 228.
- Gessese YA, Damessa DL, Amare MM, Bahta YH, Shifera AD, Tasew FS, Gebremedhin EZ. Urinary pathogenic bacterial profile, antibiogram of isolates and associated risk factors among pregnant women in Ambo town, Central Ethiopia: a cross-sectional study. Antimicrob Resist Infect Control 2017; 6: 132.
- Liu SW, Xu XY, Xu J, Yuan JY, Wu WK, Zhang N, Chen ZL. Multi-drug resistant uropathogenic Escherichia coli and its treatment by Chinese medicine. Chin J Integr Med 2017; 23: 763-769.
- Kulkarni SR, Peerapur BV, Sailesh KS. Isolation and antibiotic susceptibility pattern of Escherichia coli from urinary tract infections in a tertiary care hospital of north eastern karnataka. J Nat Sci Biol Med 2017; 8: 176-180.
- Bardoloi V, Yogeesha Babu KV. Comparative study of isolates from community-acquired and catheter-associated urinary tract infections with reference to biofilm-producing property, antibiotic sensitivity and multi-drug resistance. J Med Microbiol 2017; 66: 927-936.
- Aljanaby AAJJ. Antibacterial activity of an aqueous extract of Petroselinum crispum leaves against pathogenic bacteria isolated from patients with burns infections in Al-najaf Governorate, Iraq. Res Chem Intermed 2013; 39: 3709.
- Aljanaby AAJ. Antibacterial activity of an aqueous extracts of Alkanna tinctoria roots against drug resistant aerobic pathogenic bacteria isolated from patients with burns infections. ROMJ 2018; 7: 1-6.
- Eriksson I, Gustafson Y, Fagerström L, Olofsson B. Do urinary tract infections affect morale among very old women? Health Qual Life Outcomes 2010; 8: 73.
- Ndihokubwayo JB, Yahaya A, Desta AT, Ki-Zerbo G. Antimicrobial resistant in Africa region: Issues, challenges and action proposed. Africa Healt Motion 2013; 16: 27-31.